Diabetic patients are particularly susceptible to fungal infections due to modifications that occur in their immunological system. These modifications compromise natural defences, such as skin and nails, especially from lower limbs.
AimsAssessing the presence of dermatomycosis in lower limbs of Portuguese diabetic patients followed on Podiatry consultation. Determination of possible predisposing factors and the most frequent fungal species associated with the cases are included in the study.
MethodsA six-month prospective study was carried out in 163 diabetic patients with signs and symptoms of dermatomycosis followed by Podiatry at the Portuguese Diabetes Association in Lisbon. Samples from the skin and/or nails of the lower limbs were collected and demographic and clinical data of those patients were recorded.
ResultsTrichophyton rubrum was the most frequently isolated dermatophyte (12.1%), followed by Trichophyton mentagrophytes (7.7%) and Trichophyton tonsurans (4.4%). Our study showed positive associations between type 2 diabetes and the presence of dermatomycosis in the studied population (p=0.013); this association was also shown between the occurrence of dermatomycosis and the localization of the body lesion (p=0.000). No other predisposing factor tested was positively associated with infection (p>0.05).
ConclusionsData on superficial fungal infections in diabetic patients are scarce in Portugal. This study provides information on the characterization of dermatomycosis in lower limbs of diabetic patients.
Los pacientes diabéticos son especialmente vulnerables a las micosis debido a las modificaciones inducidas por la enfermedad en su sistema inmunitario. Estas modificaciones comprometen los sistemas de defensa naturales, como la piel y las uñas, sobre todo en las extremidades inferiores.
ObjetivosEvaluar la presencia de dermatomicosis en los miembros inferiores de pacientes diabéticos portugueses seguidos en consultas de podología y determinar los posibles factores predisponentes y las especies de hongos más frecuentes asociadas a los casos incluidos en el estudio.
MétodosSe realizó un estudio prospectivo de seis meses de duración en 163 pacientes diabéticos con signos y síntomas de dermatomicosis, atendidos por el servicio de podología de la Asociación Portuguesa de Diabetes en Lisboa. Se obtuvieron muestras de piel y/o de uñas de las extremidades inferiores y se registraron los datos demográficos y clínicos de los pacientes.
ResultadosTrichophyton rubrum fue el dermatofito más frecuentemente aislado (12,1%), seguido por Trichophyton mentagrophytes (7,7%) y Trichophyton tonsurans (4,4%). En el presente estudio ha quedado demostrada la asociación entre la diabetes de tipo 2 y la presencia de dermatomicosis en la población estudiada (p=0,013); y así mismo entre la incidencia de dermatomicosis y la localización de la lesión corporal (p=0,000). Para ningún otro factor predisponente analizado se identificó una asociación positiva con la infección (p > 0,05).
ConclusionesEn Portugal apenas se dispone de datos sobre micosis superficiales en pacientes diabéticos. El presente estudio proporciona información sobre la caracterización de las dermatomicosis en miembros inferiores de estos pacientes.
Onychomycosis and tinea pedis are recognized as very superficial diseases.28 Superficial fungal infections are increasingly being reported, particularly for individuals over 60 years of age and with a compromised health status,3 such as patients with diabetes.4,32
Diabetes mellitus is characterized by a state of relative or complete insulin depletion, leading to gross defects in glucose, fat and protein metabolism.5 It is a chronic metabolic disease with high human, social and economic implications and is actually considered one of the biggest public health issues in developing countries. The pathogenesis of diabetic foot is highly complex and complications associated with the development of infection and diabetic foot syndrome are the main cause of morbidity, non-traumatic extremity amputation, and diabetic patient mortality.19,21 Impaired sensation may cause paronychia secondary to a mycotic nail to go unnoticed, just as tinea pedis in combination with dry fissuring plantar skin that can create a portal for secondary bacterial infection.4
Several authors compared the incidence of onychomycosis in diabetic patients and individuals without the disease1,8,17,25,29,31 and concluded that the incidence is higher than in the former ones. Furthermore, one study detected an increased risk among all three major groups of organisms that can cause onychomycosis in diabetic patients: dermatophytes, yeasts, and non-dermatophyte molds.29
The World Health Organization has calculated that up to 300 million patients worldwide will be affected by diabetes in 2025.37 In Portugal, the number of adult diabetic persons estimated to be 11.7% in 2010.14
Data related to the epidemiology of dermatomycosis in diabetic patients in our country are scarce. The present study was performed in a diabetology center, and its main objective was to evaluate the fungal epidemiology in Portuguese diabetic patients with dermatomycosis in lower limbs, namely the frequency of dermatophytes isolation and their distribution, and also specific factors likely to be associated with the onset of infection in this patient group.
Materials and methodsStudy populationThe survey was performed in diabetic patients followed by Podiatry of the Portuguese Diabetes Association, in Lisbon. This descriptive study was carried on during six months, from March to August 2007. Podiatrist technicians, under the supervision of dermatologists and endocrinologists from that institution, observed diabetic patients in order to detect nail and skin lesions compatible with fungal infections and to collect samples. Dystrophic nails, subungual hyperkeratosis, yellow-brown discoloration and/or onycholysis as well as red itchy rash in soles of the feet and interdigital spaces, small pustules and scaling were the clinical criteria suggestive of onychomycosis ant tinea pedis, respectively.
Diabetic patients on topical antifungal therapy within the last one week or systemic antifungal therapy within the last four weeks were excluded of this study, in order to eliminate false negatives. Demographic and clinical data were recorded and included age, gender, type of diabetes, obesity, knowledge of previous fungal infection, occupation, underlying disease and therapies. Other questions were also approached, namely gymnasium and swimming pool attendance, difficulty in maintaining an appropriate hygiene and possession of domestic animals. The number of patients considered to evaluate each possible risk factor was variable and depended on patients’ answers to the questionnaire. In order to ensure full and even comprehension of questionnaire as well as to ensure maximum number of answers, inquiry was done to each patient by a health professional.
Sample collectionSkin and/or nails in lower limbs were collected using a sterilized scalpel to scrape the largest amount of material from the affected area, previously cleaned with an aqueous solution of ethanol 70% (in order to remove possible contaminations).38 A pre-moistened swab with saline solution was then used to wipe the scraped and clean area, and collect possible remaining fungal elements. All samples collected were sent to the Reference Mycology Laboratory at the National Institute of Health Dr. Ricardo Jorge (the Portuguese NIH).
Fungal culture and identificationSample observation and cultures were performed at National Institute of Health Dr. Ricardo Jorge. Direct microscopic examination of the samples was performed following treatment with potassium hydroxide (KOH, 30%), for not less than 20min. In addition to microscopy analysis, skin and nails scrappings were cultured on Sabouraud supplemented with chloramphenicol agar (Difco, Detroit, MI) and Mycosel agar medium (Difco, Detroit, MI) supplemented with both chloramphenicol (0.05g/L) and cycloheximide (0.5mg/mL). Swabs were inoculated into Sabouraud broth also supplemented with chloramphenicol in order to enhance the sensibility of the culture methodology. Cultures were incubated at 27°C and considered negative at the end of the third week with no growth in either medium.16 For species identification, microscopic observations were performed using tease mount or scotch tape mount and lactophenol cotton blue mount staining. Morphological identification was achieved through macro and microscopic characteristics listed in illustrated literature.7,24
Statistical analysisThe Pearson chi-square test or the Fisher Exact Test for tables 2×2 was used to compare proportions and analyze differences in species distribution. Two-sided p-values from tests were used to summarize the comparability and a 5% significance level was set (p<0.05). No statistical analysis was performed in order to treat missing data. The SPSS v15.0 program for Windows was used to perform the statistical analysis.
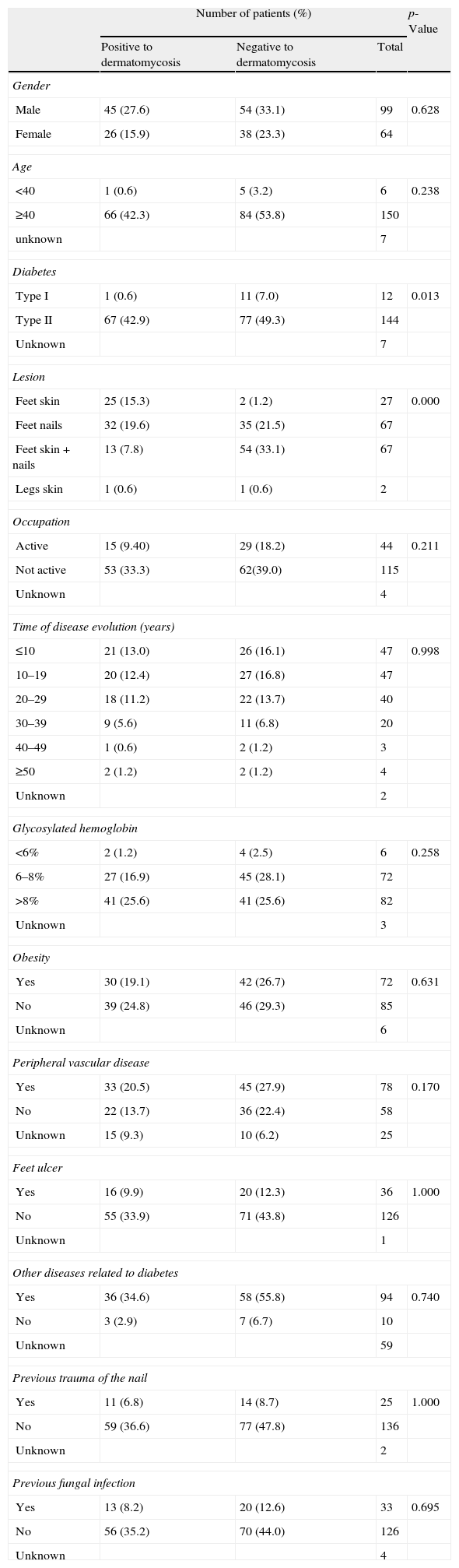
ResultsStudied populationDuring the period of study, 163 diabetic patients showing clinical signs and symptoms of dermatomycosis were screened for the presence of fungi. Thirty-three (20.2%) of these patients reported previous fungal infections in lower limbs, 99 (60.7%) of the 163 patients were male and 64 (39.3%) were female, with a male/female ratio of 1:0.65, ranging in age from 27 to 89 years old, with an average of 63.7 years old. The racial origin was: 162 Caucasian and one African. Distribution of the patients by age groups was as follows: 0–30 (n=3; 1.9%), 31–50 (n=22; 13.9%), 51–70 (n=85; 53.8%) and ≥71 (n=48; 30.4%). From those, 115 patients were not labor active (70.5%), 44 (27.0%) had a professional occupation and in four cases (2.5%) this question was not answered (Table 1).
Patients characterization and association with different risk factors.
| Number of patients (%) | p-Value | |||
| Positive to dermatomycosis | Negative to dermatomycosis | Total | ||
| Gender | ||||
| Male | 45 (27.6) | 54 (33.1) | 99 | 0.628 |
| Female | 26 (15.9) | 38 (23.3) | 64 | |
| Age | ||||
| <40 | 1 (0.6) | 5 (3.2) | 6 | 0.238 |
| ≥40 | 66 (42.3) | 84 (53.8) | 150 | |
| unknown | 7 | |||
| Diabetes | ||||
| Type I | 1 (0.6) | 11 (7.0) | 12 | 0.013 |
| Type II | 67 (42.9) | 77 (49.3) | 144 | |
| Unknown | 7 | |||
| Lesion | ||||
| Feet skin | 25 (15.3) | 2 (1.2) | 27 | 0.000 |
| Feet nails | 32 (19.6) | 35 (21.5) | 67 | |
| Feet skin+nails | 13 (7.8) | 54 (33.1) | 67 | |
| Legs skin | 1 (0.6) | 1 (0.6) | 2 | |
| Occupation | ||||
| Active | 15 (9.40) | 29 (18.2) | 44 | 0.211 |
| Not active | 53 (33.3) | 62(39.0) | 115 | |
| Unknown | 4 | |||
| Time of disease evolution (years) | ||||
| ≤10 | 21 (13.0) | 26 (16.1) | 47 | 0.998 |
| 10–19 | 20 (12.4) | 27 (16.8) | 47 | |
| 20–29 | 18 (11.2) | 22 (13.7) | 40 | |
| 30–39 | 9 (5.6) | 11 (6.8) | 20 | |
| 40–49 | 1 (0.6) | 2 (1.2) | 3 | |
| ≥50 | 2 (1.2) | 2 (1.2) | 4 | |
| Unknown | 2 | |||
| Glycosylated hemoglobin | ||||
| <6% | 2 (1.2) | 4 (2.5) | 6 | 0.258 |
| 6–8% | 27 (16.9) | 45 (28.1) | 72 | |
| >8% | 41 (25.6) | 41 (25.6) | 82 | |
| Unknown | 3 | |||
| Obesity | ||||
| Yes | 30 (19.1) | 42 (26.7) | 72 | 0.631 |
| No | 39 (24.8) | 46 (29.3) | 85 | |
| Unknown | 6 | |||
| Peripheral vascular disease | ||||
| Yes | 33 (20.5) | 45 (27.9) | 78 | 0.170 |
| No | 22 (13.7) | 36 (22.4) | 58 | |
| Unknown | 15 (9.3) | 10 (6.2) | 25 | |
| Feet ulcer | ||||
| Yes | 16 (9.9) | 20 (12.3) | 36 | 1.000 |
| No | 55 (33.9) | 71 (43.8) | 126 | |
| Unknown | 1 | |||
| Other diseases related to diabetes | ||||
| Yes | 36 (34.6) | 58 (55.8) | 94 | 0.740 |
| No | 3 (2.9) | 7 (6.7) | 10 | |
| Unknown | 59 | |||
| Previous trauma of the nail | ||||
| Yes | 11 (6.8) | 14 (8.7) | 25 | 1.000 |
| No | 59 (36.6) | 77 (47.8) | 136 | |
| Unknown | 2 | |||
| Previous fungal infection | ||||
| Yes | 13 (8.2) | 20 (12.6) | 33 | 0.695 |
| No | 56 (35.2) | 70 (44.0) | 126 | |
| Unknown | 4 | |||
Regarding clinical features related with diabetes, 12 (7.4%) patients had type 1 diabetes whereas 144 had type 2 diabetes, with an average of disease evolution of 18.4 years. Moreover, 82 patients (51.2%) had high levels of glycosylated hemoglobin (above 8%) (Table 1).
In 107 patients more than one sample were collected, generating 272 keratinized samples from all 163 diabetic patients, 113 from skin of the patients’ lower limbs and 159 from toe nails.
Seventy-one patients (43.6%) showed positive cultures to fungi. Nevertheless, only 63 (38.7%) of them were considered as having a fungal infection. Twenty-eight patients (17.2%) showed onychomycosis, 21 (12.9%) patients presented dermatomycosis in feet's skin, 13 (7.9%) patients had fungal lesions in both places and one patient (0.6%) showed fungal lesions in leg skin. Among the analyzed samples, there was a higher prevalence of lesions caused by fungi in skin of the foot whereas samples from both skin and nails lesions from the same patient showed to be negative to dermatomycosis (p=0.00). Feet ulcers were found in 36 (22.1%) patients (Table 1).
Demographic and social factors did not show positive association with the existence of fungal infection, namely regarding the gender (p=0.628), occupation (p=0.211), residence area (p=0.051) and age (p=0.238) (Table 1). Nevertheless, patients aged 70 years and older (53 patients) presented a higher incidence of dermatomycosis (55.1%) and about 75% of the patients with onychomycosis were above 60 years old (data not shown).
Clinical features and potential risk factors for the development of dermatomycosis in diabetic patients were also evaluated. Type 2 diabetes was positively associated with the development of superficial mycosis (p=0.013) whereas the other studied clinical factors did not show positive association, including obesity (p=0.631), glycosylated hemoglobin (p=0.258), other diseases related with diabetes (p=0.740), peripheral vascular disease (p=0.170), feet ulcers (p=1.000), and previous trauma of the nails (p=1.000) (Table 1).
Other independent variables such as type of antidiabetic therapy or other medications, namely topical treatments, practice of sports or swimming pool usage, use of fiber socks or sport shoes, family history of fungal infections, problems in maintaining an appropriate hygiene and possession of domestic animals did not correlate with the development of dermatomycosis (all p>0.05) (data not shown).
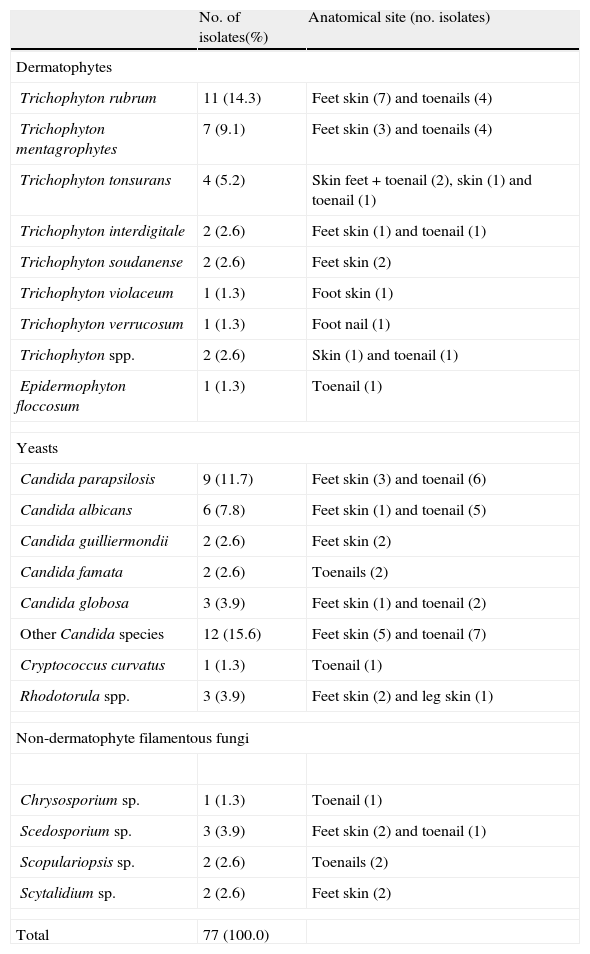
EtiologyFrom the 71 patients showing positive results to fungi, 91 fungal isolates were obtained, either from the same product (four cases) or from different body sites. Nevertheless, only 77 fungal isolates were considered in this study as the remaining isolates are saprophytes and their involvement as etiological agents is debatable (as discussed below). In six patients (8.4%), dermatophytes were collected from feet skin whereas yeasts were isolated from feet nails.
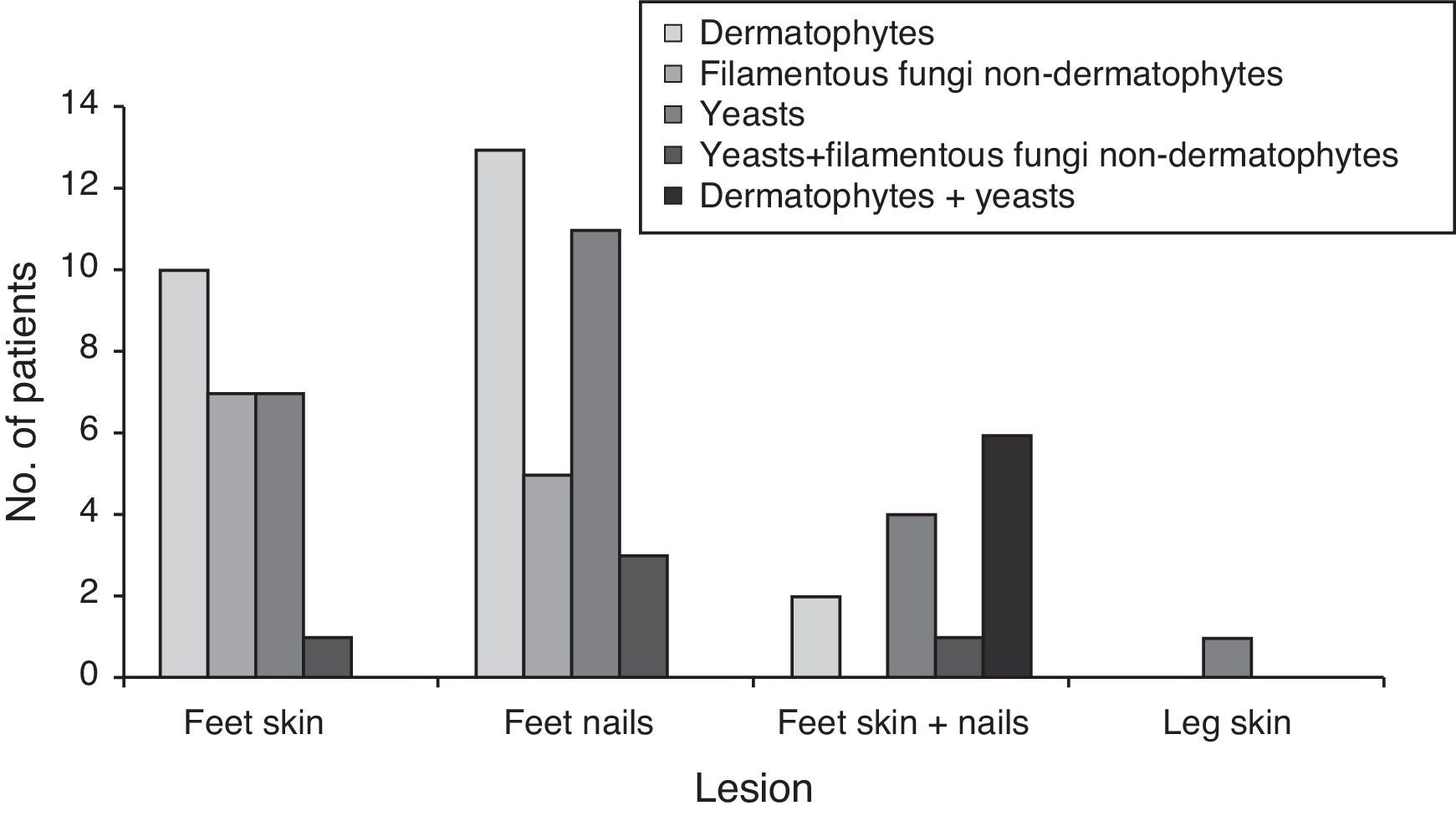
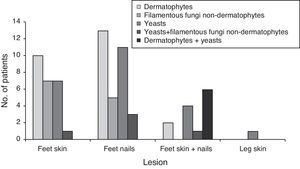
Fig. 1 and Table 2 show the distribution of the etiological agent by fungal group and site of infection, as well as the number of patients infected.
Fungal isolates collected from lower limbs of the 71 diabetic patients with samples positive to fungi.
| No. of isolates(%) | Anatomical site (no. isolates) | |
| Dermatophytes | ||
| Trichophyton rubrum | 11 (14.3) | Feet skin (7) and toenails (4) |
| Trichophyton mentagrophytes | 7 (9.1) | Feet skin (3) and toenails (4) |
| Trichophyton tonsurans | 4 (5.2) | Skin feet+toenail (2), skin (1) and toenail (1) |
| Trichophyton interdigitale | 2 (2.6) | Feet skin (1) and toenail (1) |
| Trichophyton soudanense | 2 (2.6) | Feet skin (2) |
| Trichophyton violaceum | 1 (1.3) | Foot skin (1) |
| Trichophyton verrucosum | 1 (1.3) | Foot nail (1) |
| Trichophyton spp. | 2 (2.6) | Skin (1) and toenail (1) |
| Epidermophyton floccosum | 1 (1.3) | Toenail (1) |
| Yeasts | ||
| Candida parapsilosis | 9 (11.7) | Feet skin (3) and toenail (6) |
| Candida albicans | 6 (7.8) | Feet skin (1) and toenail (5) |
| Candida guilliermondii | 2 (2.6) | Feet skin (2) |
| Candida famata | 2 (2.6) | Toenails (2) |
| Candida globosa | 3 (3.9) | Feet skin (1) and toenail (2) |
| Other Candida species | 12 (15.6) | Feet skin (5) and toenail (7) |
| Cryptococcus curvatus | 1 (1.3) | Toenail (1) |
| Rhodotorula spp. | 3 (3.9) | Feet skin (2) and leg skin (1) |
| Non-dermatophyte filamentous fungi | ||
| Chrysosporium sp. | 1 (1.3) | Toenail (1) |
| Scedosporium sp. | 3 (3.9) | Feet skin (2) and toenail (1) |
| Scopulariopsis sp. | 2 (2.6) | Toenails (2) |
| Scytalidium sp. | 2 (2.6) | Feet skin (2) |
| Total | 77 (100.0) | |
Yeasts were the most prevalent group of fungi, being isolated from lesions of 34 patients (38 isolates). Candida parapsilosis and Candida albicans were the most frequently isolated Candida species, especially from nail lesions (in 73.3% of the cases).The most prevalent dermatophyte species was Trichophyton rubrum, followed by T. mentagrophytes, T. tonsurans, Trichophyton interdigitale, Trichophyton soudanense, Trichophyton verrucosum, and Trichophyton violaceum. Two isolates from the genus Trichophyton were not identified the loss of culture's viability. One isolate of Epidermophyton floccosum was also collected from a patient's toenail. Dermatophytes were the most isolated fungi from feet skin and toenails (31 isolates). Ten patients (14.1%) showed infection only in feet skin, 13 (18.3%) only in toenails and two patients (2.8%) in both body sites (T. tonsurans in both cases).
Potential keratinophilic fungi like Scopulariopsis spp., Chrysosporium sp., Scytalidium spp. and Scedosporium spp. were also found (eight isolates) and considered as the etiological agents. One patient (1.4%) showed infection of both skin and toenails, four patients (5.6%) showed only feet skin lesions and three (4.2%) had nail lesions from where the only isolated agent was a non-dermatophyte filamentous fungi.
DiscussionLesions in lower limbs of diabetic patients could be caused by common disorders including psoriasis, lichen planus, onychogryphosis, trauma, and idiopathic dystrophic nails that may be really similar to the ones caused by fungi6 and chronic lower limb wounds are constantly exposed to bacteria and fungal organisms.26 Dermatomycosis is, nevertheless, one of the major problems concerning lower limb infections in diabetic patients
To date, few studies have examined the prevalence of onychomycosis among diabetic patients.20 This study presents the first survey on dermatomycosis in lower limbs of Portuguese diabetic patients, even though it is not representative of the national population afflicted by this disorder.
During this study, positive cultures for fungi were found in 43.6% of the patients, which is lower than the one obtained by Meyser et al.,26 whose results showed positive results in 84.6% of the analyzed patients. This discrepancy of values could possibly be explained by the lower number of individuals analyzed (78) or caused by a geographical asymmetry related to climatic and socio/cultural differences and what those represent in such infections
In our study, 22.1% of the patients showed feet ulcers, and similar value was obtained by Joseph,20 whose study revealed that feet ulceration occurs in 19% of the patients with diabetes mellitus. Furthermore, our data revealed that 44% of the patients with feet ulcers presented dermatomycosis. This value is higher than the one presented by Fata et al.,12 who obtained positive cultures in 25% of diabetic patients with ulcers in their feet. According to Bokyo et al.,2 diabetic patients with onychomycosis had an approximately three times greater risk of gangrene or foot ulcer compared with diabetic patients without it. From the 163 patients showing visible lesions, 25.1% presented onychomycosis, a similar value to the one (26%) obtained by Gupta in 199818 and higher than the ones obtained by Dogra et al. (17%)8 and by Kuvandik et al. (19.7%).23 According to Gupta, 1998,18 the development of onychomycosis was significantly correlated with age and male gender. Males were 2.99 times more likely to have onychomycosis compared with females. Our results did not show positive association with those variables despite agreeing with a higher prevalence of onychomycosis in males (1.5 times higher than females) and in ages above 60 years old (77.5%). Accordingly to Stevens34 and Elewski and Hay,10 estrogens exercise a protective role against onychomycosis, which could be the basis of such differences among dermatomycosis in male and female. Furthermore, aging could also contribute to the augmentation of dermatomycosis occurrence.36
Diabetic's skin is more susceptible to infections uncommon in non-diabetics individuals.32 Our study showed that feet skin lesions were present in 21 patients, whereas one patient showed fungal lesions in leg skin. Thirteen other patients had fungal lesions in both feet skin and nails. We observed a positive association between the appearance of skin lesion in lower limbs and the isolation of fungi.
Other variables including time of disease evolution, family history of dermatomycosis, concurrent intake of immunosuppressive therapy, and peripheral vascular disease, among others, were also analyzed and no significant correlation was found (see Table 1). Our results agree with those of Saunte et al.,32 who studied the presence of onychomycosis in a total of 271 diabetic patients. Oddly enough and contrary to our findings, other authors8,17 found positive associations among these factors and the development of fungal infection.
Some diabetic patients can be obese, which may make the act of bending over to examine their feet difficult.30 Obesity was another risk factor analyzed and despite 45.6% of our patients being obese, only 19.1% presented dermatomycosis.
In our survey, patients with type 2 diabetes showed to have higher prevalence of dermatomycosis than patients with type 1 diabetes (p=0.013), which could be possibly explained by the delay in the disease detection and following glicemic control. In a study performed by Foss et al.,13 diabetic patients without metabolic control presented higher prevalence of dermatophytosis (55.3%), when compared to the ones with controlled levels of glycosylated hemoglobin (12.5%). In our study, we also observed the same in the analyzed individuals (11.1% vs. 20.7%, respectively).
Our survey showed that dermatophytes, yeasts and non-dermatophyte keratinophilic filamentous fungi were collected in 34.1%, 41.8% and 10.4% of the analyzed diabetic patients, respectively. In contrast with this study, Eckhard et al.,9 Romano et al.,31 and Kemna and Elewski22 demonstrated that dermatophytic infection in diabetic foot patients was more common than Candida spp. Other studies,8,18 on the contrary, showed that yeasts were the most common isolate, followed by dermatophytes. According to Rich,30 and regarding onychomycosis in particular, the consensus is that there is probably not an increased incidence of dermatophyte infections of the nail unit in diabetics, but that Candida infection of the nail and surrounding area may be more prevalent in diabetics. C. albicans represented the second most frequently isolated Candida species, with 6.6% of the cases, in agreement to the value found by Kemna and Elewski.22 The most frequently isolated dermatophyte species was T. rubrum, followed by T. mentagrophytes. Our data agree with the ones published by other authors,18,23,32 with T. rubrum as the most frequently isolated. Romano et al.,31 however, found a higher percentage of T. mentagrophytes, which could reflect different specificities of populations analyzed or from the different geographic locales.
Interestingly, in the present study, we have also found other potential keratinophilic fungi as Scopulariopsis spp., Chrysosporium sp., and Scytalidium spp. Direct observation of fungal elements and abundant colony-formation of single species were considered factors for validation of the etiological agent. Aspergillus spp. and other filamentous fungi were not considered because it was not possible to obtain repeated cultures in order to confirm the etiological agent.
Although Elewski11 considers that the culture of a non-dermatophyte fungus from a sample does not definitely prove that it is the etiological agent, there is irrefutable evidence of the causative role of non-dermatophyte fungi in onychomycosis or dermatomycosis.15 Furthermore, mold species were reported as causative agents of fungal infections specifically in the diabetic foot patients.9,27,33,35
It is important to bear in mind some limitations of this study. First, the true frequency of onychomycosis in the group of patients considered, might have been underestimated due to negative cultures to fungi, since some patients who had previous antifungal treatment may yet be under antifungal inhibition. Second, the small study population and the lack of answers in some of the questions asked might have prevented some associations from reaching statistical significance. Nevertheless, this study constitutes the first report on an epidemiological survey of dermatomycosis in diabetic patients in Portugal. Our results raises awareness of the epidemiological situation of the local responsible etiological agents and risk factors involved, leading to a better prevention and recognition of mycotic foot disease in this specific group of patients.
Conflict of interestThe authors declare that there are no conflicts of interest. The authors are fully responsible for the content and writing of the paper.
We are indebted to Eleonora Paixão, for kindly providing statistical mentoring.