Although fortunately very rare in countries with a temperate climate, certain factors, such as clinical or pharmacological immunosuppression, may cause Fusarium-related fungal infections to become an emerging problem. Moreover, Fusarium is one of the most important etiological agents in exogenous endophthalmitis, which is often favored by the disruption of the epithelial barriers.
AimsThe aim of this series of clinical cases is to identify characteristic clinical findings that may allow an early diagnosis and more efficient management of this ophthalmologic emergency.
MethodsThree cases of endophthalmitis due to Fusarium solani and Fusarium oxysporum, diagnosed in 2009, 2010, and 2014 in patients from two different health regions belonging to the same health system and separated by around 43 miles, are presented. The Fusarium isolates were initially identified microscopically and the species subsequently confirmed by sequencing the elongation factor alpha (EFα) and internal transcribed spacers (ITS). Susceptibility to antifungal agents was determined using the EUCAST broth dilution method.
ResultsEvolution was poor as two of the three patients progressed to phthisis bulbi despite surgical measures and broad-spectrum antifungal antibiotic therapy.
ConclusionsIt is essential to rapidly instigate multidisciplinary measures to combat suspected endophthalmitis due to Fusarium given the poor prognosis of this type of infection.
Afortunadamente, las infecciones por Fusarium son poco frecuentes en países de clima templado; sin embargo, determinados factores como la inmunodepresión clínica o farmacológica, pueden convertirlas en un problema emergente. Fusarium es uno de los microrganismos etiológicos más importantes de la endoftalmitis exógena, favorecida habitualmente por una rotura de las barreras epiteliales.
ObjetivosEn esta serie de casos clínicos queremos identificar hallazgos clínicos característicos que puedan establecer un diagnóstico temprano y un tratamiento más eficiente de esta urgencia oftalmológica.
MétodosSe presentan tres casos de endoftalmitis por Fusarium solani y Fusarium oxysporum que se produjeron en los años 2009, 2010 y 2014, en pacientes de dos áreas de salud diferentes, pero pertenecientes al mismo sistema sanitario, las cuales distan 43 millas una de la otra. Las cepas aisladas de Fusarium se identificaron inicialmente por microscopia y su identidad se confirmó posteriormente mediante secuenciación del factor de elongación alfa (EFα) y de la región codificadora espaciadora interna (ITS). La sensibilidad a los antifúngicos se llevó a cabo por el método de dilución en caldo del EUCAST.
ResultadosSe produjo una mala evolución, ya que dos de los tres pacientes evolucionaron haca la atrofia ocular a pesar de las medidas quirúrgicas y el tratamiento antibiótico y antifúngico de amplio espectro.
ConclusionesEs importante actuar rápidamente con medidas multidisciplinarias ante la sospecha de una endoftalmitis por Fusarium por el mal pronóstico de este tipo de infecciones.
Although fungal eye infections are fortunately not common, an immunocompromised condition or the use of increasing number of drugs in patients with ophthalmologic disorders may cause these infections to become an emerging problem. Some of these ophthalmologic drugs, such as corticosteroids, long-term treatment, or the use of broad-spectrum antibiotics in damaged corneas, may be risk factors for the development of fungal endophthalmitis (FE).9,13,25 The origin and etiology of this disease can vary, with Fusarium being one of the most important etiologic agents in exogenous FE (directly from an external source such as keratitis, trauma, or intraocular surgery).21,25 A dysfunctional epithelial barrier is required for Fusarium to colonize the corneal stroma. Once inside the stroma, this microorganism produces an intense inflammatory reaction that breaks down internal barriers, thus leading to rapid tissue degradation and the characteristic clinical features of endophthalmitis.13 In this case series, we aim to identify clinical findings characteristic of Fusarium infection that may enable a prompt diagnosis and a more efficient management of this ophthalmologic condition.
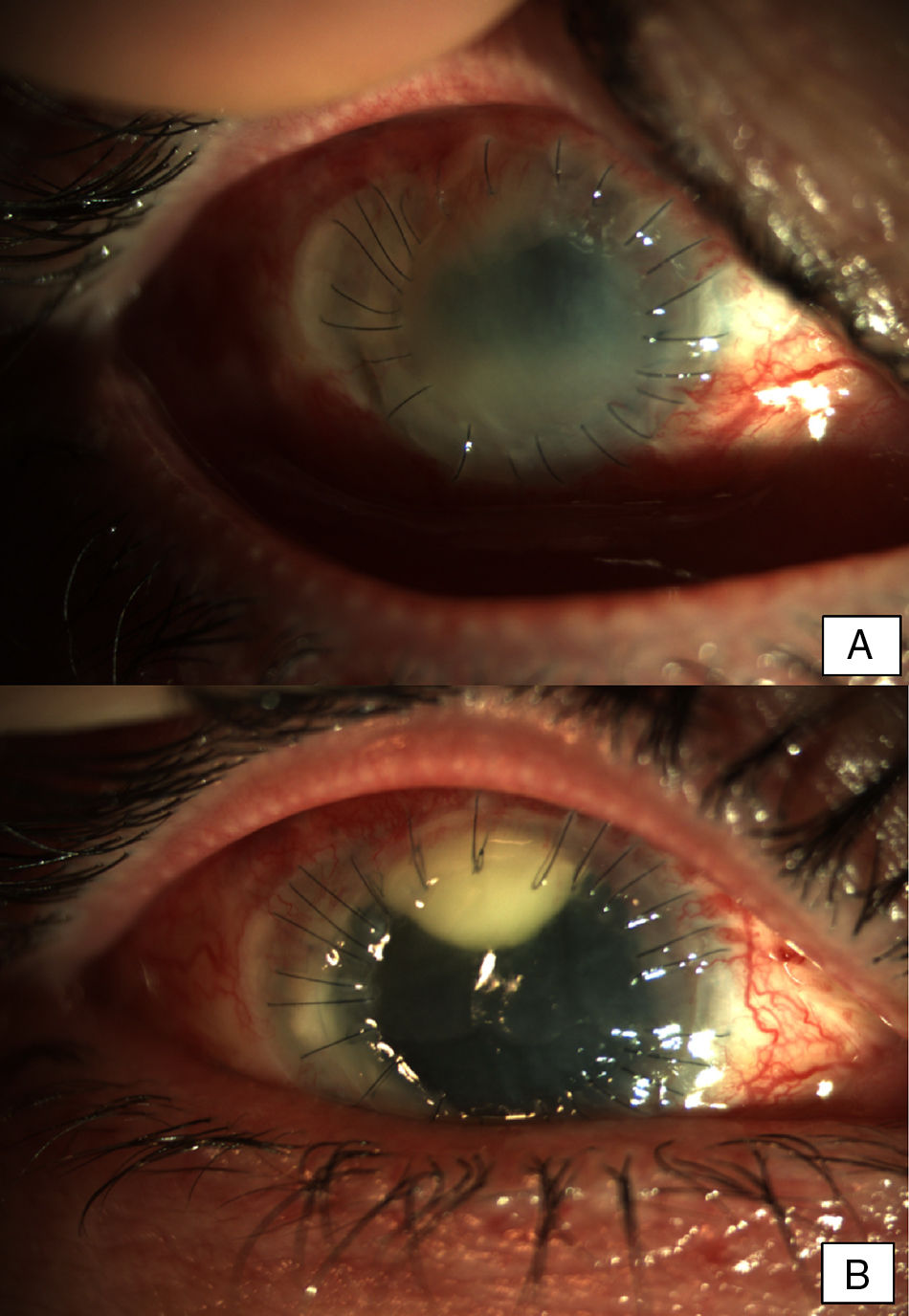
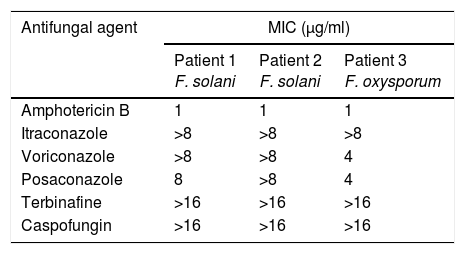
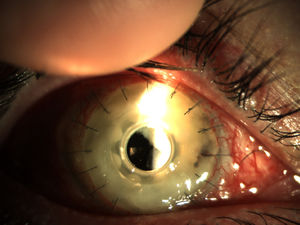
Patients and methodsPatient 1A 79-year-old woman, wearer of soft contact lenses, was sent to our casualty department by her general practitioner. She complained of having had redness and discomfort of the eyes for the past 15 days which did not improve with topical tobramycin and oral azithromycin. A central corneal ulcer with associated stromal infiltrate was found to be the cause. A corneal sample was taken and the initial result was positive for coagulase-negative Staphylococcus. As there was a strong suspicion of fungal infection, treatment with fortified topical antibiotics and topical voriconazole was prescribed. Ten days later the infection progressed to endophthalmitis, therefore posterior vitrectomy and a penetrating keratoplasty was performed. Samples of the cornea, vitreous humor, and crystalline capsule were sent for microbiological analysis, and Fusarium solani species complex was obtained in culture (Fig. 1). Antifungal susceptibility testing was performed using the EUCAST method and the MICs were as follows: amphotericin B, 1μg/ml; caspofungin, >16μg/ml; itraconazole, >8μg/ml; voriconazole, >8μg/ml; posaconazole, 8μg/ml, and terbinafine, >16μg/ml. Despite changing the treatment to amphotericin B, the graft failed and developed an exudative retinal detachment. At this point no additional interventions were considered to be necessary. The eye currently remains free from infection but in a state of phthisis bulbi (phthisis bulbi is the end-stage ocular response to trauma and/or severe ocular disease. The presentation is that of a very soft, atrophic, blind, and shrunken eye with disorganization of the intraocular structures).
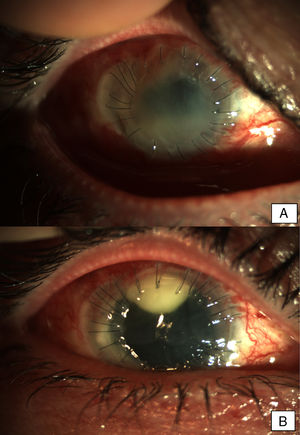
Patient 2A 56-year-old woman, wearer of soft contact lenses, was admitted to our department complaining about redness and discomfort in both eyes for the past 48h. She had been using anesthetic drops since the onset of the symptoms. Slit lamp examination showed bilateral conjunctival inflammation, a central corneal erosion, and stromal keratitis in the left eye. Treatment with topical ciprofloxacin was initiated and a conjunctival exudate sample was taken for microbiological examination. After clinical worsening, the treatment was changed to topical vancomycin and ceftazidime on the second day. Five days later, the patient showed bilateral central infiltrates with severe anterior chamber inflammation and hypopyon in both eyes. Due to the rapid evolution of the eye infection despite intensive treatment with antibiotics, and given the clinical suspicion of fungal endophthalmitis, antimicrobial treatment was extended to include topical voriconazole. The microbiological cultures were positive for Fusarium oxysporum species complex and voriconazole was replaced with topical 5% natamycin. Due to progressive stromal melting, further surgical management with intracameral ceftazidime, vancomycin, voriconazole, and an amniotic membrane graft was performed. A keratoplasty on the left eye to re-establish the corneal architecture was performed (Fig. 2A). Keratoplasty and phacoemulsification with intraocular lens implantation was carried out on the right eye two months after the onset of symptoms. The same procedure had to be repeated on the left eye nine months later (Fig. 2B). The evolution was good and a transparent keratoplasty with no signs of rejection was observed in both eyes in subsequent medical checks.
Early postoperative findings after tectonic penetrating keratoplasty in the left eye. Corneal transplant is edematous and shows fibrotic changes. Note the superior neovascularization secondary to graft failure. Early postoperative findings in the right eye after the first penetrating keratoplasty. Severe conjunctival infection with a dense superior infiltrate in the interface suggestive of infection. The transplant appears edematous and with Descemet folds in the visual axis.
A 66-year-old male with posterior polymorphous corneal dystrophy had received in his right eye six previous failed penetrating keratoplasties, cataract surgery, and one trabeculectomy for glaucoma. He had also undergone four previous failed keratoplasties, cataract surgery, limbal allograft transplantation, and trabeculectomy in his left eye. In September 2007, the patient underwent Boston type I keratoprosthesis surgery on his right eye, reaching a BCVA (best corrected visual acuity) of 20/40. During follow-up he developed cystic macular edema, which was successfully treated with intravitreous steroids and bevacizumab. In August 2008 he developed cornea melting in the inferior hemicornea, which was unsuccessfully treated with topical medroxyprogesterone and tetracyclines, therefore a new Boston keratoprosthesis type I for aphakia was repeated in October 2008, achieving a BCVA of 20/60 despite his glaucoma (disc excavation 5/6). In December 2008, he developed vitritis, which was treated with pars plana vitrectomy, with the microbiological conclusion that the vitritis was sterile. The patient was prescribed oral prednisone, topical vancomycin bid, topical tobramycin bid, topical dexamethasone bid, Combigan® bid (for glaucoma), and topical medroxyprogesterone tid.
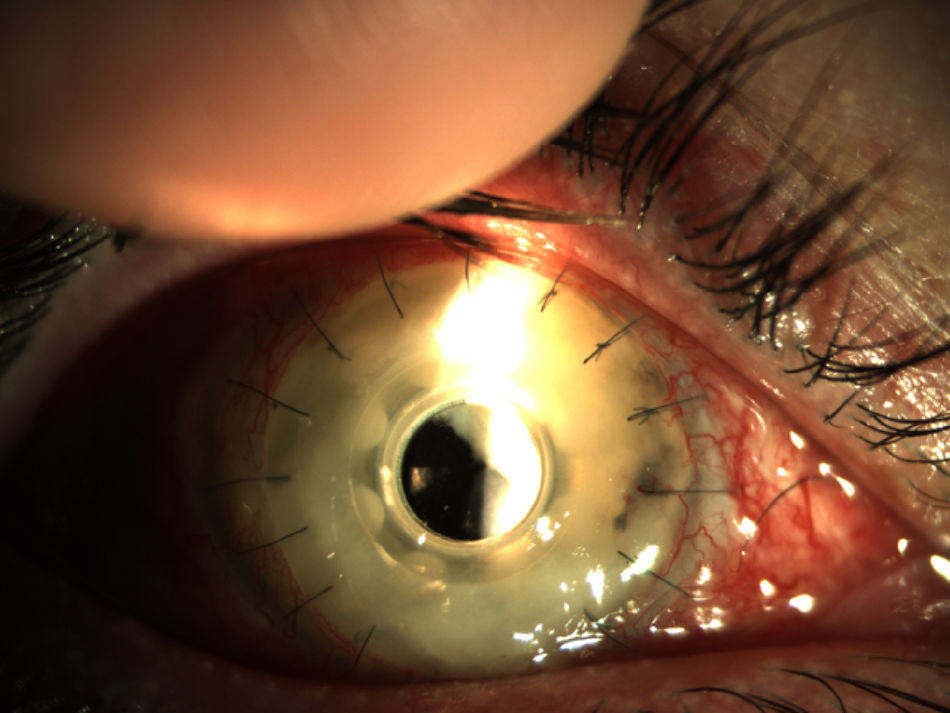
Two months later, the patient presented a corneal feather infiltrate (Fig. 3) suggestive of a vitreous fungal infection. Samples of the cornea and vitreous humor were cultured and the patient started with topical 1% voriconazole six times a day as well as vancomycin and topical medroxyprogesterone. Two days later a new pars plana vitrectomy was performed, removing the Boston keratoprosthesis and inserting a penetrating keratoplasty. The cultures yielded filamentous colonies of a fungus that was identified as F. oxysporum species complex. The patient initiated oral voriconazole and topical voriconazole, vancomycin, tobramycin, cyclosporine A, and ketorolac.
Endophthalmitis in a patient with Boston type I keratoprosthesis (Boston KPro). The whole cornea was infiltrated (keratitis) and the posterior chamber was also involved (endophthalmitis). Prompt surgery is imperative in these patients (Pars plana vitrectomy+remove the KPro and replace with a penetrating keratoplasty).
Seven days later, with a new fungal infiltrate in the keratoplasty and on the basis of the susceptibility testing results (Table 1), the patient started systemic and topical amphotericin B, which controlled the endophthalmitis but, unfortunately, the eye developed phthisis bulbi.
Fungal isolates were identified as Fusarium spp. based on the characteristics of colonies grown on potato-dextrose agar (PDA) at 35°C and microscopic examination with lactophenol cotton blue stain.
Identification of the species was done by molecular methods. The isolate was cultured in GYEP medium (0.3% yeast extract, 1% peptone; Difco, Soria Melguizo SA, Madrid, Spain) with 2% glucose (Sigma Aldrich Química, Madrid, Spain) for 24–48h at 30°C. Genomic DNA was extracted using a previously described procedure.12 DNA segments comprising a region of elongation factor alpha (EFα) and the internal transcribed spacers (ITS) were amplified using the primers EF1 (5′-ATGGGTA AGARGACAAGAC-3′), EF2 (5′-GGARGTACCAGTS ATCATGTT-3′), ITS1 (5′-TCCGTAGGTGAACCTGCG G-3′), and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) in a GeneAmp 9700 PCR system (Applied Biosystems, Madrid, Spain).14,24 The reaction mixtures contained 0.5μM of each primer, 0.2μM of each deoxynucleoside triphosphate, 5μl PCR buffer (Applied Biosystems), 2.5U Taq DNA polymerase (AmpliTaq; Applied Biosystems), and 25ng DNA in a final volume of 50μl. The samples were amplified in a GeneAmp 9700 PCR system (Applied Biosystems) using the following cycling conditions: 1 initial cycle of 5min at 94°C, followed by 35 cycles of 30s at 94°C, 45s at 47°C (EFα) or 56°C (ITS), and 2min at 72°C, with a final cycle of 5min at 72°C. The reaction products were analyzed on a 0.8% agarose gel and purified with illustra ExoProStar 1-Step (VWR International Eurolab, Spain) following the manufacturer's recommendations. Sequencing reactions were performed with 5μl of the PCR product, 1μl of primers (EF1, EF2, ITS1, or ITS4), and 4μl of DNA using a sequencing kit (BigDye Terminator Cycle Sequencing Ready Reaction, Applied Biosystems) in a final volume of 10μl. Sequences were assembled and edited using the SeqMan II and EditSeq software (Lasergene package; DNAstar, Inc., Madison, WI). Sequence analysis was performed by comparing the DNA sequences with EFα sequences from Fusarium strains obtained from the GenBank database (http://www.ncbi.nih.gov/Genbank/). The sequences were submitted to GenBank database with accession numbers: KY688091, KY688092 and KY688093 for ITS and KY886147, KY886148 and KY886149 for EF. The strains were stored in the collection of filamentous fungi of the National Center for Microbiology with the numbers CNM-CM5601, CNM-CM6230 and CNM-CM7600.
Antifungal susceptibility testingThe in vitro susceptibilities to antifungal drugs were determined using the broth dilution method, following the EUCAST method.3Aspergillus fumigatus ATCC 2004305 and Aspergillus flavus ATCC 2004304 were used as quality-control strains. The antifungal agents used in the study were amphotericin B (range 16–0.03μg/ml) (Sigma Aldrich Química), itraconazole (range 8–0.015μg/ml) (Janssen Pharmaceutica SA, Madrid, Spain), voriconazole (range 8–0.015μg/ml) (Pfizer SA, Madrid, Spain), posaconazole (range 8–0.015μg/ml) (Schering-Plough Research Institute, Kenilworth, NJ), terbinafine (range 16–0.03μg/ml) (Novartis, Basel, Switzerland), and caspofungin (range 16–0.03μg/ml) (Merck & Co., Inc., Rahway, NJ).
DiscussionFungal keratitis and its more serious associated consequences, such as FE, are a major health problem in rural regions of tropical countries, whereas the prevalence of these infections is much lower in warm climate countries.25 However, we must not overlook the possibility of this etiologic agent due to the severity and poor visual outcomes of these infections. Although Fusarium spp. is the leading cause of exogenous FE secondary to keratitis,7,19,25 several predisposing factors, such as corneal trauma or disruption of the ocular barriers, are necessary for an infection to progress. As corneal damage is a leading factor, the use of contact lenses is of particular relevance. The frequent use of these devices, the hygiene required for the proper care of the lenses and, occasionally, low clinical suspicion, may lead to a diagnosis of FE being overlooked.19 Although the origin of two of the cases herein was the use of contact lenses, the patients did not receive antifungal therapy from the beginning as some fungal infections may be clinically undistinguishable from bacterial endophthalmitis.22 As such, the specific treatment was only administrated once the clinical situation had worsened. Surgery is another factor that clearly alters the structure of the eye and may represent a risk factor for this condition. We have to take into account that fungi were isolated in 21.8% of cultures in cases with postoperative endophthalmitis.2,7 The emergence of these surgery-related infections greatly complicates the health of the patient, and outbreaks of FE due to Fusarium following surgery can sometimes appear.5 Our third case reflects this finding, with concomitant steroid and immunomodulator treatment probably increasing the risk of infection with these fungi. More rarely, outbreaks of fungal endophthalmitis may be associated with intraocular use of contaminated products, so physicians should be aware of the use of such compounds.17 A warning in this regard may come from the Microbiology Laboratory, although there are several barriers to submitting a prompt report. Thus, although Fusarium is one of the most common causes of keratitis and FE, with F. solani and F. oxysporum being the most prevalent species,7,15,16 identification may be difficult without experience as this polymorphic fungus could be misidentified or confused with contaminant molds. FE is a destructive intraocular infection that has extremely poor visual prognosis, especially when the Fusarium genus is involved, even after appropriate antifungal treatment. Therefore, rapid communication between the Microbiology Laboratory and practitioners in high suspicion cases should be facilitated as any delay in applying the right treatment may have fatal consequences.9,22 In our cases, despite a rapid beginning of the antifungal treatment, only one patient preserved an acceptable degree of vision following a rigorous and difficult treatment. Unfortunately, the remaining cases had a much worse outcome, thus confirming the poor prognosis of this disease.13
Fusarium is one of the most drug-resistant fungi, with F. solani being the most resistant species in this group.1,9 Our cases involved two F. oxysporum and one F. solani strains, in agreement with other cases in the literature.7,9 When Fusarium is identified as the cause of FE, the multi-resistant condition of this mold and the poor tissue penetration of topical antifungal agents make treatment very difficult. Amphotericin B, which has a low minimum inhibitory concentration (MIC) in vitro, has traditionally been the prescribed antifungal therapy.1,4,9 However, intravitreal injection can cause retinal necrosis, the number of necessary injections is not standardized, and refractory cases may be observed.25,26 Although natamycin has been successfully used to treat endophtalmitis by Fusarium, an intravitreal formulation is not usually available and voriconazole has a varying activity against these molds.7,26 However, despite its high MIC, voriconazole may be a treatment option as it achieves good aqueous and vitreous concentration (53% and 38%, respectively) with oral administration. Furthermore, intravitreal inoculation of voriconazole (25mg/l) is effective in refractory endophthalmitis and the topical formulation has good stability and activity over 21 days.8 In light of the above, topical voriconazole is increasingly being used by ophthalmologists, even as a first-line antifungal therapy.6,10,25 However, despite several doubts regarding its benefits, combined antifungal therapy is usually recommended as an empirically acceptable option to ensure the activity of at least one agent.11,20,23 Furthermore, combined therapy should be started as soon as the suspicion becomes high.18 Likewise, it is clear that such therapy should involve two different types of antifungals and different routes of access in FE. Thus, the simultaneous use of topical, intravitreous, and systemic drugs is recommended due to the severity of the disease and its poor prognosis. The use and combination of antifungals varied in our case series, with widespread use of voriconazole, although combined therapy was started once the diagnosis had been confirmed. A systemic approach with amphotericin B was only used in one case, although this compound does not achieve therapeutic concentrations in the eye and the final outcome was poor, mainly due to the fact that the patient was in an advanced disease state.25 It should be noted that visual outcomes depend on the severity of the infection at presentation, and the prognosis of FE will depend upon the virulence of the organism, the extent of intraocular involvement, and the timing and mode of interventions.7 In light of the above, a rapid reaction is required when the infection is suspected and, in the majority of cases, multidisciplinary measures should be included in the treatment as antimicrobial therapy is not usually sufficient if FE is already established.
Conflict of interestThe authors declare no conflict of interest.
We thank Hedapen global services for the translation and revision of the paper.