The incidence of vulvovaginal candidiasis, a common infection among healthy women primarily caused by the yeast Candida albicans, has increased significantly in recent years.
AimsThe purpose of this study was to compare the efficacy of ravuconazole (RVC) and fluconazole (FLC) in the treatment of experimental C. albicans vaginitis.
MethodsForty isolates of C. albicans were screened for their in vitro susceptibility to RVC and FLC. A strain of C. albicans that was resistant to FLC (minimum inhibitory concentration [MIC] of >64μg/ml) was selected for the in vivo study. Treatment regimens for the murine vaginal infection model were (1) 1, 5, 10, and 20mg/kg RVC once daily, (2) 20mg/kg RVC twice daily, (3) 20mg/kg FLC once daily, and (4) 20mg/kg FLC twice daily.
ResultsThe geometric means of the MIC values at 48h for all isolates tested were 0.05 and 0.5μg/ml for RVC and FLC, respectively. Regimens of either RVC or FLC at 20mg/kg twice daily were more effective to reduce the load of FLC-resistant C. albicans than single dose administration.
ConclusionsComplete eradication of C. albicans from the vagina was not observed with RVC or FLC treatment in the animal model, although RVC treatment showed a lower fungal concentration 14 days after drug administration.
En los últimos años, ha aumentado sustancialmente la incidencia de candidiasis vulvovaginal, una infección frecuente entre mujeres sanas, causada sobre todo por la levadura Candida albicans.
ObjetivosEl objetivo del presente estudio fue comparar la eficacia del ravuconazol (RVC) y del fluconazol (FLC) en el tratamiento de la vaginitis experimental inducida por C. albicans.
MétodosSe examinó la sensibilidad in vitro de 40 aislamientos de C. albicans frente a RVC y FLC. Para el estudio in vivo se seleccionó una cepa de C. albicans que fue resistente a FLC (concentración inhibitoria mínima [CIM] >64μg/ml). Las pautas de tratamiento para el modelo murino de infección vaginal fueron 1) 1, 5, 10 y 20mg/kg de RVC una vez al día, 2) 20mg/kg de RVC dos veces al día, 3) 20mg/de FLC una vez al día, y 4) 20mg/kg de FLC dos veces al día.
ResultadosPara todos los aislamientos las medias geométricas de los valores de la CIM a las 48h fueron de 0,05 y 0,5μg/ml para RVC y FLC, respectivamente. Las pautas de 20mg/kg de RVC o FLC dos veces al día fueron más eficaces para reducir la carga infectiva de C. albicans resistente a FLC que las administradas una vez al día.
ConclusionesEn el modelo animal no se eliminó completamente C. albicans del tracto vaginal estéril mediante tratamiento con RVC o FLC. Sin embargo, el tratamiento con RVC derivó en concentraciones fúngicas más bajas 14 días después de su administración.
Vulvovaginal candidiasis is an infection that affects women frequently, particularly during their childbearing years. It is caused by yeasts of the genus Candida, usually Candida albicans. C. albicans is the causative agent of vulvovaginal candidiasis in 90% of symptomatic patients, and vulvovaginal candidiasis is the second most common infection among all vaginal infections.11,31 Current treatments for Candida vulvovaginitis include a wide range of intravaginal azole preparations that are typically administered over several days,1 but many patients prefer the convenience of oral medications. Oral fluconazole (FLC) is a triazole with marked in vitro activity against Candida species. Due to its clinical efficacy and ease of administration, it is the treatment most often prescribed for this disease.30,32
However, with the increasing incidence of vulvovaginal candidiasis caused by C. albicans and the growing resistance of infections to conventional antifungals, especially FLC, there is a need to evaluate new therapeutic agents. Ravuconazole (RVC) is a novel triazole antifungal molecule developed by Eisai Co. Ltd. (Tokyo, Japan) that has a half-life of over 100h, exhibits broad spectrum activity, and has a good safety profile.3,12,17In vitro studies have demonstrated potent RVC activity against Candida spp., Cryptococcus neoformans, and other yeast species, including some strains that are not susceptible to FLC.8,23,26,28,33 Additional studies have demonstrated that RVC has in vitro activity against clinical isolates of the filamentous fungi Aspergillus, Paecilomyces, Fonsecaea pedrosoi, Cladophialophora carrionii, the dimorphic fungi Coccidioides immitis, and Histoplasma capsulatum.9,13,21,27
Martinez et al.2 developed a murine model of vulvovaginal candidiasis to evaluate the therapeutic efficacy of novel antifungals for recurrent infections caused by species of Candida resistant to conventional antifungals. RVC has been evaluated in murine and guinea pig models of infection, and has been found to be effective for the treatment of mucosal candidiasis, disseminated aspergillosis, and systemic histoplasmosis.6,7,18,25 In this study, we compared the effectiveness of RVC and FLC for the treatment of experimental C. albicans vaginitis.
Materials and methodsStrains and in vitro susceptibility testingForty isolates of C. albicans from patients with vaginal infections were sent for identification to the Departamento de Microbiología, Facultad de Medicina, Universidad Autónoma de Nuevo León, Mexico. These clinical isolates were identified by standard biochemical (API 20C AUX, Biomerieux) and microbiological procedures.24 They were stored in water at room temperature until use.
RVC (Eisai Co. Ltd. Tokyo, Japan) and FLC (Pfizer Inc., New York, USA) were obtained as reagent grade powders from their manufacturers. Isolates were evaluated for their in vitro susceptibilities by the broth macrodilution method described in the Clinical and Laboratory Standards Institute reference document M27-A3.22Candida parapsilosis ATCC 22019 and Candida krusei 6258 were included as control organisms. C. albicans isolate 03-2718 has been used in our laboratory for previous vaginal candidiasis studies.14,15,16 The minimum inhibitory concentrations (MICs) of FLC and RVC for this strain were >64μg/ml and 0.25μg/ml, respectively. Strains were maintained on Sabouraud dextrose agar slants for short-term storage and kept in 10% glycerol at −70°C for long-term storage.
Animal infection modelFive week-old BALB/c mice (weight, 18g) were purchased from Harlan Mexico. Ten mice were randomly assigned to a treatment or control group and were housed in cages containing five mice each. Food and water were provided ad libitum. All animal research procedures were approved by the University Ethics Committee. Care, maintenance, and handling of the animals followed Mexican government licensing requirements for animal experimentation, and studies were performed in duplicate.
A previously described model of recurrent vaginal candidiasis was used.2 Three days prior to infection and on days 4, 11, and 18 post-challenge, mice were given 0.5mg estradiol valerate (Delesgrogen, King Pharmaceuticals) subcutaneously to maintain pseudoestrus during the entire experiment. On the day of infection, mice were anaesthetized with 80mg/kg ketamine hydrochloride intraperitoneally and were inoculated intravaginally with 20μl of a 2×108 colony-forming units (CFU)/ml suspension of C. albicans isolate 03-2718. One day after infection, the vaginal cavity of each mouse was swabbed (prior to treatment) to ensure that the infection was consistently distributed among animals.29 The same procedure was repeated on days 6 and 20 to evaluate treatment efficacy. Each alginate swab was placed in 0.9ml of sterile saline, 10-fold serial dilutions were made, and 100μl aliquots were plated onto Sabouraud dextrose agar plates supplemented with 0.5% (w/v) chloramphenicol to determine the CFU/ml.
Drugs were administered orally on days 1 to 5 after infection. RVC was prepared fresh daily and dissolved in 0.5% carboxymethylcellulose with 10% dimethyl sulfoxide. RVC was administered in 0.2-ml doses of 1, 5, 10, or 20mg/kg once a day. One group of animals received a dose of 20mg/kg twice a day. FLC was dissolved in distilled water and was administered once or twice a day in 0.2ml doses of 20mg/kg of body weight. Control mice were infected but received no active treatment; they received the drug vehicle containing 0.5% carboxymethylcellulose and 10% dimethyl sulfoxide orally. On day 21, mice were sacrificed by cervical dislocation.
Statistical analysisComparisons were performed using the Mann–Whitney U-test, with significance set at a P-value<0.05.
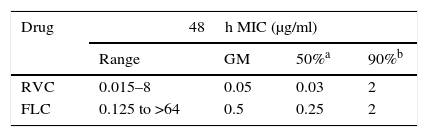
ResultsAntifungal susceptibilityThe 40 C. albicans clinical isolates were inhibited in vitro by 0.015–8μg/ml of RVC and 0.125 to >64μg/ml of FLC. The geometric means were 0.05mg/ml and 0.5μg/ml for RVC and FLC, respectively (Table 1). The concentrations that inhibited 50% of the isolates were 0.03μg/ml for RVC and 0.25μg/ml for FLC. RVC displayed stronger in vitro antifungal activity at lower concentrations than FLC. The MICs of the control strains were within the acceptable ranges for the drugs tested.4
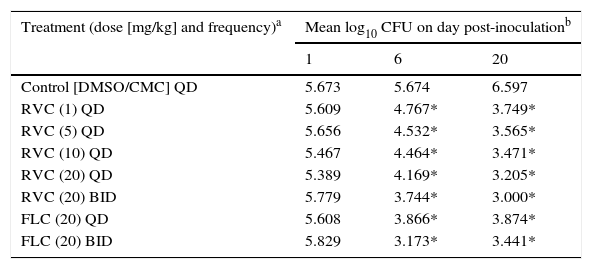
Animal modelThe effects of RVC were compared with those of FLC for the treatment of experimental vaginitis induced by a C. albicans isolate (Table 2). Compared with the vehicle-treated control, mice treated with RVC at ≥1mg/kg once daily had reduced loads of vaginal C. albicans (P=0.006 to <0.001) on days 6 and 20 postinoculation. In addition, twice-daily administration of 20mg/kg RVC resulted in larger reduction of C. albicans loads compared to single-dose administration (P=0.022). In contrast, RVC concentration did not affect C. albicans loads on days 6 and 20 postinoculation when administered at ≥1mg/kg once daily (P=0.63 to >0.87). Once- and twice-daily oral administrations of 20mg/kg FLC reduced the vaginal load of C. albicans (P≤0.001) on days 6 and 20 postinoculation.
Recovery of C. albicans strain 03-2718 from vaginas of mice treated orally with RVC and FLC.
| Treatment (dose [mg/kg] and frequency)a | Mean log10 CFU on day post-inoculationb | ||
|---|---|---|---|
| 1 | 6 | 20 | |
| Control [DMSO/CMC] QD | 5.673 | 5.674 | 6.597 |
| RVC (1) QD | 5.609 | 4.767* | 3.749* |
| RVC (5) QD | 5.656 | 4.532* | 3.565* |
| RVC (10) QD | 5.467 | 4.464* | 3.471* |
| RVC (20) QD | 5.389 | 4.169* | 3.205* |
| RVC (20) BID | 5.779 | 3.744* | 3.000* |
| FLC (20) QD | 5.608 | 3.866* | 3.874* |
| FLC (20) BID | 5.829 | 3.173* | 3.441* |
Each group had 10 mice. The mice were monitored on days 1, 6 and 20 after inoculation.
C. albicans loads at days 6 and 20 postinoculation were compared between mice treated with 20mg/kg RVC and FLC twice daily. RVC showed better in vivo activity 15 days after treatment (day 6, P=0.003; day 20, P=0.03). However, there was no difference in the fungal burden at days 6 and 20 postinoculation when 20mg/kg of RVC or FLC were given once daily (P=0.27–0.67, respectively). Complete eradication of C. albicans from the vaginal cavity was not observed with either RVC or FLC regimens.
DiscussionRVC is a novel triazole that has shown considerable activity against various fungi.20 In this study, RVC exhibited improved in vitro antifungal activity compared to FLC, which is the most widely used drug for vulvovaginal candidiasis. The antifungal activity of RVC has been demonstrated against strains of Cryptococcus neoformans isolated from HIV patients, where 100% of the isolates were susceptible to the drug.19 Although the antifungal activity of RVC against mucormycetes, such as Rhizopus, was recently confirmed, further experimentation using an animal model is required to obtain a wider perspective of its efficacy.10
For the animal model, we used a mouse reference strain for vaginal infection.5 We evaluated the in vitro activity of RVC against a C. albicans FLC-resistant isolate, as well as its efficacy during 20 days of postinfection pseudoestrus. BALB/c mice in the control group did not show a significant decrease in CFUs on days 1, 6, and 20 postinfection, confirming persistent infection in the murine model. When 20mg/kg RVC and FLC were administered twice daily, the FLC-resistant C. albicans microbial burden was significantly reduced. RVC had a prolonged duration of efficacy because it decreased the fungal burden 15 days after treatment ended. This result can be attributed to the long half-life (>100h) of RVC. In contrast, FLC has a short half-life of 20–50h. The longevity of RVC makes it a viable alternative for persistent Candida infections.
RVC is currently being evaluated in clinical trials, but the results of these trials have not yet been published. To the best of our knowledge, this is the first study evaluating the efficacy of RVC against C. albicans in a murine model of vaginal infection. Our results are particularly important because they demonstrate the efficacy of RVC against candidiasis caused by a FLC-resistant C. albicans isolate. Our results also show the usefulness of orally administered RVC for the treatment of vulvovaginal candidiasis.
Conflicts of interestAll authors declare no conflict of interest.