Serum amyloid A (SAA) is an acute phase protein that is elevated in blood during inflammation. The role of this protein in allergic diseases of airways remains unclear.
AimsThe objective of this study was to evaluate the SAA in blood, lung and bronchial cells in a murine model of bronchial hypersensitivity to Aspergillus fumigatus.
MethodsTo achieve this purpose, different groups of 5-month-old mice were housed in cages containing hay bedding that was contaminated with A. fumigatus and were kept in an isolation room for 16 days to allow for the induction of allergic airway inflammation. Subsequently, the mice were then exposed once again to Aspergillus spores at 0, 2, 8, 24 and 72h, and they were bled to acquire serum and sacrificed to obtain bronchoalveolar lavage fluid (BALF) or lung tissues for analysis. SAA levels were measured in lung, serum and BALF by dot blot assay and RT-PCR (reverse transcription polymerase chain reaction).
ResultsThe results indicated that SAA protein levels increased in both serum and lung within 2–24h after mice were exposed to Aspergillus spores. Moreover, the SAA mRNA expression levels in the lungs and BALF cells demonstrated the same trend that was observed for the protein levels through the dot blot assay; in particular, SAA mRNA levels increased within the first hour after mice were exposed to A. fumigatus.
ConclusionsIn this allergic airway model, we conclude that A. fumigatus can induce an acute inflammatory response in the airways through the stimulation of the SAA protein, increasing its levels in serum, lung tissue and BALF samples during the early hours of exposure of mice that have been sensitised for this fungus.
La proteína amiloide A sérica (AAS) es un reactante de fase aguda cuyos valores sanguíneos aumentan durante los procesos inflamatorios agudos. Todavía no se ha dilucidado el papel que desempeña en las enfermedades alérgicas de las vías respiratorias.
ObjetivosEl objetivo del presente estudio fue examinar los valores de AAS en sangre, tejido pulmonar y células bronquiales en un modelo murino de hipersensibilidad bronquial frente a Aspergillus fumigatus.
MétodosDiferentes grupos de ratones de 5 meses de vida fueron alojados en jaulas cuyos lechos de paja estaban contaminados por A. fumigatus y se mantuvieron en una sala de aislamiento durante 16 días para permitir la inducción de inflamación alérgica de las vías respiratorias. Tras este período de inducción, a las 0, 2, 8, 24 y 72h los animales se expusieron de nuevo a esporas de Aspergillus. En cada tiempo de reexposición se obtuvieron muestras sanguíneas de los animales y, acto seguido, fueron sacrificados para obtener líquido de lavado broncoalveolar y muestras de tejido pulmonar. La concentración de AAS se analizó mediante técnica de hibridación del ADN (Southern) y reacción en cadena de la polimerasa-retrotranscriptasa en muestras de suero, tejido pulmonar y células de líquido de lavado broncoalveolar.
ResultadosLos resultados del presente estudio demuestran que, al cabo de 2-24h de la exposición a A. fumigatus aumentaron los valores de proteína AAS en muestras de suero y tejido pulmonar. Además, en células de líquido de lavado broncoalveolar y muestras de tejido pulmonar los niveles de expresión de ARNm de AAS demostraron la misma tendencia, y, en particular, aumentaron al cabo de la primera hora de exposición a las esporas de A. fumigatus.
ConclusionesEn este modelo murino de alergia de las vías respiratorias, concluimos que A. fumigatus puede inducir una respuesta inflamatoria aguda de las vías respiratorias a través de la estimulación de la proteína AAS, aumentando su concentración sérica en muestras de tejido pulmonar y de líquido de lavado broncoalveolar durante las primeras horas de exposición de ratones sensibilizados frente a este hongo.
Allergic airway inflammation is one characteristic feature of asthma, with additional pathology including a reversible airway obstruction, airway hyperresponsiveness (AHR), the infiltration of eosinophils and T helper type 2 (Th2) cells into the airway submucosa, mucus hypersecretion, and airway remodelling.2 Allergic airway diseases are inflammatory disorders in which aberrant immune regulation occurs and susceptible individuals display allergen-specific responses. In these responses, inflammatory cells are recruited to the asthmatic airways or are activated in situ. These inflammatory cells include mast cells, macrophages, eosinophils, T lymphocytes, dendritic cells, basophils, neutrophils, and platelets.3
Aspergillus fumigatus is a saprophytic fungus that can survive and grow on a wide variety of organic remains. Its most common ecological niche is the ground. Because of the ease of dispersion of its conidia, A. fumigatus is one of the most ubiquitous fungi in the world.13 The small size of these conidia, which range from 2 to 3μm, allows them to remain in suspension in the environment for a long period of time; thus, humans are constantly exposed to inhaling airborne conidia, which can allow these conidia to reach the pulmonary alveoli.1 In immunocompetent patients, A. fumigatus can produce allergic bronchopulmonary aspergillosis (ABPA), allergic rhinosinusitis, and asthma.11A. fumigatus produces a significant number of allergenic molecules that react with IgE in asthmatic patients and in patients with ABPA.10
Serum amyloid A (SAA) is an acute phase protein that is elevated in the blood during infection, trauma, surgery, burn injury, tissue infarction, inflammation, neoplasia and stress.7 SAA production is primarily induced by IL-6, IL-1 and tumour necrosis factor α (TNF-α), which are multifunctional cytokines that are produced by many different types of cells in the human body.6,7 Traditionally, SAA was considered to be produced by hepatocytes and subsequently secreted into serum. However, one published study22 has demonstrated that SAA mRNA is normally expressed in the epithelial components of a variety of human organs and tissues. SAA could be released locally in certain organ-specific diseases, as demonstrated by several different groups of researchers.5,14,20 However, very few investigations have focused on the relationship between SAA and asthma. Several studies have indicated that a positive correlation exists between SAA and the prevalence of asthma and have concluded that bronchial asthma causes not only local inflammation but also systemic inflammation.8 Moreover, other authors have demonstrated that blood SAA concentrations are greater than normal in patients with asthma and allergic rhinitis.17
The objective of the current study was to evaluate whether SAA levels increase in provoked lung, blood or bronchoalveolar lavage fluid (BALF) cells in the context of a murine model of allergy airway inflammation and to discover whether SAA could be used as an inflammatory marker of allergy airway inflammation. Our hypothesis is that within several hours, the components of A. fumigatus may stimulate innate immunity through increases in the levels of the acute phase protein SAA, a factor in the initial bronchial allergy inflammation response.
Materials and methodsAnimalsFor all of the experiments in this study, we used 5-month-old, sex- and age-matched Rockefeller (RK) mice. These animals were obtained from and maintained at the Animal Facility of the Universidad Austral de Chile. During the exposure of these animals to A. fumigatus, they were placed in an isolation room with appropriate ventilation and filtering systems. This study was approved by the Bioethics Committee for the Use of Animals in Biomedical Research of the Universidad Austral de Chile.
Exposure of mice to A. fumigatus sporesDifferent groups of 5-month-old mice (eight mice per group) were housed in cages containing hay bedding that was contaminated with A. fumigatus and were kept in an isolation room for 16 days to allow for the induction of allergic airway inflammation, as described by previously published procedures.15 After 16 days of exposure to this mould, the mice were placed in a remission environment for 10 days with the purpose of having animals in basal inflammatory condition before starting the antigenic challenge. Subsequently, the mice were once again exposed to the Aspergillus spores. At 0, 2, 8, 24 and 72h after this exposure, the mice were bled to acquire serum and sacrificed with an overdose of a sodium barbital anaesthetic (Serve, USA) to obtain BALF or lung tissues for analysis as described by previously published procedures.15
Determination of serum amyloid A levels in serum and lung by immunodetectionThe determination of SAA levels in the lungs and sera of the mice was performed by the dot blot immunodetection technique, which was performed as described in previously published sources.23 Briefly, 1μl quantities of undiluted samples of serum and lung extract proteins were applied to a nitrocellulose membrane and allowed to dry completely. The membrane was then blocked for 1hour (h) with the following blocking solution: 1× Tris Buffer Solution (TBS) (40g NaCl, 12.1g Tris, pH 7.6), 5% skim milk and 0.5% Tween-20. After the blocking was completed, the membrane was incubated overnight (with constant stirring) at room temperature with goat anti-mouse SAA1 antibody (R&D Systems, USA) that had been diluted by 1:1000 in blocking solution. Following this incubation, the membrane was washed three times with phosphate-buffered saline (PBS; pH 7.2) for 5min per wash and incubated for 1h with alkaline phosphatase-conjugated bovine anti-goat IgG (Jackson ImmunoResearch Laboratories, Inc., USA) that had been diluted by 1:2000 in blocking solution. The membrane was then washed again and equilibrated for 10min with buffer for the alkaline phosphatase enzyme. Immunoreactivity was revealed using an NBT/BCIP substrate (Promega Co., USA) (containing 0.16mg/ml and 0.33mg/ml of NBT and BCIP, respectively), which was dissolved in the buffer in accordance with the manufacturer's guidelines. The samples were later digitised and the intensity levels of the pixels from the same area for each sample were quantified using the Image J software package; we then calculated the fold increase in SAA1 for each sample relative to time 0.
Determination of serum amyloid A expression levels in lung and bronchoalveolar lavage fluid cells through reverse transcription polymerase chain reactionTotal RNA Kit (TRK) lysis buffer (Omega Bio-Tek Inc., USA) was prepared by adding 14μl of β-mercaptoethanol to every 700μl of lysis buffer. A total of 700μl of autoclaved TRK lysis buffer was added to homogenised pulmonary tissue or BALF cells in a sterile 2ml Eppendorf tube. The solution was then added to a HiBind column (Omega Bio-Tek Inc., USA) in a collection tube and centrifuged at 9000×g for 60s at 15°C. The flowthrough was discarded. The column was placed in a new 2ml collection tube and washed with 500μl of RNA wash buffer I (Omega Bio-Tek Inc., USA), then centrifuged as described above, again discarding the flowthrough. Subsequently, the column was washed with 500μl of RNA wash buffer II (Omega Bio-Tek Inc., USA) that had been diluted with absolute ethanol and centrifuged at 9000×g for 60s at 15°C. A portion of the flowthrough was reused over the collection tube in the next step, whereas the excess flowthrough was discarded. In particular, the collection tube was back-washed with 350μl of wash buffer II in absolute ethanol and centrifuged as described above; the flowthrough was discarded. The collection tube was then centrifuged at 12,000×g in a column for 2minutes at 15°C to completely dry the array. The RNA column was then inserted into a 15ml centrifuge tube, and the RNA was eluted in 100μl of deionised water that had been rendered RNAse-free through treatment with 0.1% diethyl pyrocarbonate (DEPC, Sigma); the water was added directly to the column matrix, and the column was then centrifuged for 1min at 9000×g. Once the RNA was isolated, an ImProm II Kit (Promega Co., USA) was used in accordance with the manufacturer's instructions to generate cDNA. For each PCR amplification, 1μl of the cDNA that was synthesised in the previous step was used as a template; this cDNA was combined with 24μl of a mix containing all of the other necessary PCR reagents in a 0.2mL tube. The final concentration of the reaction components was as follows: 1× Taq polymerase buffer, 1mM MgCl2, 100μM of each dNTP, 0.4μM of both the sense (5′ GGG TCA AGG AAC AGA AGC A 3′) and antisense (5′ TGA AGG CAG AGG TGA AAG C 3′) oligonucleotides for SAA1 and 1U of Taq DNA polymerase (Go Taq, Promega Co., USA) in a final volume of 25μL. The amplifications were performed in a PCR Sprint Thermal Cycler (Thermo Scientific Corporation). The PCR protocol utilised an initial denaturation at 94°C for 3min, which was followed by a series of 40 cycles (95°C for 45s, 55°C for 45s, and 72°C for 45s) and a final 5min elongation step at 72°C. In addition, housekeeping genes (GAPDH; sense: 5′ CTC ATG ACC ACA GTC CAT GC 3′, antisense: 5′ GCC TGC TTC ACC ACC TTC TT 3′) were used as loading controls for the reverse transcription polymerase chain reaction (RT-PCR) amplifications. The products that were obtained for SAA1 (284bp) and GAPDH (390bp) were resolved on a 2% agarose gel, using electrophoresis in 1× TAE (10mM Tris–HCl, 0.1% acetic acid, 1mM EDTA, pH 8.0). The intensity of the PCR product band that was obtained for each gene was quantified using the Image J software package; we then calculated the SAA/GAPDH ratio.
Data analysisThe values of SAA from the dot blot results and the SAA/GAPDH ratio were calculated using the Prism software package (version 5.0; Graphpad) for graph generation and statistical analysis. The differences between groups (different sampling times) were determined with Tukey's multiple comparison test.
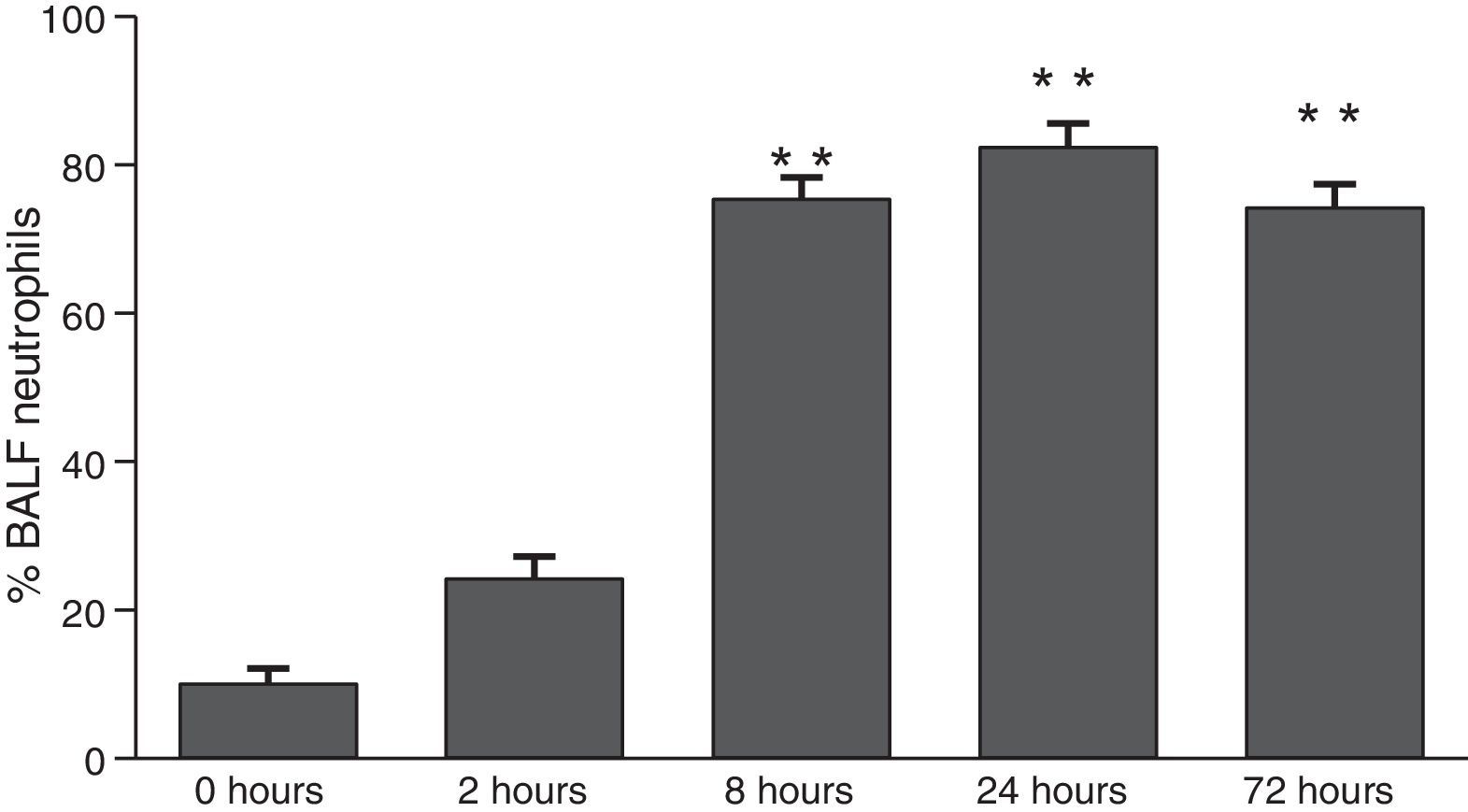
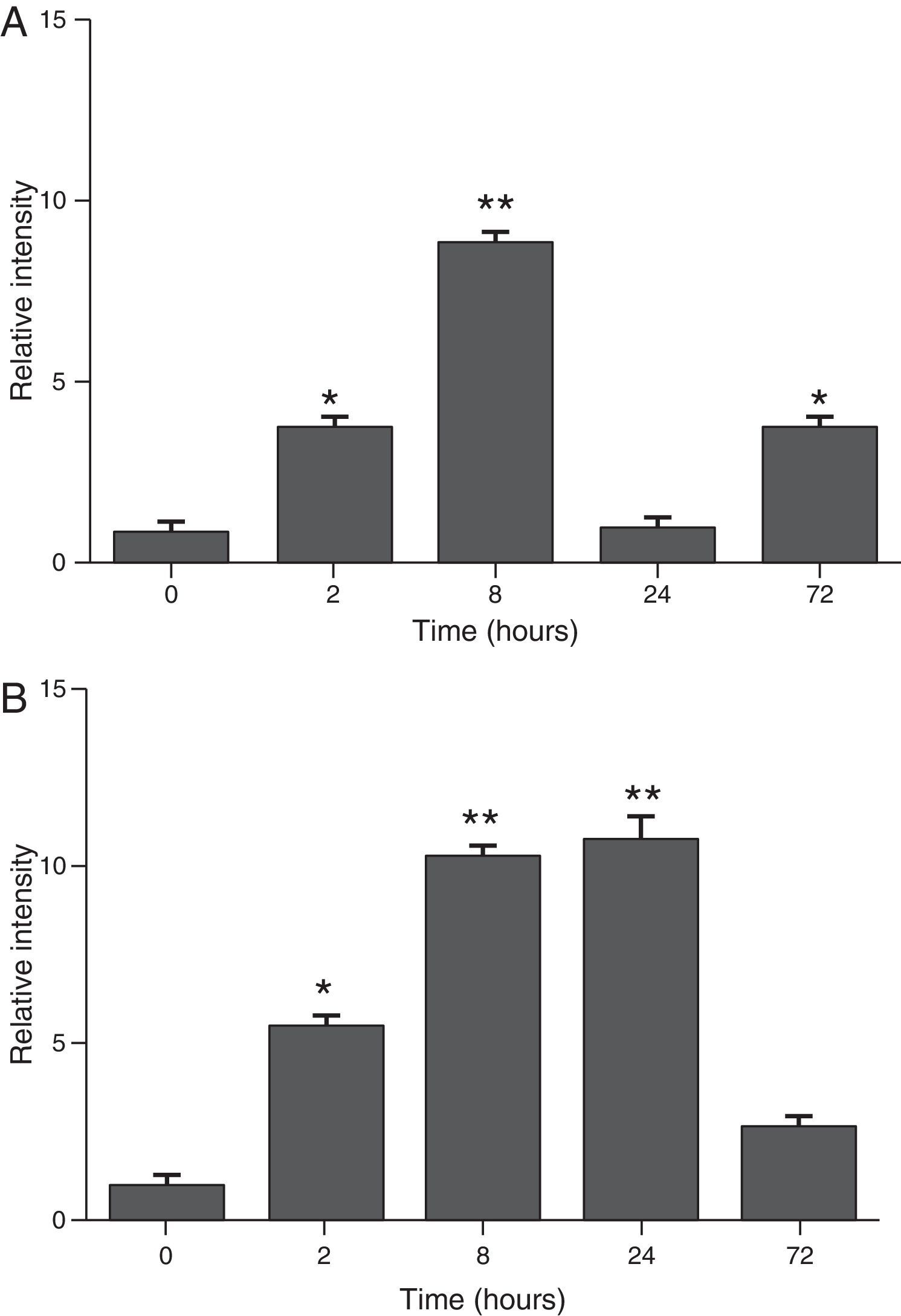
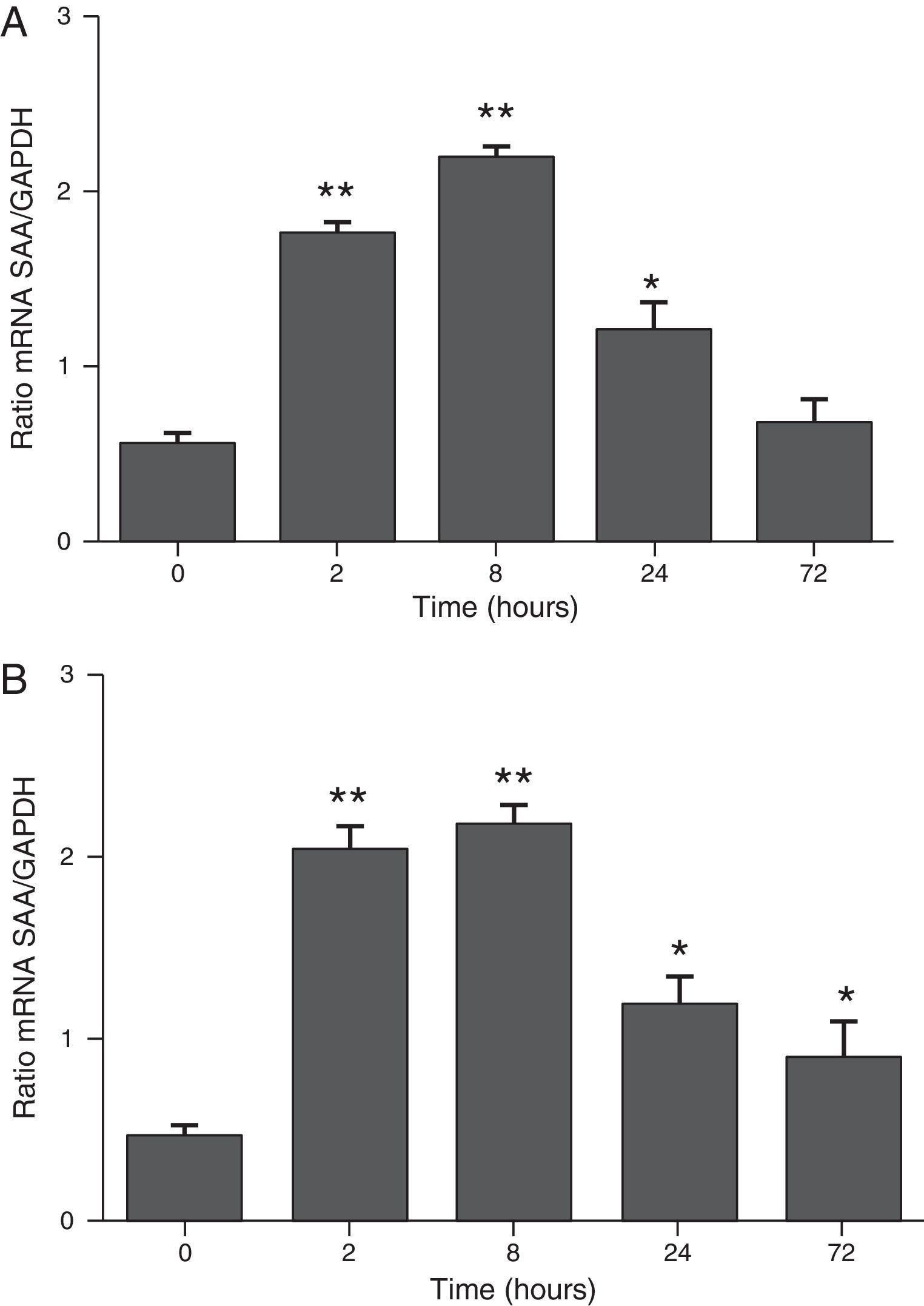
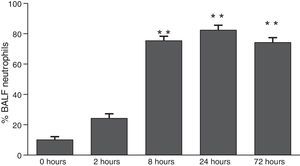
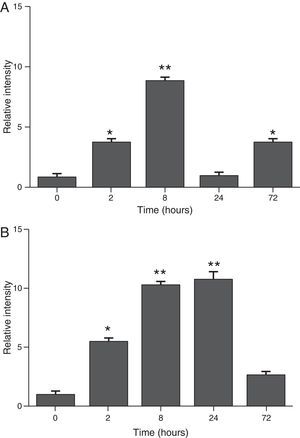
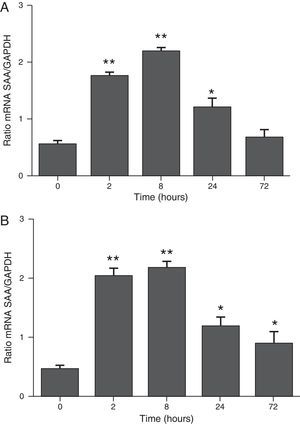
ResultsThe differential neutrophil counts in the BALF from A. fumigatus-exposed mice are shown in Fig. 1. The cells recovered from the BALF of the exposed mice exhibited a significant increase in the proportion of polymorphonuclear neutrophils after 2h. There were significantly greater proportions of neutrophils between mice that were exposed from 8 to 72h with those who were exposed to A. fumigatus for 2h. As shown in Fig. 2A, in mice that were sensitised to A. fumigatus, a subsequent exposure to the fungus spores produced an increase in blood serum SAA by 2h post exposure, with a peak in SAA levels at 8h post exposure. In these mice, pulmonary levels of SAA increased by 2h post exposure, peaking at 24h post exposure and decreasing to normal levels by 72h post exposure (Fig. 2B). Moreover, the expression of SAA mRNA in the lungs demonstrated the same trend that was observed for SAA protein levels in the dot blot assay; in particular, SAA mRNA levels increased by 8h after the exposure to A. fumigatus spores, although these levels began to decline by 24h post exposure (Fig. 3A). Similarly, the expression of SAA mRNA in BALF cells demonstrated a significant increase from baseline levels by 2h post exposure, remained constant until 8h post exposure, and had declined from peak levels by 24 and 72h post exposure. However, the SAA mRNA level at 72h post exposure remained significantly greater than the SAA mRNA level at 0h (Fig. 3B).
Determination of SAA levels by dot blot immunodetection in mice at 2, 8, 24 and 72h after exposure to A. fumigatus. (A) SAA levels in serum samples. (B) SAA levels of lung protein extracts. SAA levels are expressed in terms of relative intensity (the fold increase compared with time 0) for each experimental group of mice. The bars represent the average result from triplicate experiments for serum and lung protein extracts at each sampled time point (* and ** indicate that p<0.05 and p<0.01, respectively, in comparisons among the groups of mice that were sampled at different times after the exposure to A. fumigatus).
The determination of SAA mRNA expression by RT-PCR in mice at 2, 8, 24 and 72h after exposure to A. fumigatus. (A) The expression of SAA mRNA in BALF cells. (B) The expression of SAA mRNA in the lungs. SAA mRNA expression levels are presented in terms of the SAA1/GAPDH ratio for each experimental group of mice. The bars represent the average result from triplicate experiments for BALF cells and lung samples for each sampled time point (* and ** indicate that p<0.05 and p<0.01, respectively, in comparisons among the groups of mice that were sampled at different times after the exposure to A. fumigatus).
The protein SAA is considered to be major acute phase reactant and effector of innate immunity in all vertebrates.24 Although numerous studies have examined the mammalian acute phase response, the particular function of SAA remains unknown.9,12,21,24A. fumigatus spores can quickly stimulate immune responses and are responsible for a spectrum of respiratory ailments, ranging from pulmonary colonisation to more invasive diseases. In particular, Aspergillus species are associated with hypersensitivity in respiratory disorders that include asthma, ABPA, allergic sinusitis and hypersensitivity pneumonitis.19 In the current study, mice were exposed to fungus spores to generate a bronchial hypersensitivity response characterised with great neutrophils airways infiltration as previously published.15
In general, airway inflammation involves the activation of pathogenic-specific inflammatory cells, the modulation of transcription factors and the release of inflammatory mediators.3 Allergic asthma, which is classified as a type I hypersensitivity reaction, involves the binding of allergen-specific IgE immunoglobulins to high-affinity Fc¿ receptors (Fc¿Rs) on the surfaces of basophils and mast cells that are present in the subepithelial layer of the airways.25 A number of mediators that are released from macrophages and neutrophils are thought to play an important role in either the initiation or persistence of asthmatic inflammation.18,26 IL-1β, IL-6, IL-8, platelet-activating factor (PAF) and TNF-α are markers released from macrophages that stimulate many inflammatory cells and trigger the production of acute phase proteins.3,7,22,26 This response might cause the increase in the levels of the well-known acute phase reactant SAA in bronchial tissue that occur during airway inflammation. Our observations, which indicate the presence of high blood SAA levels during the airway inflammation response, suggest that SAA might be synthesised from other sources in addition to the airways and could therefore agree with the conjecture that asthma involves not only local inflammation but also systemic inflammation. Similar hypotheses and results have been reported by other authors, who have demonstrated that SAA levels were significantly increased in both serum and sputum samples after airway inflammation.17 These high blood levels of SAA support the notion that systemic inflammation occurs in asthma; moreover, these SAA levels could reflect the inflammatory state in asthma and may be used as a monitor of inflammation.17 Similar studies were described by Jousilahti et al.,8 who identified a positive correlation between SAA levels and the prevalence of asthma, suggesting that bronchial asthma involves both systemic inflammation and local inflammation. Furthermore, Büyüköztürk et al.4 have revealed that serum SAA concentrations are higher in patients with asthma and allergic rhinitis compared with healthy patients.
Traditionally, SAA was considered to be produced by hepatocytes and subsequently secreted into the serum. However, Urieli-Shoval et al.22 demonstrated that SAA mRNA transcripts are normally expressed in the epithelia of many organs and tissues.6,7 Therefore, SAA could be released locally in organ-specific diseases; this conjecture is supported by the fact that the increased expression of SAA on both the mRNA and protein levels has been found in atherosclerotic lesions in humans.14 In certain disorders, the SAA levels have prognostic significance; for instance, high levels of SAA in cystic fibrosis indicate the antibiotic resistance of Pseudomonas aeruginosa infections20 and a greater risk for the development of systemic amyloidosis.5
The results that were obtained in this study demonstrated that SAA levels increased in serum, lung and BALF samples in the early hours following an exposure to A. fumigatus; in particular, SAA levels were greater than normal in these samples from 2 to 72h post exposure, with peak expression at approximately 8h post exposure. Similar results were obtained in a previously published study of SAA kinetics.16 This earlier study demonstrated that a stimulus can cause SAA levels to rapidly increase in the mammalian plasma and that this increase briefly persists; in particular, it was described that the SAA protein levels in mice peaked at approximately 12h post stimulus and that these levels will remain high until approximately 72h post stimulus before steeply declining. A similar situation has been described in humans, and could indicate an overlap between SAA kinetics and the kinetics of the C-reactive protein. However, SAA plasma levels increase earlier in the acute phase response than C-reactive protein levels.16
In this allergic airway model, we conclude that A. fumigatus can induce an acute inflammatory response in the airways through the stimulation of the SAA protein, increasing its levels in serum, lung tissue and BALF samples during the early hours of exposure of mice that have been sensitised for this fungus. However, further studies are required to understand better the role of SAA protein as an inflammatory marker during the initial response to allergen exposures.
Conflict of interest statementNone of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
This work was supported by DID-UACH-S-2009-22 of Universidad Austral de Chile.