Candida species are part of the normal human microbiota. However, in recent years, nosocomial bloodstream Candida infections have emerged as a significant problem ranking the fourth common cause of fungemia in intensive care units. Although microdilution methods are the ones recommended for susceptibility testing, they are difficult to undertake in the clinical practice. Thus, an automated commercially available test is ideal.
AimsTo compare minimum inhibitory concentrations (MICs) obtained with the recently introduced Vitek 2 yeast susceptibility system card (AST-YS01) with Etest.
Methods263 clinical Candida isolates representing six species were included in the study. Categorical agreements (CA) were assessed as described elsewhere.
ResultsIrrespective of the Candida species tested, the overall CA between Vitek 2 and Etest ranged between 66.7% and 100%. In general, Etest yielded lower MICs than Vitek 2. For Candida albicans, the CA between Vitek 2 and Etest was >95% for amphotericin B, voriconazole and flucytosine, but only 89% for fluconazole. With respect to Candida glabrata, the CA was between 97% and 100%. The major errors were with Candida krusei and flucytosine and Candida kefyr and amphotericin B. Candida tropicalis susceptibility for fluconazole by Vitek 2 reported more SDD and resistant strains than Etest. Candida parapsilosis showed 100% CA against all the four antifungals tested. No very major errors were detected between the two methods.
ConclusionsVitek 2 provided comparable results to Etest with quick turnaround for the testing of Candida species susceptibilities.
Candida forma parte de la microbiota habitual del ser humano. Sin embargo, en los últimos años, las candidemias hospitalarias se han convertido en un problema significativo en las unidades de cuidados intensivos al ocupar el cuarto lugar entre las fungemias. Puesto que los métodos de microdilución, recomendados para las pruebas de sensibilidad in vitro, son difíciles de realizar en la práctica clínica, las pruebas comerciales y automatizadas son las de uso ideal.
ObjetivosComparar las concentraciones mínimas inhibidoras (CMI) obtenidas por los métodos Vitek 2 (AST-YS01) y Etest.
MétodosSe utilizaron 263 cepas clínicas de Candida, pertenecientes a seis especies. Se evaluaron los acuerdos categóricos (AC) según lo ya descrito.
ResultadosCon independencia de la especie de Candida, el AC general entre Vitek 2 y Etest osciló entre el 66,7 y el 100%. En general, Etest arrojó CMI más bajas que las de Vitek 2. Para Candida albicans el AC entre Vitek 2 y Etest fue >95% con la anfotericina B, el voriconazol y la flucitosina, pero solo del 89% con el fluconazol. Con Candida glabrata el AC fue del 97-100%. Las mayores diferencias se registraron con Candida krusei y la flucitosina, y con Candida kefyr y la anfotericina B. Los valores de sensibilidad de Candida tropicalis con el fluconazol arrojaban más cepas SDD y resistentes con Vitek 2. El AC con Candida parapsilosis fue del 100% con todos los antifúngicos testados. No se observaron grandes diferencias entre los dos métodos.
ConclusionesVitek 2 proporciona resultados comparables con los de Etest, con un tiempo rápido de respuesta respecto a las especies de Candida susceptibilidad.
Candida species are part of the normal human microbiota and are commonly found in skin, gastrointestinal tract, and genitourinary system.13 Patients with certain risk factors are predisposed to develop deep-seated or mucosal Candida infections. These factors include treatment with broad spectrum antibiotics, prolonged use of central venous catheters, persistent neutropenia, the administration of corticosteroids or other immunosuppressive agents, HIV infection and diabetes mellitus.12
In recent years, nosocomial bloodstream Candida infections have emerged as a significant problem ranking the third to fourth most common cause of fungemia in many intensive care units.21 Moreover, epidemiology of Candida infections is rapidly changing, where non-Candida albicans species are becoming predominant. Clinical data collected from 2019 patients with candidemia obtained from the Prospective Antifungal Therapy (PATH) Alliance database registry revealed that the prevalence of non-C. albicans Candida species was 54.4%.9C. albicans is regarded as a more aggressive and virulent species, and if left untreated mortality rates can be high because of its dissemination to other organs. In one multicenter prospective study in USA, the mortality associated with C. albicans was 47% for adults and 29% for children (<13 years).14 Fortunately, unlike Candida glabrata, almost all the strains of C. albicans are susceptible to fluconazole, which is usually the preferred therapeutic agent for candidemia in clinically stable patients. Among other species of the genus, Candida tropicalis, C. glabrata and Candida parapsilosis are the main species associated with candidemia.18 In elderly patients, C. glabrata is the second most common cause of candidemia, and because of its reduced susceptibility to fluconazole it can be more difficult to treat.17 However, in pediatric patient population (<13 years), C. parapsilosis is often a more common cause of candidemia after C. albicans (34% versus 49%) or even a leading cause in some European studies.14,20 In Kuwait, among neonatal patients between 2004 and 2010, C. parapsilosis was the predominant species causing candidemia, followed by C. albicans, whereas in a 10-year retrospective study (1996–2005) representing different patient populations, C. albicans was more common (39.5%) than C. parapsilosis (30.6%).1,8 In all the candidemia studies reported from Kuwait, the prevalence of C. glabrata was <6%.
Although the microdilution methods recommended by the Clinical and Laboratory Standards Institute (CLSI)4 are the reference methods for performing antifungal susceptibility testing of Candida species, a number of commercially available alternative methods have been developed, as they are easy to perform, less time consuming and breakpoints may be interpreted easier. In this study, we have evaluated the performance of Vitek 2 AST-YS01 susceptibility card in comparison with Etest (bioMérieux, France).
Material and methodsCandida isolates and identificationA total of 263 clinical isolates of Candida were included in the study. They were obtained over a six-year period (2008–2013) and stored frozen at −80°C on Cryo-Beads (AES Laboratory, Bruz, France), after a routine phenotypic identification, in the Fungal Culture Collection of the Mycology Reference Laboratory, Department of Microbiology. The isolates came from blood, urine, vagina, sputum and other specimens. The isolates were sub-cultured and identified by germ tube test, colony color on CHROMagar Candida (Becton Dickinson/BBL, Sparks, Madison, USA) and by Vitek 2 YST cards and/or ID32C strips (bioMérieux).11 The species identification was as follows: C. albicans (n=127), C. glabrata (n=67), C. tropicalis (n=39), Candida krusei (n=12), C. parapsilosis (n=12) and Candida kefyr (n=6).
Antifungal susceptibility testingEtestEach Candida isolate was freshly sub-cultured on Sabouraud dextrose agar supplemented with chloramphenicol. One to two colonies were suspended in saline (NaCl 0.85%) and turbidity was adjusted to 0.5 McFarland standard. Petri dishes (150mm) containing RPMI-1640 medium supplemented with 2% glucose and pH adjusted to 7.0 with MOPS (0.165M, pH 7.0) buffer were used for susceptibility testing. Employing a sterile cotton swab, yeast suspension was evenly spread onto the surface of the medium. Etest procedure was performed as recommended by the manufacturer (bioMérieux). Petri plates were allowed to dry for 10–15min before applying the Etest strips. Minimum inhibitory concentration (MIC) values were recorded after 24–48h incubation at 35°C when sufficient growth was visible. The MICs were read at the point of intersection between the zone of inhibition and the Etest strip. For amphotericin B and flucytosine, the MICs were read at the point of complete inhibition (100%). The MICs for azoles (fluconazole and voriconazole) were read at the first point of significant inhibition (80% of visible growth). Interpretive susceptibility breakpoints were those recommended by the CLSI 2012 document (M27-S4).4 Reference strains of C. albicans (ATCC 90028) and C. parapsilosis (ATCC 22019) were used as quality controls for Etest. For the Vitek 2 system, the manufacturer includes two reference strains, C. parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) in the system.
Vitek 2 yeast susceptibility testThe Vitek 2 card AST-YS01 is FDA approved. The susceptibility tests were performed according to the manufacturer's recommendations. A yeast suspension was adjusted to 2 McFarland using the DensiCheck (bioMérieux). Each suspension was diluted by transferring 280μl to a tube containing 3ml of saline solution. After inserting the card with the yeast suspension, it was incubated for 10–26h and read automatically. The results were expressed as MICs. The Vitek 2 AST-YS01 card contains serial dilutions ranging from 1 to 64μg/ml for fluconazole and flucytosine, from 0.12 to 8μg/ml for voriconazole, and from 0.25 to 16μg/ml for amphotericin B.
Susceptibility breakpointsCLSI clinical breakpoints for the susceptibility patterns of C. albicans, C. tropicalis and C. parapsilosis to fluconazole, susceptible-S; susceptible dose-dependent-SDD, and resistant-R isolates, were ≤2μg/ml, 4μg/ml, and ≥8μg/ml, respectively, and for C. glabrata SDD ≤32μg/ml and R, ≥64μg/ml (CLSI, 2012, document M27-S4)4. All isolates of C. krusei were considered resistant to fluconazole irrespective of the MICs. For C. kefyr, the epidemiological cutoff value (ECV) was 1μg/ml. For voriconazole, S, SDD, and R breakpoints for C. albicans, C. parapsilosis and C. tropicalis were ≤0.12μg/ml, 0.25–0.5μg/ml, and ≥1μg/ml, and for C. krusei ≤0.5μg/ml, 1μg/ml, and ≥2μg/ml, respectively. Due to the lack of clinical breakpoints, the ECV was used to determine S (WT) and R (non-WT) for voriconazole and C. glabrata (≤0.5/≥1μg/ml). Likewise, ECVs were used for fluconazole and C. kefyr (Kluyveromyces marxianus) (≤1/>1μg/ml) and for voriconazole and C. kefyr (≤0.015/>0.015μg/ml).11 These susceptibility values were used to determine categorical agreement (CA).11,15,16,19 With regard to amphotericin B, the following cutoff values as proposed by EUCAST were used: susceptible (<1μg/ml) and resistant (≥1μg/ml).2,5 For flucytosine, the following interpretive breakpoints were used: susceptible (≥4μg/ml), intermediate (8–16μg/ml), and resistant (≥32μg/ml).5
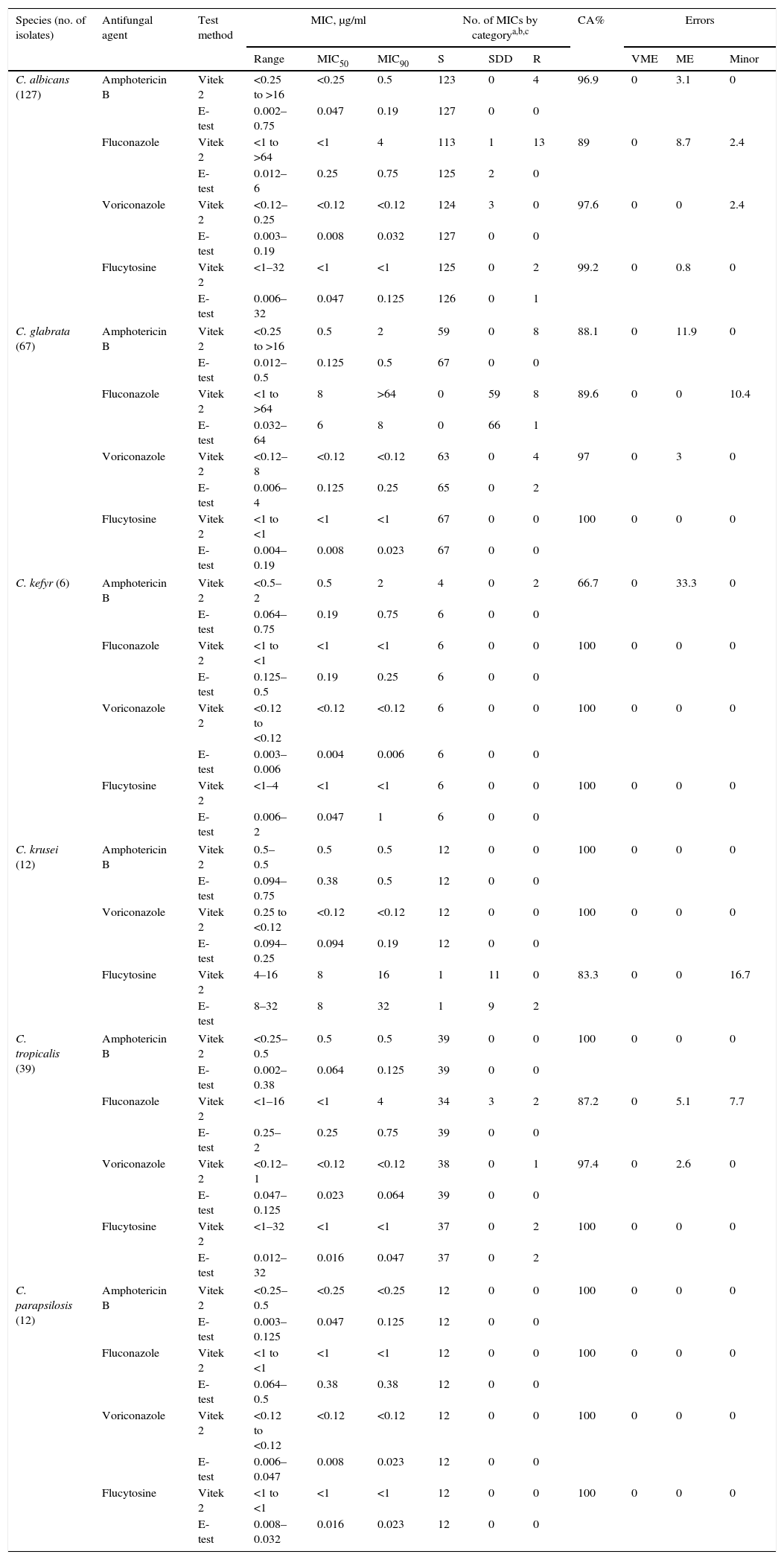
Analysis of resultsThe MIC results obtained with the VITEK 2 yeast susceptibility test were compared to those with Etest read at 24–48h. Discrepancies among MIC endpoints of more than two dilutions were used to calculate the essential agreement (EA). Interlaboratory and intralaboratory agreement, assessed with the 10-isolate reproducibility panel, was defined when MIC results were within a three-dilution range. Categorical agreements were assessed as described elsewhere.3,16 Very major errors were identified when the Etest MIC indicated R, and the VITEK 2 MIC indicated S. Major errors were identified when the isolate was classified as R by the VITEK 2 and S by the Etest. Minor errors were determined when the results of one of the test methods was either S or R and that of the other was SDD.16 The concentration of the antifungal drug inhibiting 50% of the strains (MIC50) and 90% of the strains (MIC90) is shown in Table 1.
Categorical agreement between Vitek 2 yeast susceptibility system MICs and Etest MICs for 263 Candida isolates.
| Species (no. of isolates) | Antifungal agent | Test method | MIC, μg/ml | No. of MICs by categorya,b,c | CA% | Errors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | S | SDD | R | VME | ME | Minor | ||||
| C. albicans (127) | Amphotericin B | Vitek 2 | <0.25 to >16 | <0.25 | 0.5 | 123 | 0 | 4 | 96.9 | 0 | 3.1 | 0 |
| E-test | 0.002–0.75 | 0.047 | 0.19 | 127 | 0 | 0 | ||||||
| Fluconazole | Vitek 2 | <1 to >64 | <1 | 4 | 113 | 1 | 13 | 89 | 0 | 8.7 | 2.4 | |
| E-test | 0.012–6 | 0.25 | 0.75 | 125 | 2 | 0 | ||||||
| Voriconazole | Vitek 2 | <0.12–0.25 | <0.12 | <0.12 | 124 | 3 | 0 | 97.6 | 0 | 0 | 2.4 | |
| E-test | 0.003–0.19 | 0.008 | 0.032 | 127 | 0 | 0 | ||||||
| Flucytosine | Vitek 2 | <1–32 | <1 | <1 | 125 | 0 | 2 | 99.2 | 0 | 0.8 | 0 | |
| E-test | 0.006–32 | 0.047 | 0.125 | 126 | 0 | 1 | ||||||
| C. glabrata (67) | Amphotericin B | Vitek 2 | <0.25 to >16 | 0.5 | 2 | 59 | 0 | 8 | 88.1 | 0 | 11.9 | 0 |
| E-test | 0.012–0.5 | 0.125 | 0.5 | 67 | 0 | 0 | ||||||
| Fluconazole | Vitek 2 | <1 to >64 | 8 | >64 | 0 | 59 | 8 | 89.6 | 0 | 0 | 10.4 | |
| E-test | 0.032–64 | 6 | 8 | 0 | 66 | 1 | ||||||
| Voriconazole | Vitek 2 | <0.12–8 | <0.12 | <0.12 | 63 | 0 | 4 | 97 | 0 | 3 | 0 | |
| E-test | 0.006–4 | 0.125 | 0.25 | 65 | 0 | 2 | ||||||
| Flucytosine | Vitek 2 | <1 to <1 | <1 | <1 | 67 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.004–0.19 | 0.008 | 0.023 | 67 | 0 | 0 | ||||||
| C. kefyr (6) | Amphotericin B | Vitek 2 | <0.5–2 | 0.5 | 2 | 4 | 0 | 2 | 66.7 | 0 | 33.3 | 0 |
| E-test | 0.064–0.75 | 0.19 | 0.75 | 6 | 0 | 0 | ||||||
| Fluconazole | Vitek 2 | <1 to <1 | <1 | <1 | 6 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.125–0.5 | 0.19 | 0.25 | 6 | 0 | 0 | ||||||
| Voriconazole | Vitek 2 | <0.12 to <0.12 | <0.12 | <0.12 | 6 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.003–0.006 | 0.004 | 0.006 | 6 | 0 | 0 | ||||||
| Flucytosine | Vitek 2 | <1–4 | <1 | <1 | 6 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.006–2 | 0.047 | 1 | 6 | 0 | 0 | ||||||
| C. krusei (12) | Amphotericin B | Vitek 2 | 0.5–0.5 | 0.5 | 0.5 | 12 | 0 | 0 | 100 | 0 | 0 | 0 |
| E-test | 0.094–0.75 | 0.38 | 0.5 | 12 | 0 | 0 | ||||||
| Voriconazole | Vitek 2 | 0.25 to <0.12 | <0.12 | <0.12 | 12 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.094–0.25 | 0.094 | 0.19 | 12 | 0 | 0 | ||||||
| Flucytosine | Vitek 2 | 4–16 | 8 | 16 | 1 | 11 | 0 | 83.3 | 0 | 0 | 16.7 | |
| E-test | 8–32 | 8 | 32 | 1 | 9 | 2 | ||||||
| C. tropicalis (39) | Amphotericin B | Vitek 2 | <0.25–0.5 | 0.5 | 0.5 | 39 | 0 | 0 | 100 | 0 | 0 | 0 |
| E-test | 0.002–0.38 | 0.064 | 0.125 | 39 | 0 | 0 | ||||||
| Fluconazole | Vitek 2 | <1–16 | <1 | 4 | 34 | 3 | 2 | 87.2 | 0 | 5.1 | 7.7 | |
| E-test | 0.25–2 | 0.25 | 0.75 | 39 | 0 | 0 | ||||||
| Voriconazole | Vitek 2 | <0.12–1 | <0.12 | <0.12 | 38 | 0 | 1 | 97.4 | 0 | 2.6 | 0 | |
| E-test | 0.047–0.125 | 0.023 | 0.064 | 39 | 0 | 0 | ||||||
| Flucytosine | Vitek 2 | <1–32 | <1 | <1 | 37 | 0 | 2 | 100 | 0 | 0 | 0 | |
| E-test | 0.012–32 | 0.016 | 0.047 | 37 | 0 | 2 | ||||||
| C. parapsilosis (12) | Amphotericin B | Vitek 2 | <0.25–0.5 | <0.25 | <0.25 | 12 | 0 | 0 | 100 | 0 | 0 | 0 |
| E-test | 0.003–0.125 | 0.047 | 0.125 | 12 | 0 | 0 | ||||||
| Fluconazole | Vitek 2 | <1 to <1 | <1 | <1 | 12 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.064–0.5 | 0.38 | 0.38 | 12 | 0 | 0 | ||||||
| Voriconazole | Vitek 2 | <0.12 to <0.12 | <0.12 | <0.12 | 12 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.006–0.047 | 0.008 | 0.023 | 12 | 0 | 0 | ||||||
| Flucytosine | Vitek 2 | <1 to <1 | <1 | <1 | 12 | 0 | 0 | 100 | 0 | 0 | 0 | |
| E-test | 0.008–0.032 | 0.016 | 0.023 | 12 | 0 | 0 | ||||||
S, SDD, and R, respectively, were those of the CLSI (2012) for fluconazole and C. albicans and C. tropicalis (≤2/4/≥8μg/ml), and C. glabrata (–/≤32/≥64μg/ml); for voriconazole and C. albicans and C. tropicalis (≤0.12/0.25–0.5/≥1μg/ml) and C. krusei (≤0.5/1/≥2μg/ml).
Due to the lack of breakpoints the epidemiological cutoff value was used to determine S (WT) and R (non-WT) for fluconazole and C. kefyr (≤1/>1μg/ml) and for voriconazole and C. kefyr (≤0.015/>0.015μg/ml), for voriconazole and C. glabrata (≤0.5/≥1μg/ml), respectively (Pfaller and Diekema19).
Due to the lack of CLSI breakpoints for amphotericin B, isolates with MIC <1μg/ml were considered susceptible (S) and ≥1μg/ml as resistant (R). For flucytosine, the following interpretive breakpoints were used: susceptible (≥4μg/ml), intermediate (8–16μg/ml), and resistant (≥32μg/ml).
ME: major errors; VME: very major errors.
A summary of the antifungal susceptibility data comparing Etest with Vitek 2 AST-YS01 cards is presented in Table 1. Regardless of the Candida species tested, the overall CA between VITEK 2 and Etest of the 263 isolates ranged between 66.6% and 100%. In general, Etest yielded lower MICs than Vitek probably because of the narrow range of dilutions used in Etest strips when compared to Vitek cards. With C. albicans the CA for amphotericin B, voriconazole and flucytosine was >95%, but only 89% for fluconazole, where 13 isolates were resistant to fluconazole with Vitek (≥8μg/ml). With respect to C. glabrata, the CA was 97% and 100% for voriconazole and flucytosine, respectively and <90% for fluconazole and amphotericin B. The major errors (83.3%) were with C. krusei and flucytosine, where two isolates were read as R by Etest and SDD by Vitek 2. Furthermore, two major errors were detected with C. kefyr and amphotericin B, R by Vitek and S by Etest (33.3%). C. tropicalis susceptibility reading for fluconazole by Vitek reported more SDD and R strains than Etest. With respect to C. parapsilosis there was 100% CA for all the four antifungals tested (Table 1). Not checking many isolates of C. kefyr, an emerging pathogen of hematologic and other immunocompromised patients, implies some limitations in the study.3,6 So far, the available antifungal susceptibility data is limited to only few strains. In a study by Pfaller et al., a comparison of fluconazole MICs determined by CLSI BMD and Vitek 2 AF03 IUO yeast susceptibility card was performed on three isolates of C. kefyr and revealed an EA of 66.7%.15,16 In a study of 13 strains from different specimens (blood, urine and stool) of 8 patients, MICs of fluconazole, flucytosine and micafungin were found to be quite variable, suggesting resistance in several of the isolates.7,10
In conclusion, Vitek 2 AST-YS01 card provides a reliable alternative to Etest with a quick turnaround time for in vitro susceptibility testing of Candida species. Since most of the major hospitals in Kuwait are using Vitek 2 AST-YS01 card for susceptibility testing, the information provided herein reinforces the utility of this method.
Conflict of interestThe authors declare no conflicts of interest.