Fungal infections have increased in critical care patients, causing high morbidity and mortality.
AimsDescribe the frequency and responsible fungal species involved in bloodstream fungal infection from 2001 to 2007 in tertiary care level hospitals belonging to a surveillance network in Colombian cities.
MethodsData were collected from a microbiology surveillance network based on 27 hospital laboratories in five Colombian cities. Data were entered into a Whonet® version 5.4 database. Fungemia data were analyzed according to location (Intensive care unit -ICU- vs. non-ICU services). Frequency over time was also described.
ResultsFungal infections corresponded to 4.1% of all bloodstream infections. Candidemia represented 3.7% and 5.2% of all isolates in non-ICU and ICU services, respectively. Over 99% of the isolates were yeasts, and Candida albicans was the most frequently isolated organism in and out of the ICU, showing a decreasing trend in the last few years. In the adult ICU and non-ICU services, the second organism most frequently isolated was C. tropicalis, while C. parapsilopsis was the most frequent in the pediatric and neonatal ICU, also showing an overall decreasing trend. Cryptococcus neoformans was the fourth mycotic organism most frequently identified.
ConclusionsIn Colombia, epidemiology of fungal infections seems to be changing. C. albicans is the principal agent causing bloodstream fungal infection, but an increase of non-albicans species has been observed as well as high frequency of C. neoformans.
Las infecciones micóticas han aumentado en los pacientes críticos y son responsables de una alta morbilidad y mortalidad.
ObjetivosDescribir la frecuencia de presentación y las especies de hongos responsables de las infecciones del torrente sanguíneo en una red de vigilancia de hospitales de tercer nivel en varias ciudades de Colombia en el periodo entre 2001 y 2007.
MétodosLos datos fueron obtenidos a partir de los laboratorios de microbiología de 27 hospitales de 5 ciudades colombianas. Estos datos fueron incorporados a una base creada en Whonet® versión 5.4 de la OMS. Se analizaron los datos de fungemias de acuerdo a la localización (servicios de cuidado intensivo -UCI- y servicios no-UCI) y se describe la tendencia en el tiempo.
ResultadosEn 27 hospitales de la red las fungemias correspondieron al 4,1% de los hemocultivos positivos, y las candidemias representaron el 3,7 y el 5,2% de todos los aislamientos en los servicios no-UCI y UCI, respectivamente. Las levaduras fueron los agentes etiológicos responsables en más del 99% de los casos, y Candida albicans fue el microorganismo más identificado tanto en la UCI como fuera de ella, con una menor frecuencia en los últimos años del estudio. En UCI de adultos y en los servicios no-UCI el segundo microorganismo más frecuente fue Candida tropicalis, mientras que en la UCI pediátrica y la neonatal fue Candida parapsilopsis. Cryptococcus neoformans fue el cuarto hongo más frecuentemente identificado.
ConclusionesEn Colombia la epidemiología de las infecciones micóticas parece estar cambiando. C. albicans es el principal agente causal de fungemias pero se observa un incremento en el resto de especies del género y una alta frecuencia de C. neoformans.
Worldwide, invasive mycoses have become an emerging problem and are a common issue related to hospital acquired infections. They affect mainly patients who are in the intensive care units (ICU).4,7,15 In the past few years, the frequency of infection caused by yeasts has increased significantly due, in part, to the increase of patients susceptible to this type of infections, particularly adult and pediatric critical care patients, HIV infected persons, immunosuppressed patients, transplants recipients, and people with other predisposing factors.2
Important epidemiological changes have also been observed in the prevalence of various species of yeasts and resistance frequency to some first-line antimycotic agents, especially fluconazole.5,9,15,30 It has also been observed that the frequency of various species changes according to the type of patient, the type of ward in which the patient is hospitalized,13 underlying condition(s), and particularly the geographical region.8 In South America, a study that included clinical isolates collected between 1997 and 2002 showed the predominance of C. albicans over other species.10
The goal of this work is to show the frequency and species of fungi responsible for bloodstream infections occurring during a tertiary hospital surveillance network in Colombian cities.
Methods and materialsParticipating hospitalsData were collected from the microbiological surveillance network including 27 reference hospitals in the following Colombian cities: Bogotá (capital city of Colombia), Ibague and Neiva (Southwest of the country), Manizales (mountainous area adjacent to the coffee country), and Cucuta (near the border with Venezuela). The participating hospitals are all tertiary health care facilities that agreed voluntarily to forward monthly information reports.
Data collectionParticipating microbiological laboratories sent monthly reports, which were entered into a Whonet database (World Health Organization WHO-initially version 5.1 and then version 5.4) utilizing the BackLink 2 (WHO) program to transfer information from automated microbiological identification systems: VITEK® (Biomerieux, Lyon, France) and MicroScan® (Dade Behring, Sacramento, USA). In those laboratories using manual microbiological identification systems, the collected information was directly entered into the Whonet program. Data were classified according to origin in: intra-hospital wards, ICU, and treatment outside of hospital settings (emergency rooms, outpatient consultation, etc.).
Microbiology quality controlLaboratories included in the Whonet® 5.4 program were subjected to an external quality program assessment conducted by the microbiology laboratory of the National Health Institute.
FungemiaBloodstream infections were selected from the database, and only those corresponding to mycotic isolates were chosen. The study exclusively analyzed information pertaining to isolation in the intra-hospital care and ICU settings. Only the first positive blood culture isolate for each patient was used, and subsequent positive culture isolates from patients were excluded.
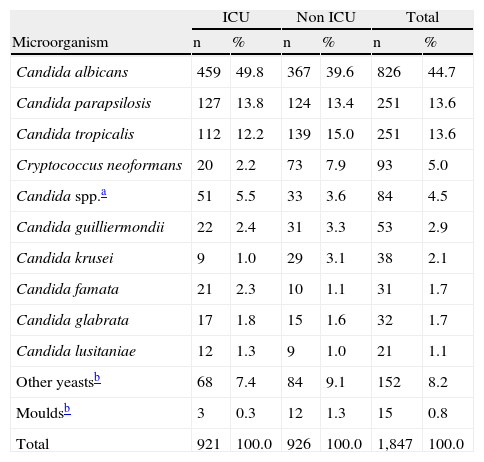
ResultsDuring the surveillance term between 2001 and 2007, 45,026 positive blood cultures were reported by the microbiology laboratories of the participating institutions. Out of these, 60.7% (27,331) came from hospital services different from the ICU (non-ICU), and the remainder 39.3% (17,695) came from ICU services. From the total isolates obtained, bloodstream infection caused by fungi represented 4.1% (1,847). Out of the latter, 49.8% (921) of the isolates came from the ICU area. 99.2% (1,832) of the identified isolates corresponded to yeasts. Table 1 shows the most frequently identified organisms and their location.
Frequency of fungal isolates by service type.
| ICU | Non ICU | Total | ||||
| Microorganism | n | % | n | % | n | % |
| Candida albicans | 459 | 49.8 | 367 | 39.6 | 826 | 44.7 |
| Candida parapsilosis | 127 | 13.8 | 124 | 13.4 | 251 | 13.6 |
| Candida tropicalis | 112 | 12.2 | 139 | 15.0 | 251 | 13.6 |
| Cryptococcus neoformans | 20 | 2.2 | 73 | 7.9 | 93 | 5.0 |
| Candida spp.a | 51 | 5.5 | 33 | 3.6 | 84 | 4.5 |
| Candida guilliermondii | 22 | 2.4 | 31 | 3.3 | 53 | 2.9 |
| Candida krusei | 9 | 1.0 | 29 | 3.1 | 38 | 2.1 |
| Candida famata | 21 | 2.3 | 10 | 1.1 | 31 | 1.7 |
| Candida glabrata | 17 | 1.8 | 15 | 1.6 | 32 | 1.7 |
| Candida lusitaniae | 12 | 1.3 | 9 | 1.0 | 21 | 1.1 |
| Other yeastsb | 68 | 7.4 | 84 | 9.1 | 152 | 8.2 |
| Mouldsb | 3 | 0.3 | 12 | 1.3 | 15 | 0.8 |
| Total | 921 | 100.0 | 926 | 100.0 | 1,847 | 100.0 |
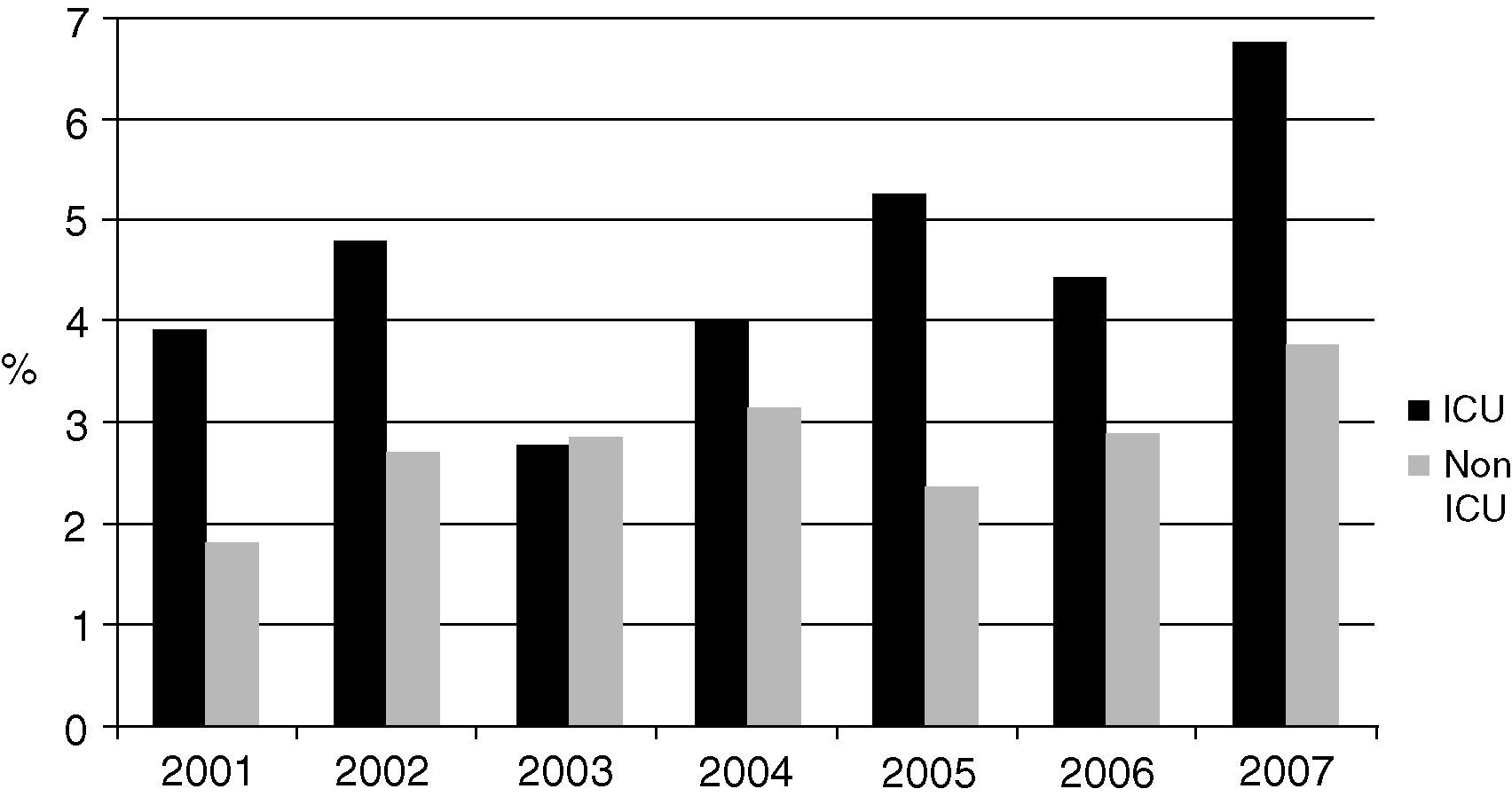
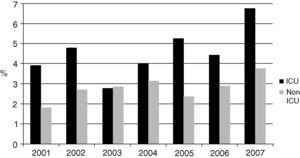
The most frequently isolated genus was Candida, which represented 87.8% (1,622) of all the mycotic isolates and 88.5% of the yeast isolates. Candida species corresponded to 87.8% and 84.6% of fungi in the ICU and non-ICU, respectively. Candida isolates represented 3.6% of the total number of organisms identified in the bloodstream. Fig. 1 shows the proportion of Candida species isolates compared to all the bloodstream infections over the years of study.
Candida species frequency ranked sixth in the non-ICU isolates (after negative coagulase staphylococci -SCN-, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa) and fifth among the most frequent ICU isolates (after SCN, S. aureus, K. pneumoniae, and E. coli), exceeding the frequency of other organisms such as Acinetobacter baumannii and P. aeruginosa.
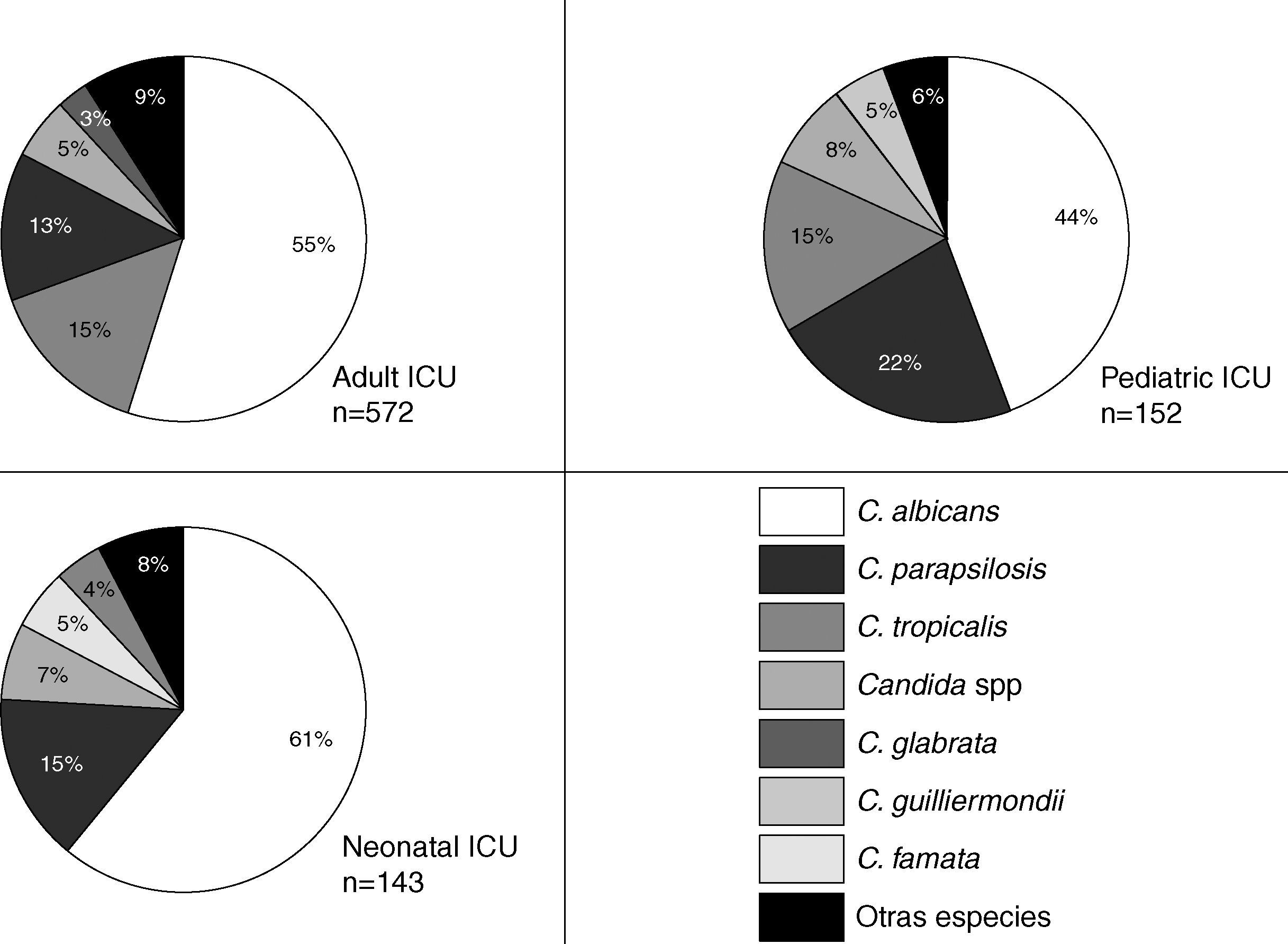
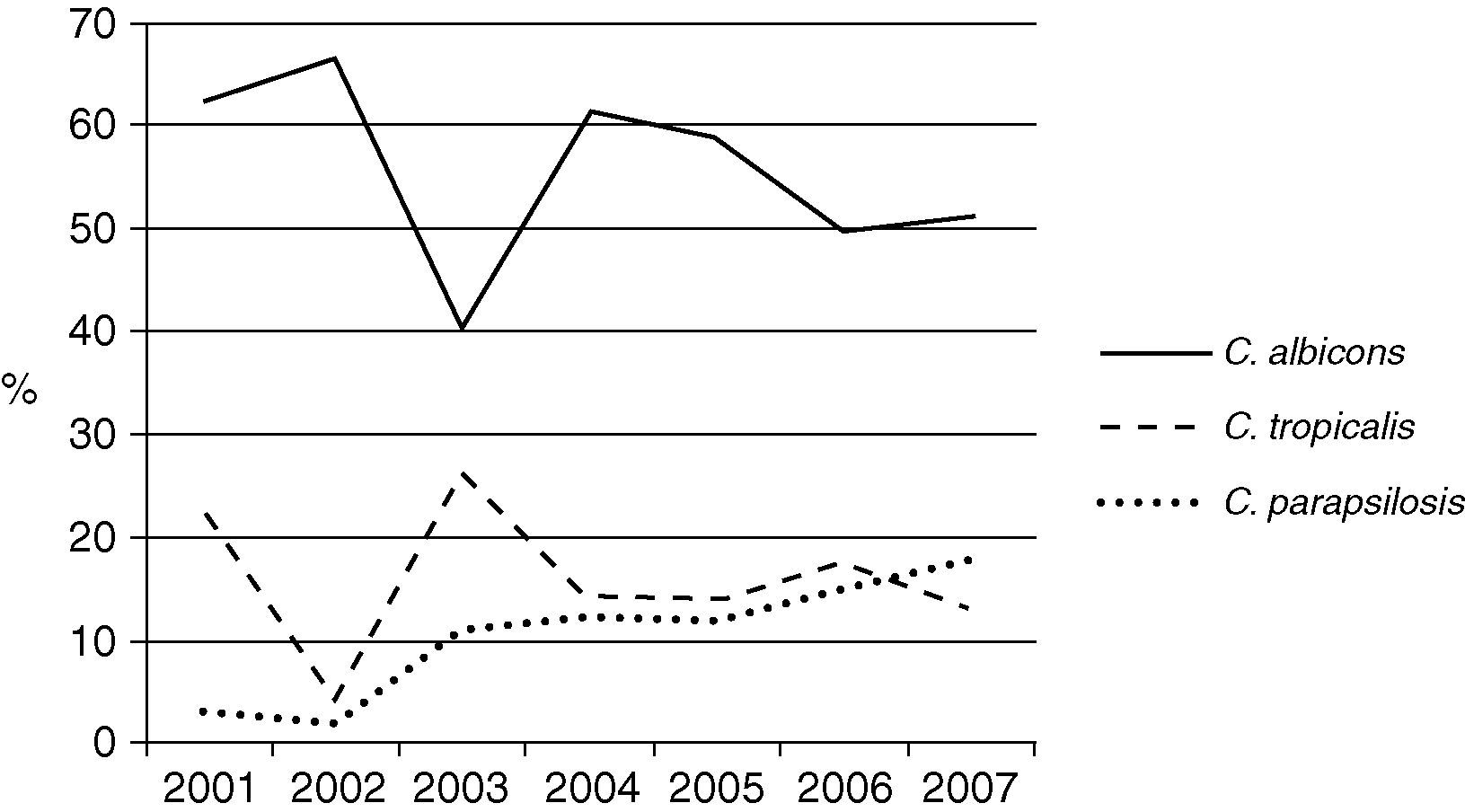
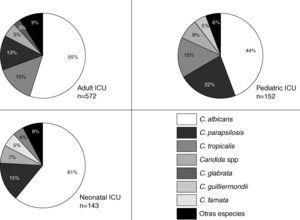
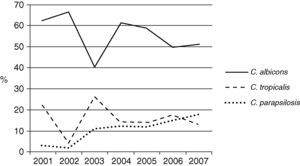
The Candida species most frequently identified corresponded to C. albicans, which represented 47.5% of the candidemia cases in the non-ICU services and 54.1% of the candidemia cases in the ICU. No differences were observed in the proportion of Candida species identified in the non-ICU services during the surveillance period. C. albicans was identified in 38.6% to 51.4% of the isolates over the surveyed years without a clear increasing or decreasing trend. Thus, 68.3% (629 isolates) of the mycotic isolates obtained in the ICU area originated in the adult ICU, 16.7% (154 isolates) in the pediatric ICU, and 15% (138 isolates) in the neonatal ICU. In the ICU, 92.2% of the fungal isolates identified corresponded to the genus Candida. Fig. 2 shows the distribution of the main Candida species in various ICU. In the three clinical settings, C. albicans was the most frequently identified species, even though it occurred in less than 50 percent of the instances in the pediatric ICU. C. parapsilosis was the second most frequently identified species in the pediatric and neonatal ICU, while C. tropicalis was so found in the adult ICU. Over time, the prevalence of the principal Candida species has changed in the adult ICU as shown in Fig. 3. The proportion of C. parapsilosis isolates exceeded that of C. tropicalis during the last year of observation, and a trend for the C. albicans isolates to decrease in frequency was observed.
Overall, Cryptococcus neoformans was fourth in the number of fungal isolates (Table 1). Other species of Cryptococcus were C. laurentii (n=7), C. albidus (n=4), C. terreus (n=2), C. ater and C. uniguttulatus (one isolate each one).
Other genera or species identified in mycotic isolates with lower frequency included Rhodotorula (n=25), Sporobolomyces (n=20), Trichosporon (n=16), Saccharomyces (n=10), Hansenula (n=8), C. catenulata (n=7), Fusarium (n=7), C. zeylanoides (n=6), C. stellatoidea (n=5), C. lipolytica (n=4), Pichia (n=4), Aspergillus (n=3), C. kefyr and C. rugosa (n=2, each one), Cladosporium (n=2), C. humicola, Cunninghamella, Geotrichum, Histoplasma, Kluyveromyces, Mycelia sterilia, Myxozyma and Penicillium (one isolate each one). There were 23 unidentified yeast isolates.
DiscussionThis study shows the epidemiology of bloodstream fungal infections in tertiary hospitals in Colombia in terms of species distribution and type of ward where the isolates were obtained. C. albicans is the most frequently identified mycotic organism in the bloodstream, as it has been observed in many regions of the world. In the late 1990's, the Sentry surveillance study data showed that the frequency of C. albicans among isolates responsible for bloodstream infection varied in the world as follows: 45% in Latin America, 55% in the United States of America, 50% in Europe, and 60% in Canada.27 In the majority of settings (various types of ICU and non-ICU services), C. albicans was the yeast identified with the highest frequency.24,31,33 In Spain, a comparison made between two periods of time, the 1990's and the current decade, showed a change in frequency for C. albicans from 60% of the isolates in the first period to 49% of the isolates in the second period.16 Overall, an increase in non-C. albicans Candida species was observed. This change was evident in our hospitals’ adult ICUs, while the same trend was not observed in the neonatal or pediatric ICUs. The reasons for these changes may be multiple. First, there has been an increase in the number of high-risk patients, namely, patients infected with HIV and Cryptococcus, immunosuppressed patients due to diverse causes and types of medication, and patients needing use of invasive devices and antibiotics.20 The Spanish study showed a different frequency of risk factors in the observed periods.1 Other studies have shown that various risk factors are related to the appearance of NCA species such as: central catheter duration, number of antibiotics used,5 and type of surgery performed.1,6 Second, however, the majority of studies suggest that one of the most important factors for the selection of NCA strains is the use of fluconazole.5,6 In countries such as the United States and France, the use of fluconazole has been connected to the appearance of C. glabatra as the second agent causing candidemia,8,19,28 exceeding the frequency of C. parapsilosis and C. tropicalis isolates. If the low frequency of C. glabrata, compared to other countries, is related to a lower consumption of fluconazole is unknown. The type of patient also determines the identified species. Studies conducted in the pediatric and neonatal ICU showed that the frequency of C. albicans may be different and that C. parapsilosis and C. tropicalis are the most frequently identified species.22,24,31 Frequency similar to the high frequency of C. albicans found in Colombian neonatal ICU has been seen in studies from other latitudes.29
This study shows important changes in the epidemiology of Candida species. In general, Candida isolates in the bloodstream ranked fifth among the ICU isolates and sixth in frequency for the non-ICU setting. In the United States, Candida species ranks fourth place in the frequency of agents causing bloodstream infections,22 a situation which is similar in Greece,34 while in Switzerland it ranks seventh.19 Again, the reason for this change may be related to the type of patients admitted to ICUs. An increase in frequency of yeast infections has been observed in pediatrics.11
Reporting epidemiologic changes may also have clinical relevance. In the region, the largest study that evaluated susceptibility of the species identified in Colombia, Ecuador, and Venezuela to fluconazole showed that susceptibility to fluconazole higher than 90% was identified only among C. albicans and C. parapsilosis isolates.10 NCA isolates other than C. parapsilosis had a considerable increase in the number of resistant and dose-sensible dependent isolates. Hence, in the presence of a mycotic agent in the bloodstream, the probability of having good susceptibility to fluconazole was as low as 58 percent (that is, the sum of C. albicans and C. parapsilopsis proportions), a fact which makes the use of higher fluconazole doses, or alternative antifungal agents, advisable; similar recommendations have been generated in other geographical areas.35 Another small study conducted in Bogotá, whose isolates mainly corresponded to C. albicans and C. tropicalis, showed these species’ good susceptibility.25 However, the recommendation for empirical treatment for the region is not clear, given the emergence of C. parapsilosis isolates observed in the ICU. An increase in C. parapsilosis isolates in those areas where caspofungine is used has been observed.32
Bloodstream fungal infections entail high mortality,14 and it has been found that delays in the use of antimycotic medication increases mortality significantly.17,23 Taking into account the growing frequency revealed in our study, coverage of these organisms must be initiated in high-risk factor patients even before obtaining blood culture results, keeping in mind that blood culture sensitivity in patients with invasive fungal infection may be close to 50 percent.26 Even though we lack high sensitivity diagnostic methods, the use of predictive scales may help identify those patients with higher infection probability.3
Our study also reports a relatively high frequency of Cryptococcus spp. isolates. The frequency of this genus varies geographically and also varies with the frequency of high-risk individuals susceptible to cryptococosis. In Colombia the main risk group is formed by HIV-infected patients.18 In Hungary, the Cryptococcus spp. infection frequency in the bloodstream was 43%21 during the 1990's, while in Chile it corresponded to 10% of all the mycotic isolates identified from blood early in the current decade.12 Species of Cryptococcus other than C. neoformans were also found, but the clinical significance of these findings is unknown.
The most important constraints of our study are its retrospective nature and the poor sensitivity of blood cultures. These facts prevent the confirmation of identified species and probably cause the underestimation of the problem magnitude. Also, the limited number of time points does not allow the identification of a real trend for the frequency of fungemia or the interpretation of the changes observed over time.
In conclusion, our study shows changes in the frequency of various Candida species in Colombia and an increase in the number and proportion of bloodstream isolates; it also shows a decrease in frequency of C. albicans and an increase in other species, particularly C. parapsilosis in adult ICU patients.
Conflict of interestThe authors do not disclose any conflicts of interest.
FundingThis study was conducted under the auspices of the Research Division of the National University of Colombia (Bogota campus) through a research Grant (Graduate Thesis Support Grant 2006 code 5621.)
Participating hospitals and institutions (2001-2007): Universidad Nacional de Colombia. (Aura Lucía Leal Castro, Javier Eslava Schmalbach, Giancarlo Buitrago Gutiérrez, Carlos Saavedra Trujillo, Sonia Isabel Cuervo), Hospital Simón Bolívar (Carlos Álvarez, Gustavo Aristizábal, Constanza Correa), Hospital Universitario de San Ignacio (Carlos Álvarez, Jorge A. Cortés, José Roberto Tamara), Hospital San José (Paola Jiménez, Patricia Guzmán, Claudia Fajardo Uribe), Hospital Santa Clara (José Roberto Tamara, Luz Mila López, Gloria Inés Gallo), Fundación Hospital San Carlos (Jaime Saravia, Jorge A. Cortés), Hospital Militar Central (Matilde Méndez, Carlos Pérez), Hospital Universitario Clínica San Rafael (Carlos Saavedra Trujillo, Jaime Alberto Patiño, Clemencia Ávila, Martha Pulido), Fundación Cardioinfantil (Álvaro Arango, Patricia Bravo), Instituto Nacional de Cancerología (Sonia Isabel Cuervo, Diana Bermúdez, Claudia Patricia Arroyo Ariza), Clínica del Niño (Tailandia Rodríguez, Mauricio Luna, Martha Martínez, Martha Uzeta), Clínica San Pedro Claver (Carlos Alquichire, Martha Cecilia Ruiz, Aura Lucía Leal Castro), Hospital de Kennedy (Romelia Villa), Hospital de la Misericordia (Jaime Alberto Patiño, Myriam Lucia Galeano, Patricia Rincón), Clínica del Occidente (Martha L. Salinas, Elkin Lemus, Norma Montoya), Clínica Jorge Piñeros Corpas, Saludcoop (Carlos Alquichire, Carlos Díaz Granados, Ceneth Deaza), Fundación Santa Fe de Bogotá (Clara Luz Rico, Blanca Stella Vanegas, Guillermo Prada), Clínica Infantil Colsubsidio (Giovanni Rodríguez Leguizamón, Deise Yadira Rojas, Patricia Jiménez Gonzáles, Claudia Cecilia Dueñas Gaitán), Hospital El Tunal (Elkin Lemus, Narda Olarte, Alberto Valderrama, Julia Etella Quijano, Martha Isabel Garzón), Centro Policlínico del Olaya (Catherine Rojas, Humberto Beltrán, Sandra Pedraza, Ana Isabel Sánchez, Sandra Burgos), Hospital Universitario La Samaritana (Johanna Osorio, Beatriz Cuevas, Lucy Guzmán), Instituto Nacional de Salud (Elizabeth Castañeda, Claudia Agudelo), Asociación Colombiana de Infectología (ACIN)-Capitulo Central, Sistema de Vigilancia de la Resistencia Bacteriana, Área de Vigilancia en Salud Pública, Secretaría Distrital de Salud (María Patricia González, Daibeth Elena Henríquez). Thanks to Ana María Rubio for her support preparing preliminary versions of the text.