A case of fungemia with interstitial lung compromise caused by Malassezia sympodialis is reported in an obese pediatric patient on long-term treatment with inhaled corticosteroids for asthma. The patient was hospitalized due to a post-surgical complication of appendicitis. The patient was treated with amphotericin B for 3 weeks, with good clinical evolution and subsequent negative cultures.
Presentamos un caso de fungemia con compromiso intersticial causado por Malassezia sympodialis en un paciente pediátrico obeso, asmático y con tratamiento prolongado con corticosteroides inhalados, que fue hospitalizado debido a una complicación posquirúrgica de una apendicectomía. El paciente fue tratado con anfotericina B durante 3 semanas con buena evolución clínica y negativización de los cultivos subsecuentes.
Malassezia species are components of the normal microbiota of human skin and of many warm-blooded animals but, for still unknown reasons, these yeasts are able to change their saprophytic state and invade the stratum corneum as pathogens.2,3,10,11,15 These yeasts have been involved in the pathogenesis of a variety of dermatological disorders. Furthermore, in the immunocompromised host, including critically ill neonates, Malassezia may cause a number of invasive infections ranging from very mild to life-threatening ones.2,6,21
We present a case of fungemia with interstitial lung compromise caused by Malassezia in a pediatric patient.
CaseA 7-year-old male patient afflicted with both obesity and moderate asthma, with long-period inhaled corticosteroids and under beta-2-agonist therapy, was hospitalized in July 2009 with acute abdomen. The diagnosis revealed phlegmonous appendicitis. Twenty-four hours after surgery, evidence of acute lower respiratory tract infection (ALRI) caused by a respiratory syncytial virus (RSV) was observed. RSV was detected by indirect immunofluorescence (IFI) of nasopharyngeal aspirate. As a complication, resulting from severe coughing, the patient showed diaphragm eventration. The patient underwent reoperation and was treated with ceftriaxone 100mg/kg/day and metronidazole 30mg/kg/day EV. Five days later, due to poor clinical and infection recovery, a new antibiotic scheme with imipenem 50mg/kg/day and vancomycin 40mg/kg/day was indicated. Prior to antibiotic scheme, samples drawn from conventional blood and urine cultures were negative.
In spite of the antibiotic treatment the patient developed moderate respiratory distress, high fever and low oxygen saturation level. Auscultation revealed decreased air entry, bilateral subcrepitant rales and disseminated sibilant bronchi. The abdomen was bloated, depressible upon deep palpation and the surgical wound showed no signs of phlogosis.
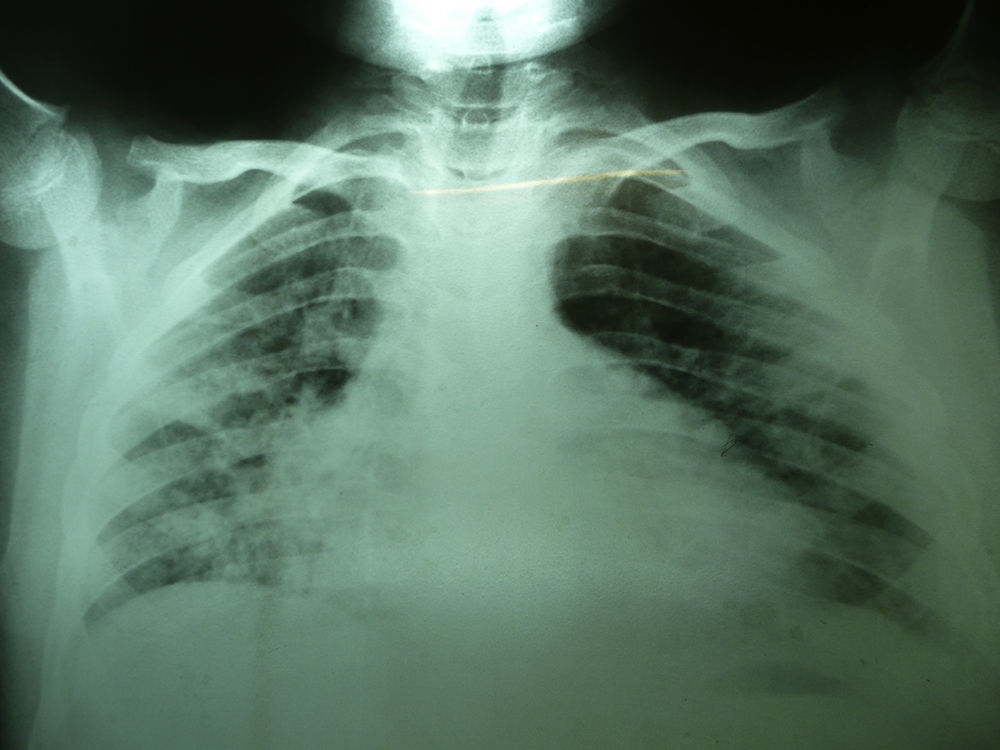
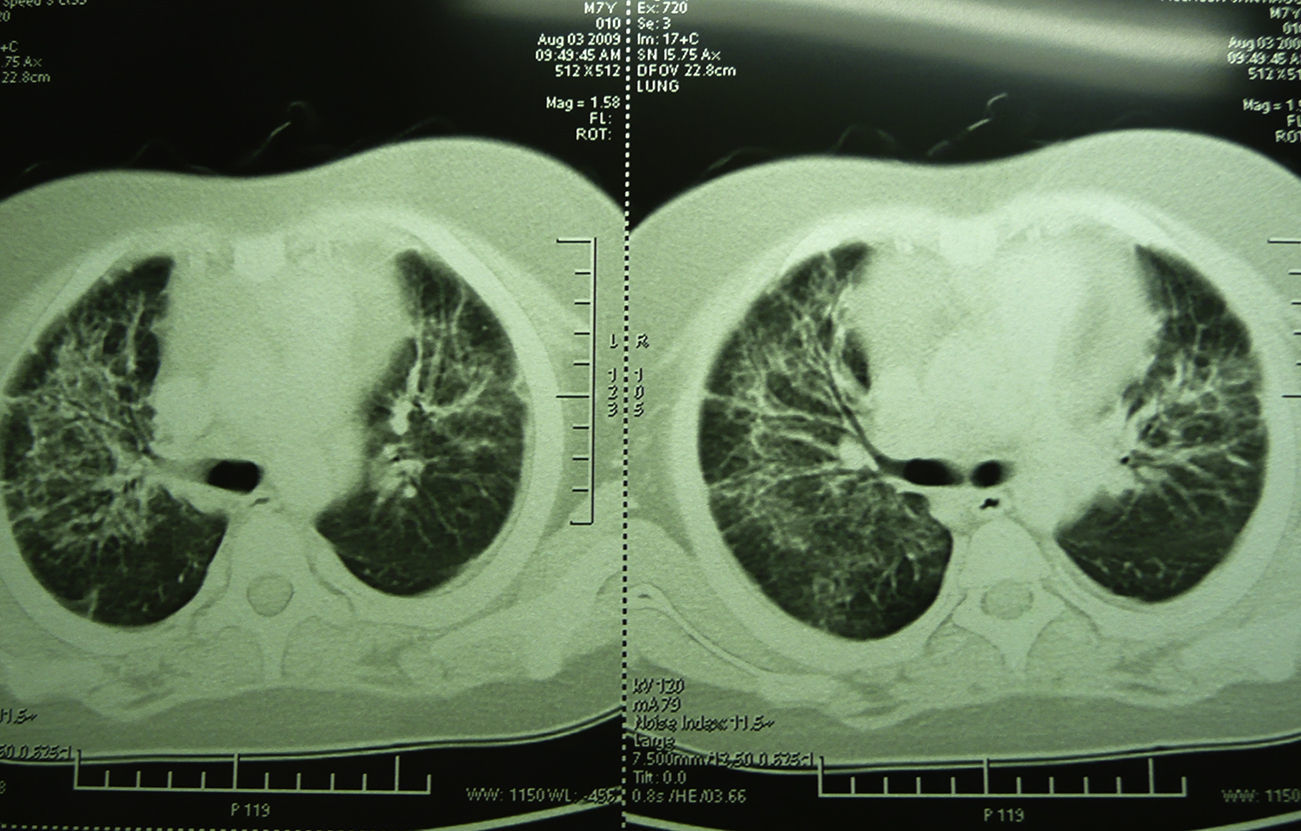
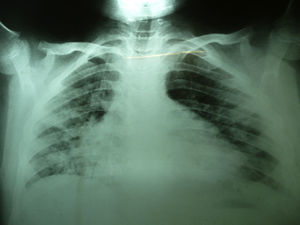
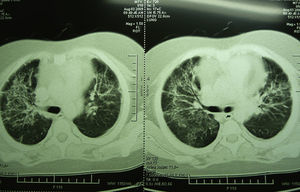
Laboratory studies showed the following counts: leukocytes (21,900/mm3), 74% segmented, 1% band neutrophils, 1% eosinophils, 22% lymphocytes and 2% monocytes. The hemoglobin concentration was 11mg/dl, platelet count 167,000/mm3, and the sedimentation rate was >120mm. The X-ray showed diffuse bilateral alveolar-interstitial infiltrates (Fig. 1). The CT scan also revealed pleural effusion of 16mm (Fig. 2).
After 24 days of hospitalization, due to moderate respiratory distress with manifestations consistent with multifocal pneumonia and oxygen requirement through nasal cannula, the patient was admitted in ICU (intensive care unit). Imipenem and vancomycin intake was interrupted. Treatment with beta-2 agonist, hydrocortisone, ranitidine, ciprofloxacin was initiated intravenously.
Prior to indicating ciprofloxacin treatment, new samples were collected for conventional urine and blood cultures and catheter tip culture. To exclude fungal infection, blood culture was performed by lysis-centrifugation. In order to rule out other etiologies, samples of respiratory secretions were obtained serially and the PPD test was carried out. As broad-based budding yeasts were observed on stains in material obtained from lysis-centrifugation, a differential medium for Malassezia was included. Five days later, a lipophilic yeast growth was obtained; 48h later Malassezia sympodialis was identified. Amphotericin B deoxycholate 1mg/kg/day was indicated (accumulated dosage 20mg/kg). No adverse effects with amphotericin B were observed.
Immunodeficiency markers were studied: HIV testing, protein electrophoresis, immunoglobulin dosage, lymphocyte subsets and complements were performed. These studies showed normal parameters. All other microbiological studies including PPD test were negative.
As a consequence of this finding, this fungus bearing was sought. Malassezia sympodialis was also isolated from the patient's skin. The patient's clinical history reported hyperpigmented lesions of the skin recorded since 2005, located mainly in the inguinal area, assumed as superficial mycoses and treated with topical antifungals but with poor response.
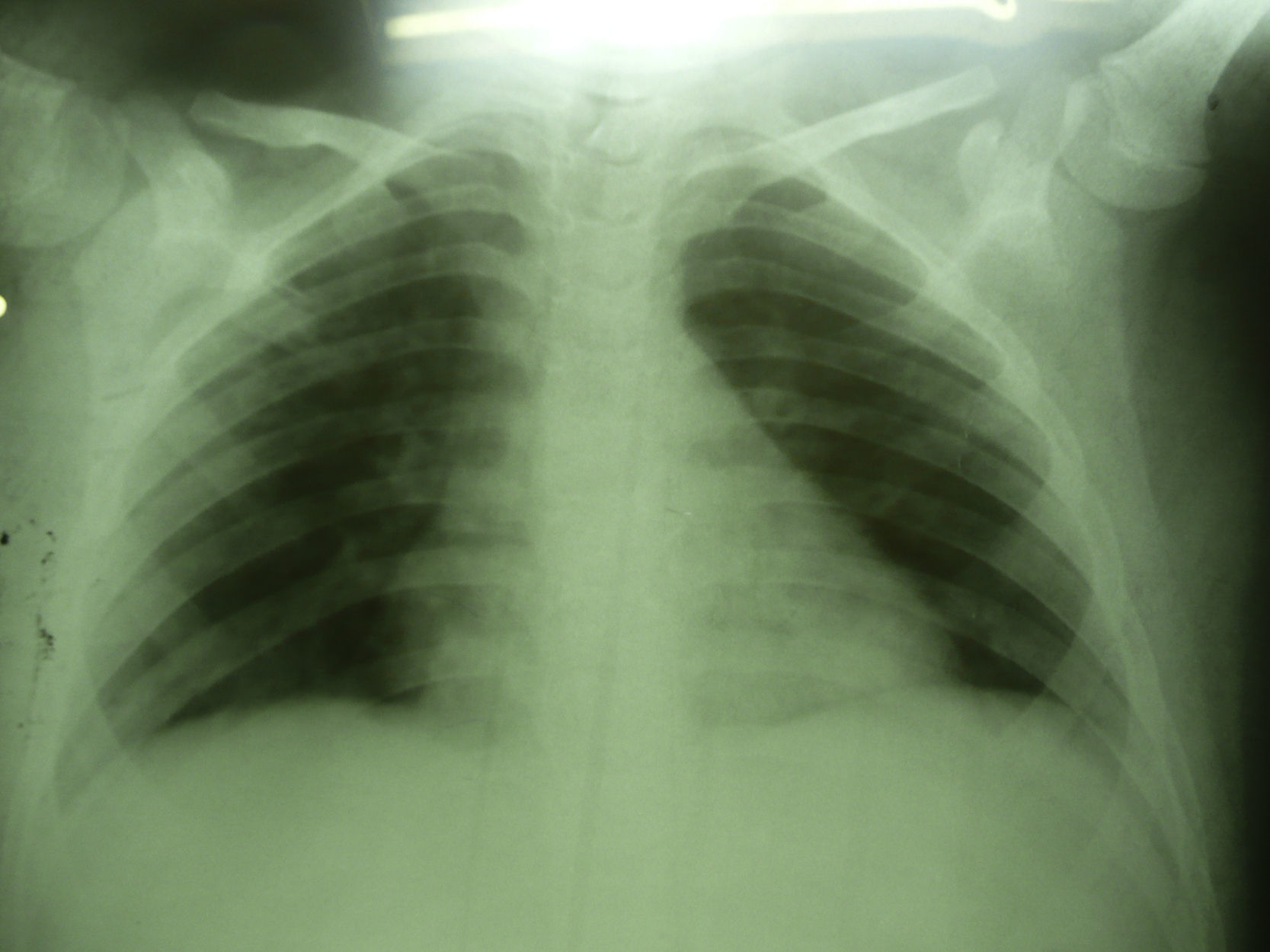
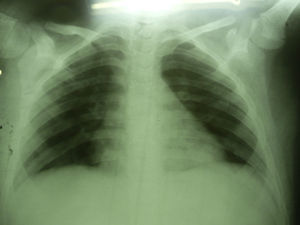
Seven days after starting antifungal treatment new samples for lysis-centrifugation blood culture were collected, which were negative. The patient's condition evolution was favorable; he responded to the 21-day antifungal treatment positively. Imaging studies were normal 3 months later (Fig. 4).
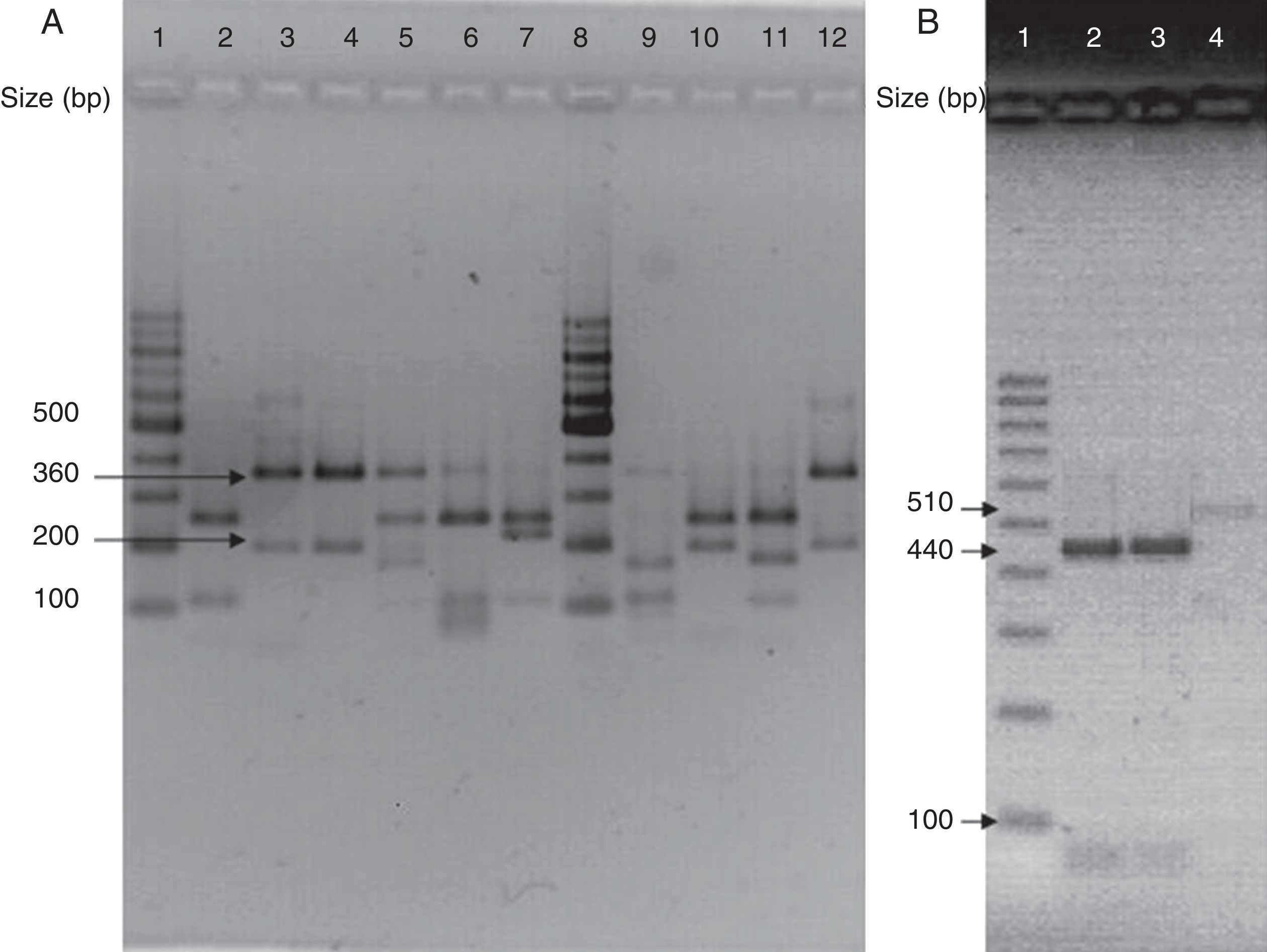
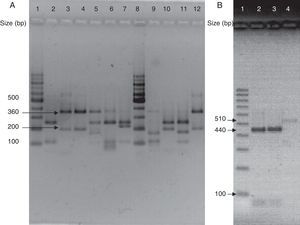
(A) Visualization of 26S rDNA products after digestion with Cfo I (Lane 1–12: 100bp ladder, CBS 7019 M. furfur, CBS 7222 M. sympodialis, CBS 9169 M. dermatis, CBS 9558 M. nana, CBS 7956 M. slooffiae, CBS 10533 M. pachydermatis, 100bp ladder, CBS 9725 M. yamatoensis, CBS 9432 M. japonica, CBS 7876 M. obtusa, and IMR-M-735). (B) Digestion with Mbo I (Lanes 1–4: 100bp ladder, IMR-M-735, CBS 7222 M. sympodialis and CBS 9169 M. dermatis).
The patient was never under mechanical ventilation.
Blood culture and molecular identificationBlood cultures were performed on modified Dixon's medium at 32°C for 7 days.9Malassezia isolate (IMR-M-735) was identified by PCR-RFLP.13
For DNA extraction, cellular lysis was performed by a boiling-thermic shock combination. One loopful of yeasts was suspended in 80μl of distilled water and boiled for 5min at 100°C, then frozen for 10min at −70°C and finally boiled again for 5min at 100°C. The crude DNA suspension (extract) was stored at −20°C, until its use for the analysis.
Amplification was performed by using generic primers, forward 5′-TAA CAA GGA TTC CCC TAG TA-3′ and reverse 5′-ATT ACG CCA GCA TCC TAA G-3′. The primers successfully amplified the target part of 26S rDNA from all tested Malassezia strains, providing a single PCR product of the expected size (approximately 580bp).13 Amplified DNA products were subjected to restriction fragment length polymorphism (RFLP) using Cfo I (Promega) in a first identification step. Whenever the restriction pattern produced by M. sympodialis or Malassezia dermatis appeared, additional Mbo I (Fermentas) was used (Fig. 3).7 CBS 7019 Malassezia furfur, CBS 7222 M. sympodialis, CBS 9169 M. dermatis, CBS 9558 Malassezia nana, CBS 9432 Malassezia japonica, CBS 10533 Malassezia pachydermatis, CBS 9725 Malassezia yamatoensis, CBS 7876 Malassezia obtusa and CBS 7956 Malassezia slooffiae were included as reference strains.
DiscussionMalassezia colonizes the skin and scalp of healthy individuals. This colonization increases from adolescence, when the sebaceous glands increase their activity and the concentration of lipids in the skin raises.2,3,11,15 On the other hand, these opportunistic yeasts may cause invasive infections in immunocompromised patients.3–6,21,23 The first case reported of peritonitis caused by lipophilic yeast was in 1979 by Wallace.22 In our case, overweight and long-term use of inhaled corticosteroids, as well as surgical stress, were factors that allow our patient to be considered as an immunocompromised patient.
Malassezia infections are more common in warm climate regions.2 Our city is located in a subtropical region with a high annual average temperature and humidity, which facilitate colonization by Malassezia in its inhabitants. As evidenced by outbreak investigations, the cutaneous commensal biota of the patient or healthcare workers is the usual source of the infecting organisms. This colonization by M. sympodialis could be one of the causes that may lead to subsequent infection. In addition, it has been reported that genetic factors may predispose to dermatological diseases related to Malassezia.2,10 Our patient might have some sort of susceptibility, taking into account his long-lasting history of mycoses (lasting for years). A history of prior cutaneous infections caused by the same fungus makes it almost impossible to think of a possible horizontal transmission of infection.
Malassezia sepsis and pneumonitis have been described in children and adults with central venous catheters and in patients with preceding surgery, such as in our case.6,17–19,21Malassezia fungemia is usually associated with lipid-solution administration through venous catheters.1,5,8,12,18,20,21,23 But reports evaluating the colonization of central venous catheters by Malassezia species have demonstrated a colonization rate of 0.7% in unselected hospitalized adults despite not receiving parenteral lipid nutrition.21 Our patient was not on intralipids but his skin colonization, the prolonged hospitalization, the broad-spectrum antibiotic treatment and the central venous catheter could have acted as risk factors, but not the parenteral nutrition. We agree with some authors who emphasize that colonization and the presence of a central line appear to be mandatory prerequisites for fungemia and that the administration of parenteral lipids may act as a facilitating factor.5,21
Unfortunately, the catheter could not be cultured for the isolation of Malassezia, since the patient was referred exclusively for bacteriological studies. It was probably colonized by the same M. sympodialis found in the child's skin, but epidemiological typing to identify clonal relations of isolates was not made.
In critically ill patients, clinical status is difficult to determine. In infants hospitalized in intensive care units with central catheters, parenteral nutrition with lipids and broad spectrum antibiotics treatment, who develop fever, pulmonary infiltrates, leukocytosis, thrombocytopenia and negative conventional cultures for bacteria and fungus, Malassezia should be thought as one of the likely etiologic agents.1,5,18,20 However, individual cases lacking the typical risk factors have been reported.14,24 In adults, when fever is the only clinical symptom presented by the patient,5 and some may be asymptomatic, a differential diagnosis should be conducted to elucidate other opportunistic infections.20 Systemic spread appears to be limited to the lungs.17 When dealing with critically ill patients, it is difficult to observe the pathological role of Malassezia, since several aggravating factors are combined.23
Our patient developed high fever and respiratory distress with hypoxemia. Initially, we took into account the probable fungal etiology because of his history of prolonged hospitalization, broad-spectrum antibiotic therapy and both abdominal surgeries. On the other hand, microbiological studies confirmed viral infection with RSV and viral infections can favor or predispose the fungal infection. Subsequently, persistent fever and negative cultures suggested the performance of a differential culture for the isolation of Malassezia. Although the frequency of Malassezia infections is low, this highlights the importance of determining its likely etiology and preparing the laboratory appropriately for differential diagnosis, since this fungus can only be isolated in special culture media not used in laboratory routine.3,9
Treatment includes catheter and lipid-solution administration removal and antifungal therapy.21,23 Our patient was treated with amphotericin B for 3 weeks, with good clinical response confirmed by subsequent negative cultures. Surely, the removal of central venous catheter in this case was a cornerstone of the treatment.16
The majority of invasive infections reported in literature have been associated with M. furfur and M. pachydermatis. The first is a lipophilic yeast commonly isolated from humans and the latter, M. pachydermatis, is a zoophilic yeast not lipo-dependant, occasionally isolated from human skin.6,17–19,21 This is a relevant case since we have not found any report of sepsis and interstitial lung compromise caused by M. sympodialis, one of the lipo-dependent species of the genus.. Also, it is essential to take into account other predisposing factors than the sole presence of parenteral lipidic nutrition.
The revision of the genus Malassezia opened up new questions about the pathogenicity of Malassezia species. Further studies will be necessary to avoid subdiagnosis and to understand the relationship of these species with the environment and with associated pathologies.