Intracranial fungal masses are uncommon diseases, but their incidence is increasing, most often due to the prolonged survival of patients with different immunodeficiencies. The management of patients with intracranial fungal masses included stereotactic biopsy for diagnosis, partial or radical surgery excision and prolonged antifungal therapy.
AimsWe report the case of a 51-year-old diabetic man with a history of psoas abscess due to Candida albicans 1 year before the onset of neurological symptoms, including headache and generalized tonoclonic seizures.
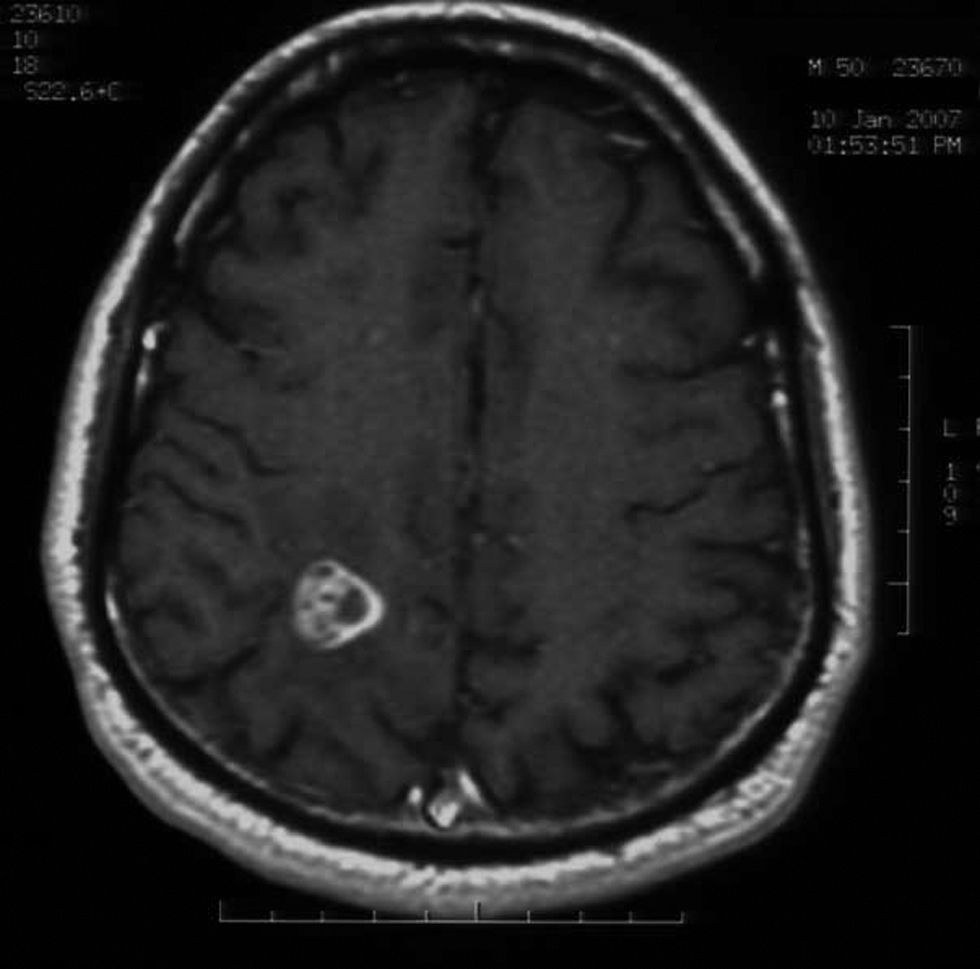
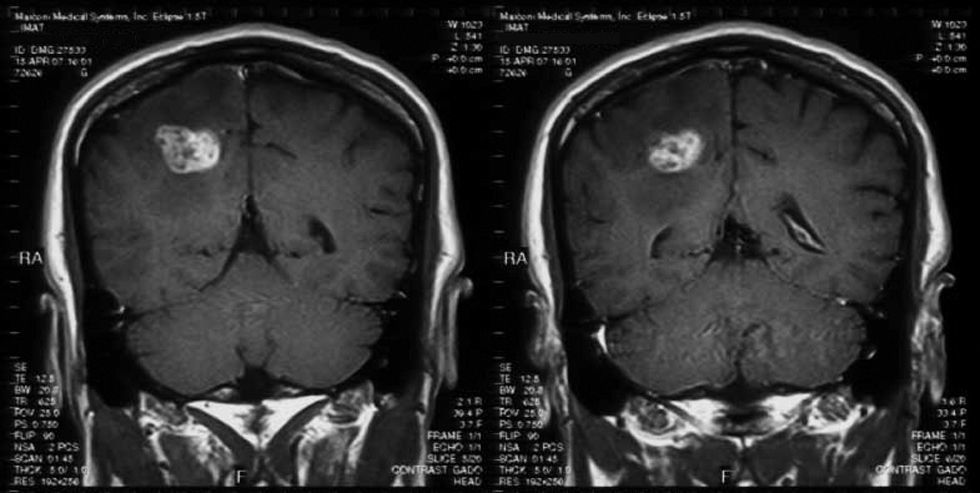
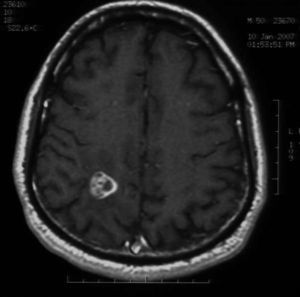
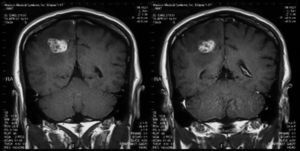
MethodsMagnetic resonance imaging showed a single lesion located in the right parietal lobe with mass effect, surrounding edema and enhancement after injection of gadolinium. The material was purulent.
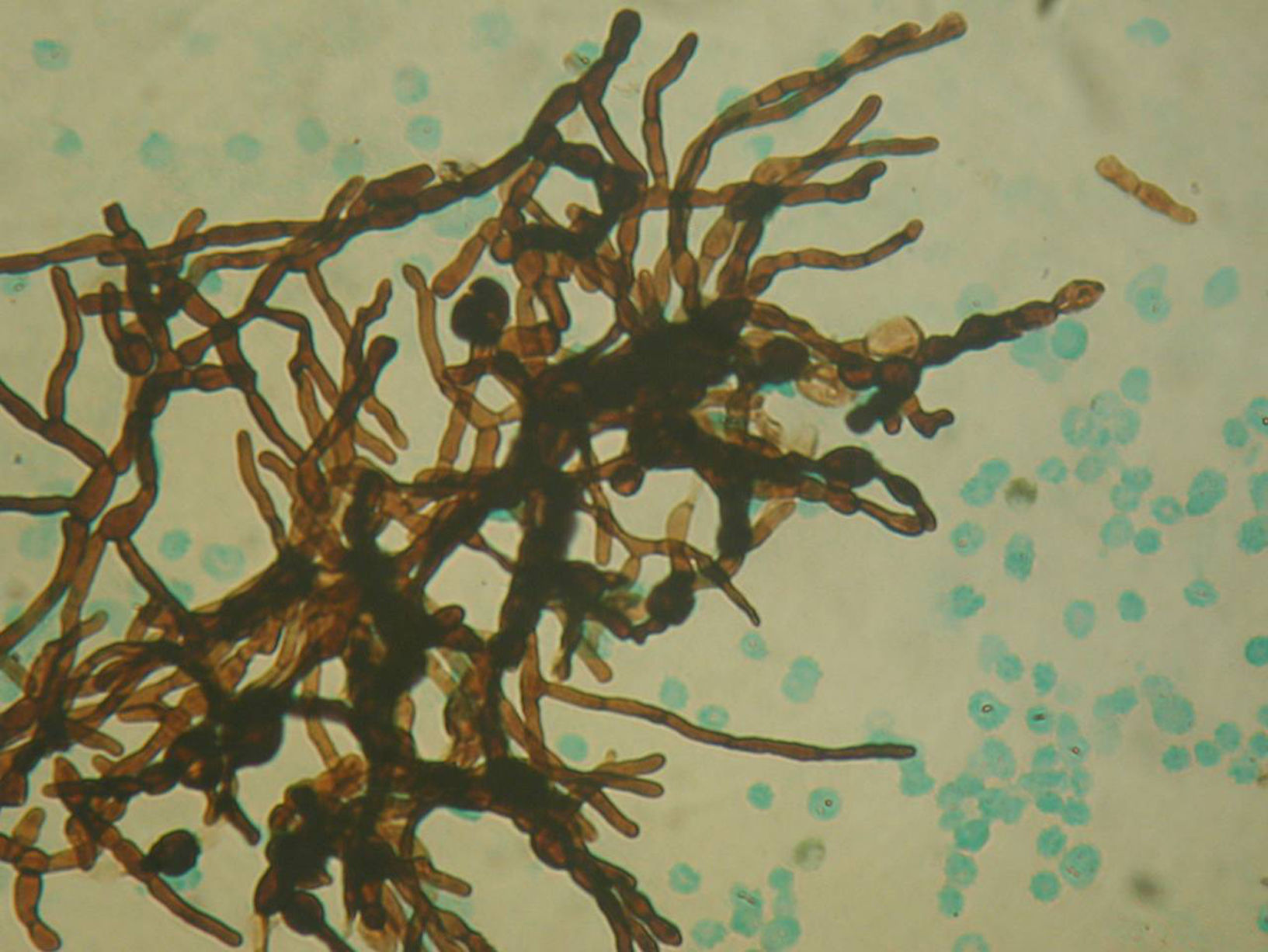
ResultsDirect microscopic examination showed hyaline, branched and septate hyphae compatible with fungal elements.
ConclusionsFungal infections, especially due to Candida species, should be considered in diabetic patients with parenchymal brain abscesses. Radical excision followed by prolonged antifungal therapy based on fluconazole or amphotericin B is necessary to improve the prognosis of this type of patients.
Las masas cerebrales de etiología fúngica son complicaciones poco frecuentes, pero su incidencia se ha incrementado en relación directa con la prolongación de la supervivencia de los pacientes con diferentes inmunodeficiencias. El enfoque clínico de estos pacientes incluye la biopsia estereotáctica de la lesión con fines diagnósticos, la resección quirúrgica parcial o total y el tratamiento antifúngico prolongado.
ObjetivosSe describe el caso de un paciente diabético con antecedentes de absceso del psoas por Candida albicans un año antes del inicio de los síntomas neurológicos (que incluyeron cefaleas y convulsiones tónico-clónicas generalizadas).
MétodosUna resonancia nuclear magnética mostró una lesión única, localizada en el lóbulo parietal derecho, con refuerzo del contraste, edema perilesional y efecto de masa sobre las estructuras adyacentes. Se efectuó resección quirúrgica completa de la lesión.
ResultadosEl examen macroscópico de la misma mostró material purulento que en el examen microscópico directo evidenció la existencia de hifas hialinas, ramificadas y tabicadas compatibles con elementos fúngicos.
ConclusionesLas infecciones micóticas, en especial aquellas causadas por hongos del género Candida deben incluirse en el diagnóstico diferencial de los abscesos cerebrales en pacientes diabéticos. El tratamiento quirúrgico seguido de la administración prolongada de antifúngicos como fluconazol o anfotericina B es fundamental para mejorar la evolución y el pronóstico de estos pacientes.
Intracranial fungal masses are uncommon diseases but their incidence is increasing, mostly due to the prolonged survival of patients with different immunodeficiencies. AIDS, cancer chemotherapy, hematopoietic stem cell transplantation and solid organ transplantation also contribute to the increased risk of invasive fungal infections.3,7,19 The use of vascular catheters, exposure to broad-spectrum antimicrobial agents, prolonged stay in intensive care units and parenteral nutrition are recognized as risk factors for candidemia.17 Additionally, fungal infections of the central nervous system (CNS) account for a high proportion of opportunistic infections in patients with HIV-AIDS disease.24
Candida, Aspergillus and Cryptococcus are the most common fungal pathogens responsible for the majority of brain abscesses in immunocompromised patients.1
Case reportA 51-year-old man with history of psoriasis and type-2 diabetes, treated with metformin but with poor glycemic control, was admitted to our hospital, reporting 2 months of headaches and generalized tonic–clonic seizure episodes. He referred to a previous hospitalization 1 year before, owing to a psoas abscess due to Candida albicans. He did not receive statins as hypolipidemic drugs or anti-TNF drugs for psoriasis. Physical examination revealed fever (38°C), tachycardia (102beats/min) and weight loss (approximately 10kg in the previous 3 months). The patient also presented a focal neurological syndrome involving paresis with weakness of his left arm. Lung auscultation was normal; abdominal examination revealed hepatomegaly; the spleen was not palpable.
Relevant laboratory findings were anemia with hematocrit 31%, hemoglobin 9g/mL, leukocytes 6100/mm3 (76% of PMN), platelet count 131000/mm3 and hyperglycemia (196g/dL) with plasma glycosylated hemoglobin (HbA1c) >7.0g%. Renal function evaluated by the creatinine clearance was normal. Liver enzyme levels, coagulation tests and chest radiograph were normal. Blood and urine cultures for bacteriae, mycobacteriae and fungi were negative. A transthoracic echocardiogram was performed with no signs of vegetations in cardiac valves. Abdominal ultrasonography showed hepatomegaly and findings consistent with non-alcoholic hepatic steatosis.
Videocolonoscopy was normal. Computed tomography of thorax, abdomen and pelvis was also normal. Brain magnetic resonance imaging (MRI) showed a single lesion located in the right parietal lobe with mass effect, surrounding edema and enhancement after injection of gadolinium (Figs. 1 and 2). MRI of the dorsolumbar spine was normal. Lumbar puncture was not performed because of the presence of cerebral edema and signs of mass effect on the middle line structures.
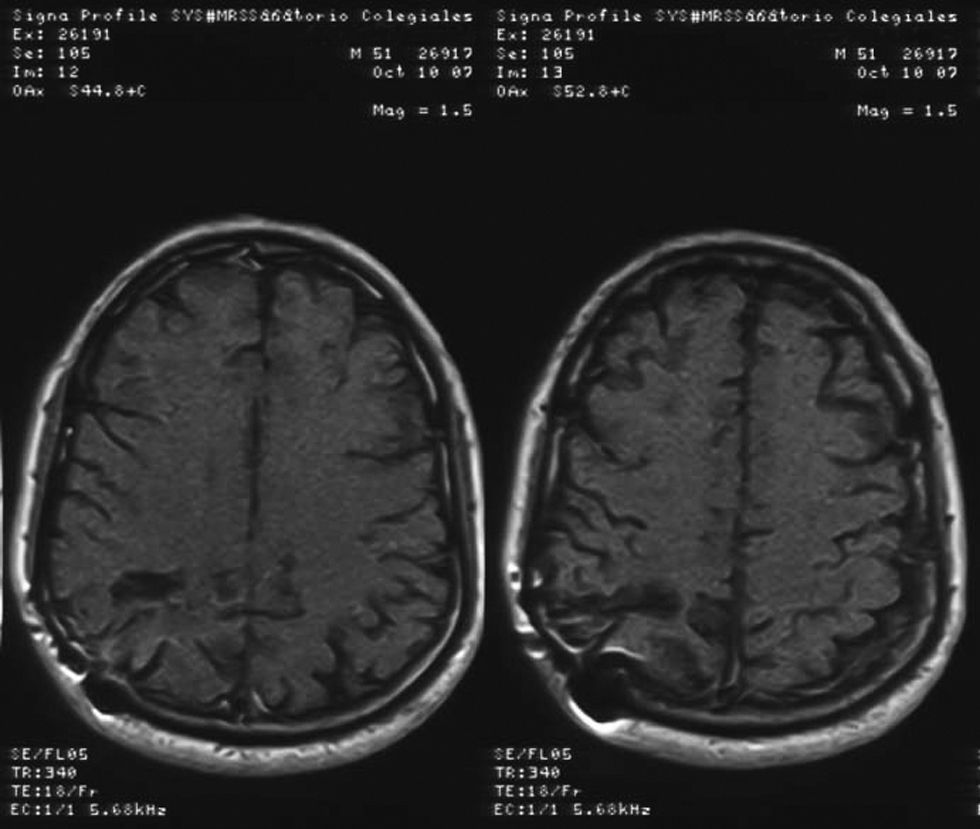
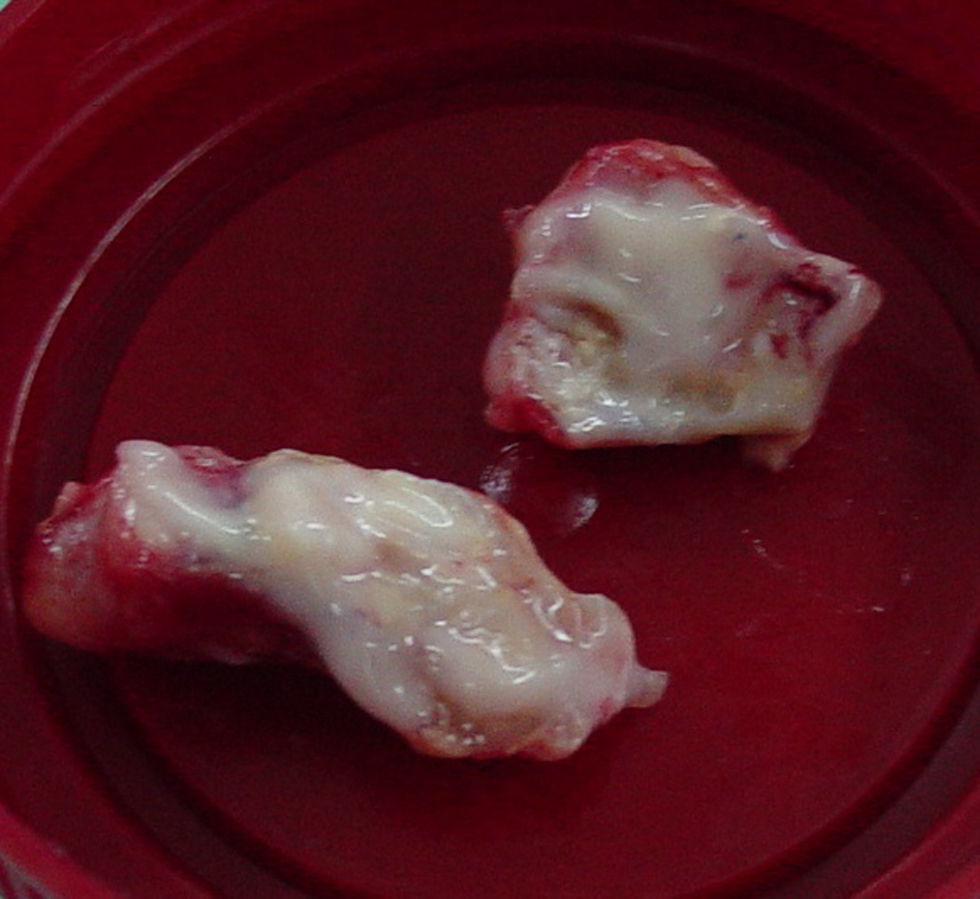
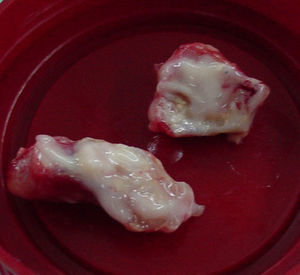
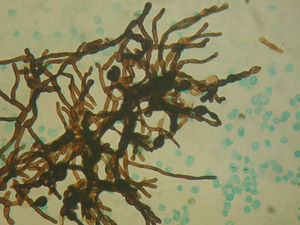
Image guided stereotactic surgical excision of the cerebral abscess was performed (Fig. 3). The material was purulent (Fig. 4); direct microscopic examination showed hyaline, branched and septate hyphae compatible with fungal elements. Grocott stain showed septate hyphae (Fig. 5). Ziehl–Neelsen, Gram and Giemsa smears were negative. Culture of abscess material was negative. Biopsy pathological findings include wide inflammatory infiltrate with predominance of neutrophilic leukocytes.
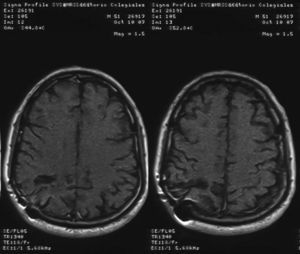
Initial treatment involved manitol as antiedematous osmotic diuretic and a combination of intravenous amphotericin B deoxicolate 0.8mg/kg/day and fluconazole 800mg/day for 1 month. He did not receive corticosteroids as cerebral antiedematous because of the risk of making the glycemic control worse. The total accumulation dose of amphotericin B was 1500mg. After surgery, the patient was treated with antifungal therapy based on oral fluconazole at doses of 600mg/day for 4 months, with a good clinical and radiological evolution. Two years after the onset of neurological symptoms, he is still in good clinical condition without neurological sequelae.
DiscussionFungal infections of CNS are not common and they are often secondary to a focus located in the lung or the digestive tract.
Neurofungal infections, meningitis or abscesses are associated with extremely high mortality caused by delayed onset of therapy, severe underlying disease and multiresistant fungal organisms.20
One of the most frequent predisposing diseases for disseminated fungal infections is diabetes and, most recently, these complications are associated with immunocompromised status.12. The rising incidence of diabetes mellitus has resulted in an increased number of cases of candidaemia, intracranial fungal masses, granulomas and abscesses, due to Candida species.21C. albicans, still represents the predominant species.23 Other risk factors for candidaemia and cerebral abscess include the presence of intravascular catheters, admission to intensive care unit, parenteral nutrition, multiple antibiotics, prolonged corticosteroid therapy, cancer chemotherapy, solid organ transplantation, AIDS and neutropenia.17
Candida is the fourth most common cause of bloodstream infections, and epidemiological data indicate that at least 72% of all nosocomial fungal infections and 8–15% of all nosocomial bloodstream infections are caused by Candida species.16 Additionally, autopsy series show that 1–6% of patients with systemic candidosis also have CNS involvement. This finding suggests that neurocandidiasis is frequently misdiagnosed.18
Although intracranial fungal masses can be seen at any age, the majority of patients are in the third, fourth or fifth decade of their lives, as in our patient.
These complications have also been reported in neonates, infants and young children.15
Cryptococcosis is the most frequent fungal infection of the CNS, followed by aspergillosis and candidiasis.12 Coccidioidal meningitis is also frequently observed in endemic areas.
Clinically, fungal infections of the CNS may be presented as disseminated meningoencephalitis or as located brain lesions or abscesses. Clinical manifestations of candidiasis include mucocutaneous involvement (especially genital, oropharyngeal and esophagical lesions), cutaneous lesions in cases of chronic mucocutaneous candidiasis, and systemic or disseminated compromise.
Generally, invasive or disseminated candidiasis involves multiple organs. Neurocandidiasis is rarely observed during the course of disseminated candidiasis, and abscess formation in the brain is a severe complication in patients with different risk factors. Endocarditis, endophtalmitis and brain abscess are the most severe locations of candidaemia.15 Schelenz and Grandsden22 analyzed 128 cases of Candida bloodstream infections and the authors detected only 6% of patients with these serious complications.
Intracranial fungal masses due to Candida spp. usually develop by the haematogenous spread of fungus from a source of infection generally located at the digestive or the urinary tracts. Primary candidiasis of the brain or meninges is rare; however, central nervous system involvement is reported in 18–52% in disseminated candidiasis.11,14 The duration of neurological symptoms can vary from a few days to several months, as in the patient we present.2,9.
Fungal abscesses are hypointense on T1 weight and hyperintense on T2 with a well-defined ring enhancement after gadolinium administration. These neuroimaging characteristics are similar to those observed for abscesses due to bacterial, mycobacterial and parasite pathogens.4,5 However, in immunocompromised patients, fungal abscesses can appear as patchy or punctate T2 hyperintense lesions with frequent absence of enhancement post-gadolinium.10,12,13 Fungal infections, including candidiasis, can rarely cause cerebrovascular involvement, usually associated with large vessel vasculitis by invasion or embolization.6
Njambi et al.20 observed 20 cases of fungal meningitis and/or cerebral abscesses within the last 25 years. The most common etiologic agent was Candida spp., accounting for 9 of 20 patients. The authors point out an extremely high mortality due to the delay in the onset of a specific therapy, severe underlying diseases and multiresistant fungal organisms.
Diagnosis of disseminated or invasive candidiasis remains difficult and is generally confirmed by direct microscopic examination of the drained pus achieved during surgical excision of the abscesses. Cultures are frequently negative, as in our patient. Although it is true that the candidial etiology of the brain lesion in our patient was not confirmed, the fungal nature of the process is doubtless due to the presence of hyaline hyphae in the direct microscopic examination (Fig. 5). As the patient was diabetic and had a history of psoas abcess due to C. albicans, that etiology was regarded as the most possible one. The presence of hyaline hyphae is not the most habitual microscopic aspect of the Candida fungi, but these may produce true hyphae as well as pseudohyphae and blastoconidia. This is an interesting aspect of our case and can be explained by the host inflammatory responses in fungal infections. The Th1/Th2 responses have potentially important implications in the pathogenesis of immune reconstitution syndrome in immunodeficient patients. However, the immunopathogenesis of CNS fungal infections remains incompletely studied. Host defense mechanisms can influence the severity of fungal infections, and the clinical form of the disease depends on the patient's immune response. In the immunocompromised host, haematogenous dissemination may happen and fungal invasion of the CNS can occur.8 The Aspergillus galactomannan test is useful only for oncohematological patients with neutropenia. The glucan test is not available in Argentina due to its high cost and poor demand. Finally, the cryptococcal latex test was not carried out since the microscopic findings of the cerebral abcess were not correlated with such etiology.
Treatment of cerebral Candida infection is normally based on a combination of amphotericin B deoxycolate and 5-fluorocytosine as first-line therapy. In our country, 5-fluorocytosine is not available. In patients without response to amphotericin B or those who present severe adverse events associated with this therapy, fluconazole alone or in combination with 5-fluorocytosine might be used.23 Novel antifungal drugs, such as caspofungin, penetrate poorly into the CNS especially if the blood–brain barrier is intact. However, voriconazole shows a good CNS concentration and might be used in patients with refractoriness or incompa- tibility to amphotericin B,18 although voriconazole has not been proven to be superior to fluconazole in the treatment of C. albicans infections, which was the species identified in the psoas abcess. Voriconazole use would have been justified in the event of therapeutic failure or resistance to fluconazole. The aim at recommending a prolonged treatment with fluconazole was to eliminate any candidiasis residual focus in a diabetic patient.
In conclusion, uncontrolled diabetes mellitus is a significant risk factor for disseminated candidiasis. A rising trend of invasive candidiasis, including neurocandidiasis, can be observed in patients with diabetes mellitus.