During the past decades there has been an increase in cryptococcal infections caused by the basidiomycetous yeast species Cryptococcus gattii sensu lato, among humans and animals that live in endemic regions in Australia, Europe and the Americas. Unlike human cryptococcosis, little epidemiological data are available about C. gattii sensu lato infections in horses.
Case reportA fatal case of a disseminated C. gattii sensu lato infection in an 11-year-old Arabian gelding imported from South Africa into the United Arab Emitares is reported. Tissue samples were studied by conventional mycology procedures and the obtained cryptococcal isolate was molecularly characterized by mating-type determination, amplified fragment length polymorphism (AFLP) fingerprinting, and multi-locus sequence typing (MLST). Phylogenetic analysis was performed to investigate the geographic origin of the cryptococcal isolate. The isolate was identified as Cryptococcus deuterogattii (AFLP6/VGII), mating-type α. Phylogenetic analysis showed that it was closely related to another C. deuterogattii isolate from the Middle East.
ConclusionsA second case of a C. deuterogattii infection in the Middle East is described. It is likely that the horse acquired the infection in the Middle East, as the isolate is closely related to that of a recent human case from that region.
Durante las dos últimas décadas, las infecciones criptocócicas causadas por el hongo levaduriforme basidiomiceto Cryptococcus gattii sensu lato se han incrementado entre los seres humanos y los animales que viven en regiones endémicas de Australia, Europa y América. A diferencia de la criptococosis humana, existen muy pocos datos epidemiológicos disponibles sobre las infecciones por C. gattii sensu lato en los caballos.
Caso clínicoSe expone el caso de una criptococosis diseminada fatal por C. gattii sensu lato en un caballo árabe castrado de 11 años de edad, importado desde Sudáfrica a los Emiratos Árabes Unidos. Las muestras de tejido analizadas por métodos microbiológicos convencionales permitieron el aislamiento de un criptococo que fue posteriormente caracterizado por técnicas moleculares para la determinación del tipo sexual, la obtención del perfil AFLP (amplified fragment length polymorphism) o polimorfismo de tamaño de fragmentos amplificados, y la tipificación por secuenciación multilocus (multi-locus sequence typing [MLST]). Se llevó a cabo un análisis filogenético para investigar el origen geográfico del criptococo aislado. Mediante PCR y AFLP el aislamiento fue identificado como Cryptococcus deuterogattii (AFLP6/VGII) y tipo sexual α. El análisis filogenético mostró que el aislamiento se encuentra muy próximo a otro único aislamiento de C. deuterogattii de Oriente Medio.
ConclusionesEste es el segundo caso descrito de infección por C. deuterogattii en Oriente Medio. Parece que el caballo adquirió la infección en aquella región, ya que el aislamiento muestra una relación muy próxima con otro de un caso reciente en un ser humano de esa región.
Cryptococcosis is a fungal disease that affects humans and animals and is caused by members of the Cryptococcus gattii species complex, a basidiomycetous pathogen that was until two decades ago rarely reported outside tropical and subtropical climate zones.28 Since the onset of several outbreaks, as well as the environmental isolation of C. gattii sensu lato in temperate climate zones, it has become apparent that this pathogen has a larger geographical distribution than the one previously thought.2,8,10,22,25 Until a decade ago it was generally believed that Eucalyptus trees were the primary environmental niche of C. gattii sensu lato, but this was refuted by large-scale environmental sampling that showed that trees in general are the niche.8,16Cryptococcus species are a common human pathogen, and similarly can affect a wide variety of domestic and wild animals that reside in endemic areas such as Australia, Europe and the Americas.6,17,20,24,29
The C. gattii species complex can be divided into five genotypes through molecular techniques such as PCR fingerprinting, PLB1 and URA5 restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP) fingerprinting, multi-locus sequence typing (MLST), and whole-genome sequencing.12,13,23 The C. gattii species complex has recently been taxonomically revised and the five genotypes have been raised to the species level as C. gattii sensu stricto (genotype AFLP4/VGI), Cryptococcus bacillisporus (genotype AFLP5/VGIII), Cryptococcus deuterogattii (genotype AFLP6/VGII), Cryptococcus tetragattii (genotype AFLP7/VGIV) and Cryptococcus decagattii (AFLP10/VGIV).12Cryptococcus gattii sensu stricto and C. deuterogattii have a global distribution, with the former being involved in small outbreaks in Mediterranean Europe and the latter as the cause of large outbreaks in Canada, the Pacific Northwest of the USA, and Western-Australia.2,4,10,22Cryptococcus bacillisporus, C. decagattii and C. tetragattii are mostly involved in cryptococcosis among immunocompromised subjects; the latter is geographically restricted to Africa and India while the other two species are predominantly known from the Americas.7,13
Little epidemiological data are available with respect to C. gattii sensu lato infections among horses, although a recent study showed that it has a greater frequency than infections caused by C. neoformans.9,26,27 Here we present a fatal case of disseminated cryptococcosis in an Arabian horse in the United Arab Emirates (this case report has been presented at the 10th International Equine Infectious Diseases Conference, Buenos Aires, Argentina, April 4–8, 201615).
An otherwise healthy 11-year-old Arabian gelding was presented with chronic respiratory disease, bloody nasal discharge and neurological signs, including ataxia and blindness. Based on the clinical evaluation, a Giemsa-stained nose-smear, and a trans-tracheal lavage cytology, all showing that the lungs and the central nervous system were affected, the diagnosis of disseminated cryptococcosis was made. An initial antifungal treatment with fluconazole (5mg/kg PO SID) was started; after few days, the treatment was switched to amphotericin B (0.5mg/kg dissolved and administered in 1 liter saline with 1% dextrose IV every other day). However the horse continued worsening and was euthanized two months later. Necropsy revealed enlarged lungs with massive edema and grayish consolidation of the entire right lung containing a large and septate jelly-like mass (15cm in diameter), and a jelly-like mass (5×2×2cm) in the brain near the choroid plexus (see figures in reference15). No lesions were seen at the conchae. During necropsy samples from all organs, including the brain, were taken for histology, bacterial and fungal culture. Tissue sections from liver, spleen, lung, brain, kidney and choanae were plated onto Sabouraud dextrose agar (Oxoid, Basingstoke, United Kingdom) and incubated aerobically at 30°C for 48h. Samples from these organs were also plated onto blood agar (Oxoid), Brilliant green phenol lactose sucrose agar (Merck, Kenilworth, NJ, USA) and Nutrient agar (Oxoid), and were incubated aerobically at 37°C for 48h. The identification of the bacteria and the isolated yeast were performed by using the VITEK 2 Compact, VITEK GN and API 20Strept (bioMérieux, Marcy-l’Étoile, France). Massive fibrinopurulent inflammation with numerous round yeast-like organisms with broad halo, typical of a cryptococcal infection, was found with the histology of the septate jelly-like lung mass as well as in the brain mass. Numerous Cryptococcus-like yeast colonies were observed onto the Sabouraud dextrose agar inoculated with lung and brain biopsies, but not from choanae, kidney, liver and spleen specimens. Additionally, Klebsiella pneumoniae subsp. pneumoniae and Streptococcus equi subsp. zooepidemicus were isolated from brain, choanae, kidney, liver, lung and spleen.
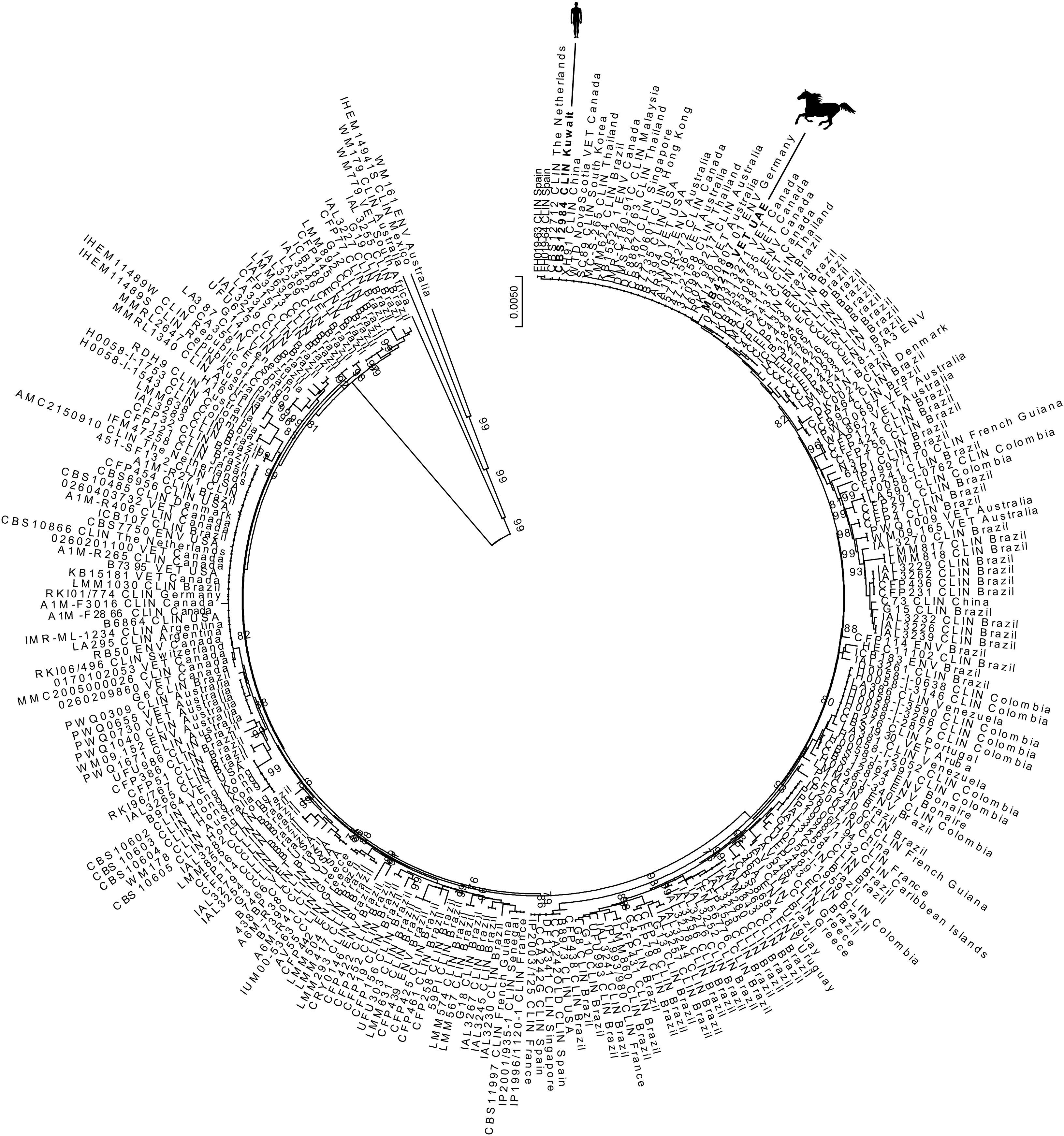
The cryptococcal isolate MB4219, identified as C. neoformans by VITEK 2, was subsequently subjected to molecular characterization by mating-type determination of the STE12 gene and genotyping by AFLP fingerprinting as previously described.12 The isolate was found to be mating-type α and, surprisingly, belonged to the C. gattii species complex as the genotype was found to be AFLP6/VGII, representing C. deuterogattii. The number of cryptococcal infections due to C. gattii sensu lato in the Middle East are sparse, with only two clinical cases known so far. Molecular characterization of the isolate obtained from an immigrant-worker in Kuwait showed that C. deuterogattii was the culprit of the disease in this man.1 The other human C. gattii sensu lato infection came from Egypt, and the stored isolate CBS10583 was later on identified as C. neoformans.21 Similarly to the Egyptian clinical case, the C. gattii sensu lato cultured from Eucalyptus camaldulensis trees in the Gharbia Governorate, Egypt were only phenotypically characterized and as these isolates are not stored it remains unknown to which genotype and/or species they really belong.19 To further elucidate the geographic origin of the equine C. deuterogattii isolate, a 7-loci MLST was performed as described before (Genbank accession numbers KX499486–49949311,12,14). A primary MLST dataset of globally collected C. deuterogattii isolates was generated by combining data from published studies.1,3,5,11,12,14,18,23 A 1000× bootstrapped neighbor-joining analysis showed that the equine isolate MB4219 was closely related to the clinical isolate CBS12984 from Kuwait, as well as to a cluster of environmental veterinary and clinical isolates that had a global origin which is known as the ‘minor Vancouver Island Outbreak genotype’ (Fig. 1).
The chestnut gelding was born in South Africa in 2001 and arrived in the United Arab Emirates via the United Kingdom in September 2008; since then the Arabian horse remained in the United Arab Emirates. So far there are no reports from South Africa and the United Kingdom of C. deuterogattii infections. In South Africa C. gattii sensu stricto (genotype AFLP4/VGI) and C. tetragattii (genotype AFLP7/VGIV) are commonly found among HIV-infected subjects, while in the United Kingdom C. gattii sensu lato has been rarely encountered.7,12,13 As the single C. deuterogattii human case from the Middle East involved an immigrant worker from the Philippines who lived and worked three consecutive years in Kuwait, it was at that time concluded that the patient acquired the infection in his homeland prior to traveling to Kuwait.1 However, the C. deuterogattii isolates of the human case from Kuwait and the equine case from the United Arab Emirates are, based on MLST data, genetically closely related (Fig. 1). This indicates that C. deuterogattii might have a local niche in the Middle East that yet needs to be discovered, which highlights the importance of environmental surveillance for pathogenic microorganisms in this region.
The authors would like to thank Prof. Dr. María Francisca Colom (Alicante, Spain) for preparing the Spanish-language abstract.