In recent years the need has arisen to study and develop (or re-discover) foods that have nutritional characteristics as well as specific functions, such as improving health and/or reducing the risk of disease. For this reason knowledge of the nutritional value of food is important to promote greater consumer acceptance. In Mexico huitlacoche (also, cuitlacoche) has traditionally been prized as a delicacy since the time of the Aztecs and is currently being studied as a potential functional food and as a producer of natural bioactive substances that are used in fortifying foods.
AimsTo present an updated review about the properties of the huitlacoche (corn smut) as functional food.
MethodsA bibliographic search was performed and data were discussed.
ResultsThe data of the works reviewed here show that huitlacoche contains many compounds that confer to it unique organoleptic and nutraceutical characteristics.
ConclusionsThe content of bioactive substances in huitlacoche supports the proposal that this is a good functional food as well as producer of compounds to enrich other foods.
Durante los últimos años ha surgido la necesidad de elaborar, estudiar o redescubrir los alimentos que, además de proporcionarnos los beneficios nutricionales que los caracterizan, puedan cumplir una función específica, como mejorar la salud o reducir el riesgo de contraer enfermedades. Por este motivo, el conocimiento del valor nutrimental de los alimentos es importante para que estos tengan una mejor aceptación entre los consumidores. En México, el huitlacoche o cuitlacoche ha sido tradicionalmente apreciado como una delicia culinaria desde la época de los aztecas, y actualmente se está estudiando su potencial como alimento funcional y como productor de sustancias bioactivas naturales, que puedan ser utilizadas en la producción de alimentos fortificados.
ObjetivosPresentar una revisión actualizada acerca de las propiedades del huitlacoche (carbón del maíz) como alimento funcional.
MétodosSe realizó una investigación bibliográfica y los datos fueron discutidos.
ResultadosLos datos de los trabajos revisados aquí indican que el huitlacoche contiene muchos compuestos que le confieren características organolépticas y nutracéuticas únicas.
ConclusionesEl contenido de sustancias bioactivas en el huitlacoche apoya la propuesta de que éste es un buen alimento funcional, además de producir compuestos para enriquecer otros alimentos.
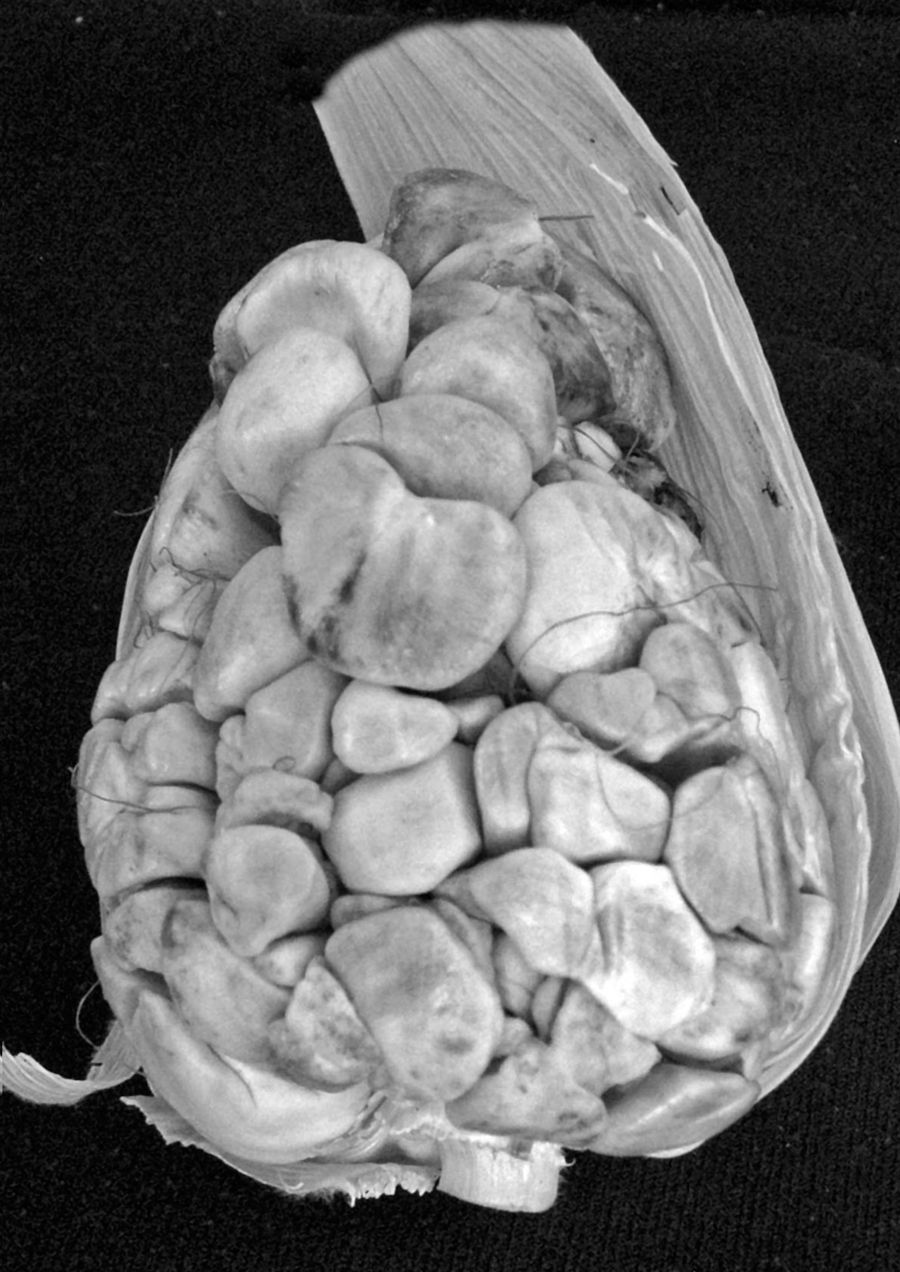
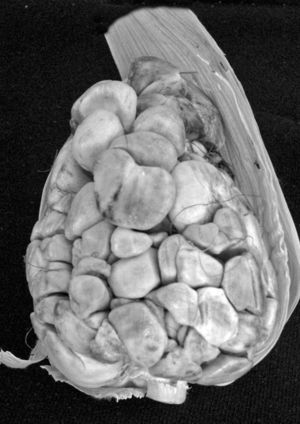
The dimorphic fungus known as Ustilago maydis is responsible for the formation of corn smut, characterized by the formation of galls or tumors principally in ears, but also in stems and leaves of the plant host (Zea mays)4 (fig. 1). This disease is usually considered a world-wide disease; nevertheless it has been used as food in Mexico since pre-Columbian cultures24. As a result its popular name, huitlacoche or cuitlacoche comes from the word Nahuatl (the language of the Mexicas or Aztecs) “cuitlacochin” or “cuitlacuchtli” that means “degenerate corn on the cob”. Also the significance of the word “cuitlatl” is “excrement”, and that of “cochi” is “black” or “dark”. In folk tales there are other interpretations of this term, including “crow filth” and “sleeping filth”. In Nahuatl, this disease was also designated “popoyauh” or “popoyotl”, meaning “burnt corn”. For the Mexican excrement was not only a waste product in the physical sense, it also had a spiritual sense in which it was considered a distillation of food, which explains the root of the word. Indeed, “cuitlatl” comes from the name of a Mexica (Aztec) emperor named Cuitlahuac36,39.
The use of huitlacoche as food has spread to the point that it is currently a culinary delight of international chefs due to the unique mixture of components that produce its flavor, aroma, and organoleptic characteristics20. Determination of total proteins, amino acids, dietary fiber, carbohydrates and unsaturated fatty acids have been made through nutritional studies, identifying huitlacoche as a functional food that produces bioactive substances, the latter of which can be used to create fortified food products. Huitlacoche is currently available in Mexico during the months of July and August, but in order to be marketed internationally it must be able to be produced year round, which requires the development of forms of production that can result in an economically feasible and nutritionally functional product of agreeable aroma, color and flavor.
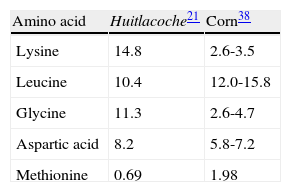
Composition of free amino acids, volatile compounds and fatty acids, and their effect on the generation of flavorThrough derivatization studies, it has been determined that huitlacoche contains almost all the essential and many non-essential amino acids. The most abundant amino acid is lysine, corresponding to 14.84% of the total amino acids, followed by leucine, glycine, and aspartic acid (collectively representing 29.97% of the total amino acids in the sample (table 1 table 1)21. Also huitlacoche contains three of the four amino acids, which have an important effect in producing the umami flavor (that detected by the tongue principally because of the presence of glutamic, aspartic, tricholomic and ibotenic acid), resulting from their function as precursors or direct participants in the generation of this. Only ibotenic acid is missing from huitlacoche21.
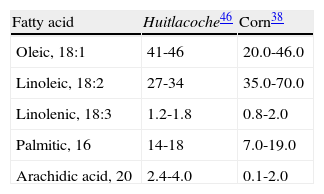
Aroma extract dilution analysis (AEDA) and gas chromatography-olfactometry of static head-space analysis (GCO-SH) was used to identify the principal volatile compounds that confer the characteristic aroma of huitlacoche. Such compounds are hexanal, octanal, decanal, (E,E)-nona-2,4-dienal, (E)-undeca-2-enal, vanillin and sotolon20. The fact that the aromatic compounds of huitlacoche are principally aldehydes could owe itself to the oxidation of fatty acids, such as oleic or linoleic acid, which are the principal fatty acids of this food, constituting 40 and 30% of the total fatty acids, respectively (table 2 table 2)20,45. Linolenic and linoleic acids conforms one of the sources of two essential fatty acids, omega-3 and omega-6, respectively9. The high content of essential fatty acids suggests a high nutritional value for huitlacoche, and could be due to the fact that corn is one of the cereals with the greatest content of fats and with a good proportion of the essential unsaturated fatty acids as well (table 2)38.
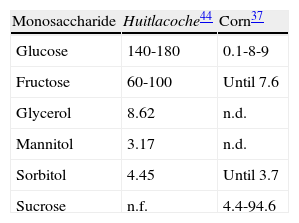
Content of mono and polysaccharidesAnother factor that affects the organoleptic characteristics of a food product is the content of carbohydrates. In a study conducted by Valdez-Morales et al44, 8 monosaccharides and 8 alditols were identified in samples of huitlacoche, of which two monosacharides, glucose and fructose, were the most abundant, together constituting approximately 81% of the total carbohydrates. Galactose, xilose, arabinose and manose were found in lesser proportions (table 3 table 3). Glycerol, glucitol and mannitol were the most representative of the alditols observed in the samples of huitlacoche22.
The high content of glucose and fructose in huitlacoche, even more than the extra-sweet varieties of corn (table 3), suggests that a good proportion of this monosaccharide is a result of the infection of the fungus44. This idea is supported by the recent description of a novel high-affinity sucrose transporter (srt1) of U. maydis, which is expressed only during the infection of the host and its main role is the direct utilization of sucrose, that is in high levels in maize (table 3), without prior extracellular hydrolysis into monosaccharides37,47. The same could be true for the production of glycerol and mannitol. On the other hand, disaccharides like trehalose and sucrose were not detected in huitlacoche (table 3)22,44.
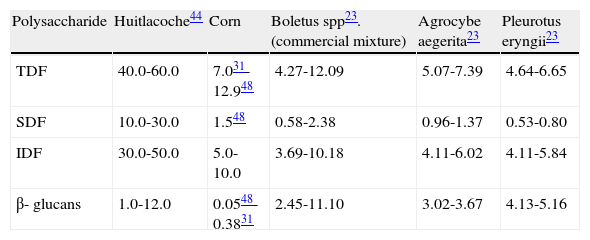
Regarding the content of polysaccharides huitlacoche contains, as do other edible mushrooms, either homoglycans and heteroglycans which are part of the dietary fiber of a food32,44. B-glucans are mushroom polysaccharides with a variety of structures, water-soluble or insoluble and that possess antitumor and immunostimulating properties. β-(1→3) backbone and β-(1→6) branch points are the most known antitumor structures49. β-glucans activates the complement and improve the response of the macrophages and killer cells. They can also be anti-oncogenic due to their protector effect against genotoxic compounds and because of their anti-angiogenic effect1,32. The content of β-glucans in huitlacoche is higher (between 1.0 to 12.0% of dry weight)44 than that reported in corn (0.05-0.038%)31,48 and similar to other edible mushrooms (2.45-11.10%)23 (table 4 table 4). Among different maize genotypes tested to produce huitlacoche, corina cajete and biznaga creole, two types of creole corn, showed a high content of β-glucans and are proposed for the production of huitlacoche rich in β-glucans44. The content of total dietary fiber (TDF), soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) are higher in huitlacoche than in corn (table 4), despite the fact that for example TDF in huitlacoche significantly decreases from 52% in the raw product to 49% when cooked44. The content of those dietary fibers in other edible mushrooms as Boletus spp. (commercial mixture), Agrocybe aegerita and Pleurotus eryngii23 tend to be lower than in huitlacoche (table 4).
Content of dietary fiber and β-glucans in huitlacoche, corn and other edible mushrooms, % of dry weight
| Polysaccharide | Huitlacoche44 | Corn | Boletus spp23. (commercial mixture) | Agrocybe aegerita23 | Pleurotus eryngii23 |
| TDF | 40.0-60.0 | 7.031-12.948 | 4.27-12.09 | 5.07-7.39 | 4.64-6.65 |
| SDF | 10.0-30.0 | 1.548 | 0.58-2.38 | 0.96-1.37 | 0.53-0.80 |
| IDF | 30.0-50.0 | 5.0-10.0 | 3.69-10.18 | 4.11-6.02 | 4.11-5.84 |
| β- glucans | 1.0-12.0 | 0.0548-0.3831 | 2.45-11.10 | 3.02-3.67 | 4.13-5.16 |
IDF: insoluble dietary fiber; SDF: soluble dietary fiber; TDF: total dietary fiber.
Polyphenols are compounds characteristic of plants that have more than one phenol group per molecule11. Quercetine and kaempferol are polyphenols with low molecular weight that promise to be effective anti-cancer agents because of scavenging reactive oxygen species (ROS) and increasing the anti-cancer activity of drugs like dacarbazine (DTIC), ascorbic acid and N-acetyl-cystein (NAC)5,43.
Although it has not been demonstrated that huitlacoche contains these compounds, the causal agent, U. maydis, produces enzymes like tyrosinase (E.C. 1.14.18.1) and laccase (E.C.1.10. 3.2) that catalyze the polymerization of hydroxy and mono phenols. While these enzymes were initially used in bioremediation, they have been utilized in the production of aggregates of relatively low molecular weight of quercetine and kaempferol, which have an anti-oxidant capacity greater than that of their monomeric precursors. It has been found that low concentrations of the systems of laccase-quercetine or laccase-kaempferol and tyrosinase-kaempferol have a strong effect on the inhibition of ROS and the lipoperoxidation of the membranes of a cell line of hepatocytes7.
In addition, a symptom of the infection of corn by U. maydis is the production of anthocyanins3, that are natural pigments characteristic of plants and are used as colorings and antioxidants in foods and pharmaceutical preparations, which are also polyphenolic compounds30.
Indolic compoundsIndoles such as ascorbigen are found principally in foods like cabbage, broccoli and other crucifers. Experimental evidence suggests that these compounds confer a protector effect against cancer, especially breast cancer, since they inhibit estrogen type receptors34.
Although the route of biosynthesis of indolic pigments and of production of auxines, such as indole acetic acid (IAA), has been described in U. maydis35,50 it is still unknown if these elements confer some functional and/or organoleptic characteristics to huitlacoche. Nevertheless, the production of IAA in huitlacoche is important for the formation of the plant tumor that is part of the infection35.
Protease productionCurrently the proteolytic system of U. maydis is partially known26, although in the study of proteases greater attention has been given to its function in relation to cellular physiology, these enzymes also favor the production of functional metabolites in foods and are involved in the generation of flavor2. It is known that various foods are source of peptides, which are inactive within the sequence of the native protein, but upon being liberated by enzymatic hydrolysis (in the intestine or in the process of production), can have beneficial biological effects on the organism. For instance, they act as antioxidants of lipoproteins and as regulators of biological processes17,29.
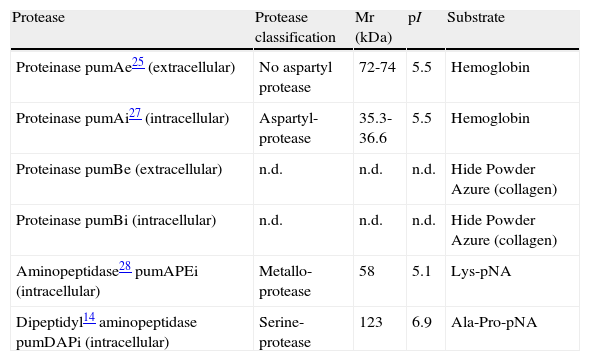
In the case of U. maydis, four proteolytic activities have been detected (table 5 table 5) in the FB1 and FB2 haploid strains. The pumAe (extracellular), pumAi (intracellular) proteinases and the aminopeptidase pumAPE that have been purified and biochemically characterized25,27,28 moreover pumAi has been related to dimorphic transition28. The characteristics of dipeptidyl aminopeptidase (pumDAP) were determined from a recombinant enzyme obtained by the heterologous expression of gen dap2 of U. maydis in Pichia pastoris14.
Biochemical characteristics of the U. maydis proteases
| Protease | Protease classification | Mr (kDa) | pI | Substrate |
| Proteinase pumAe25 (extracellular) | No aspartyl protease | 72-74 | 5.5 | Hemoglobin |
| Proteinase pumAi27 (intracellular) | Aspartyl-protease | 35.3-36.6 | 5.5 | Hemoglobin |
| Proteinase pumBe (extracellular) | n.d. | n.d. | n.d. | Hide Powder Azure (collagen) |
| Proteinase pumBi (intracellular) | n.d. | n.d. | n.d. | Hide Powder Azure (collagen) |
| Aminopeptidase28 pumAPEi (intracellular) | Metallo-protease | 58 | 5.1 | Lys-pNA |
| Dipeptidyl14 aminopeptidase pumDAPi (intracellular) | Serine-protease | 123 | 6.9 | Ala-Pro-pNA |
n.d.: not determined.
In many cases proteases of microbial origin, like neutral and alkaline proteinases, papain, and others, are added to food to improve the quality of its hydrolyzed products2. The proteases described in U. maydis could participate in the generation of these peptides in huitlacoche or contribute in some way to increase its nutritional value. Lysine is a limiting factor in the nutritional value of corn, but huitlacoche is one of the foods with the greatest content of this amino acid. Of course, all sources of nutritional proteins tend to supply functional peptides17.
With the availability of the U. maydis genome15 the search for and study of codifying genes of other proteases is relatively easy. Given the relevancy which is now recognized regarding the consumption of foods that contain compounds with antioxidant activity, the identification of proteolytic enzymes that generate these products is becoming very important.
Biosynthesis of glycolipids and biosurfactantsBiosurfactants, which are biological molecules with surfactant or tensoactive properties produced on surfaces of microorganisms or secreted by the same, have various advantages over chemical surfactants, including low toxicity, high biodegradability, environmental compatibility, selectivity and specific activity at high temperatures, as well as extreme pH and salinity16.
In conditions of nitrogen starvation, U. maydis produces great quantities of two biosurfactants, derived from two classes of glycolipids: ustiliagic or celobiosid lipid acid (UA) and ustilipid or lipid mannosylerythritol lipid (MEL)12,18,19,42. The secretion of UA is critical in the antagonic effect of U. maydis on Botrytis cinerea when they are co-inoculated on tomato leaves. In the same sense, MEL from Candida antartica also presents antimicrobial activity42. Accordingly, the use of biosurfactants as agents of biocontrol could be a new field of action for these metabolites.
Huitlacoche productionHuitlacoche is sold raw, in prepared food or processed in Mexican local markets during July and August (the second half of the rainy season), reaching between 400 and 500 tons of product per year10. However the introduction of this food into the international market, in countries as US, Japan, China and some of the European Community, as France, Spain and Germany for example13, requires the development of techniques that will allow for the production of large quantities during the whole year.
The factors that can affect the production of huitlacoche are the efficient production of the inoculant, the timing of inoculation and harvest, the characteristics of the corn hybrid which are used, and the strains of U. maydis utilized33,46. For instance, the taste, aroma and nutritional value of huitlacoche are factors that are dependent on the variety of corn and the state of development in which the fungus is harvested44.
For production of huitlacoche, the inoculant must be obtained in a controlled process. First, two sexually compatible strains of U. maydis are kept separate in a medium of acidic potato dextrose agar (aPDA) and cultivated separately in 50mL of potato dextrose broth (PDB), under constant stirring at room temperature for 18 to 24h, to obtain the preinoculant. Then 0.5mL of this preinoculant is cultivated in 100mL of PDB under constant stirring for 12 to 18h in order to obtain the inoculant. For plant inoculation, the basidiospores of each strain are adjusted to a concentration of 106 cells/mL of medium and then mixed together33.
In a report about the production of biomass by the Taguchi method, the pH of the medium was determined to be 7, the velocity of stirring 200rpm, and the glucose concentration in the medium 40g/L. These are the most important factors in the production of the biomass of the FBD12 U. maydis strain. By using the before mentioned conditions, a maximum biomass of 15.67g/L was obtained in 48h6.
The inoculation of the mixture of sexually compatible strains in the corn plants is administered by means of an injection of the suspension of spores in the silk-channels of the corn or in the leaves once the silk has emerged40. Sweet corn hybrid varieties are the most susceptible to infection. Field studies suggest that the infection is more severe when the plants are inoculated between 4-8days after the mid-silk growth state33, which can be tested by pressing a grain of corn with a fingernail to see if a milky substance comes out. Maximum yields of huitlacoche and the best quality product (including color and flavor) are obtained 16 to 17days post inoculation33,45. Pollination will reduce the formation of galls up to 50%8,41. In spite of the fact that the control of polinization can be costly, it is necessary for quality control of the commercial production of huitlacoche.
Taking all of these factors into account can make the commercial production of huitlacoche functional, thus offering the international market with a food of fine organoleptic qualities year round.
Conflict of interestNone declared.
We thank Bruce Allan Larsen for reviewing the use of English in this manuscript.
The present study received support from CONACYT-84085, SIP-IPN 2010-0514, SIP-IPN- 2009-0402 and ICyTDF-Cd. Sostenible-2010, PICSO 10-95. M. Juárez-Montiel received a scholarship from CONACYT and is a PIFI-IPN and TELMEX. L. Villa-Tanaca and C. Hernández-Rodríguez received grants from COFAA-IPN and EDI-IPN. L. Villa-Tanaca was hired by the “Program of excellence of the Section of Research and Postgraduate studies of the IPN”.