Cases of superficial and invasive mycoses caused by emerging species of Candida have been increasingly reported over the last thirty years. The production of hydrolytic enzymes plays a central role in the fungal infective process. In Candida infections the secretion of both proteases and phospholipases are well-known virulence attributes.
AimsTo determine the protease and phospholipase production from 58 human clinical isolates of Candida obtained from individuals with cutaneous candidiasis seen in the Human and Veterinary Diagnostic Mycology Sector from Universidade Federal Fluminense (UFF), Brazil, from November 2008 to August 2009.
MethodsFungal identification was performed using biochemical tests. Proteolytic activity was detected on agar plates containing bovine serum albumin, and phospholipase production was determined on egg-yolk plates.
ResultsThe Candida species isolated were Candida parapsilosis (27.59%), Candida famata (18.96%), Candida albicans (15.52%), Candida haemulonii (12.06%), Candida ciferri (8.62%), Candida guilliermondii (6.90%), Candida tropicalis (5.17%) and Candida lipolytica (5.17%). All isolates of C. albicans produced both protease and phospholipase. As regards the isolates of non-C. albicans Candida species, 53.06% and 4.08% were able to produce protease and phospholipase, respectively. For example, the majority of isolates of C. parapsilosis (15/16) produced protease, while 40% of C. ciferri isolates (2/5) were phospholipase producers. This study shows, for the first time, that C. ciferri and C. haemulonii strains were able to produce protease.
ConclusionsCollectively, our results showed that different species of Candida isolated from cutaneous lesions were able to produce proteases and/or phospholipases, which are multifunctional molecules directly involved in the infectious process of these fungi.
Los casos de micosis superficiales e invasoras relacionados con las especies emergentes de Candida se han reportado progresivamente durante las últimas tres décadas. La producción de enzimas hidrolíticas juega un papel central en varios contextos de la patogenicidad fúngica. Con respecto a la infección por Candida, la secreción de proteasas y fosfolipasas son atributos de virulencia bien conocidos.
ObjetivosDeterminar y comparar la producción de proteasa y fosfolipasa de 58 aislamientos clínicos humanos de diferentes especies de Candida obtenidos de pacientes con candidiasis cutánea, atendidos en el Sector de Diagnóstico Micológico Humano y Veterinario de la Universidad Federal Fluminense (UFF), durante el período de noviembre de 2008 a agosto de 2009.
MétodosLa identificación de las especies de Candida se realizó mediante pruebas bioquímicas, la actividad proteolítica se detectó en placas de agar que contenían albúmina de suero bovino y la actividad fosfolipasa se determinó utilizando el método de la placa de yema de huevo semi-cuantitativa.
ResultadosLas especies aisladas fueron Candida parapsilosis (27,59%), Candida famata (18,96%), Candida albicans (15.52%), Candida haemulonii (12,06%), Candida ciferri (8,62%), Candida guilliermondii (6,90%), Candida tropicalis (5,17%) y Candida lipolytica (5,17%). Todos los aislamientos de C. albicans produjeron tanto proteasa como fosfolipasa. El 53,06% de los aislamientos de Candida no-C. albicans fueron capaces de producir proteasa y el 4,08% fosfolipasa. La mayoría de los aislamientos de C. parapsilosis (15/16) produjo proteasa, mientras que el 40% de los aislamientos de C. ciferri (2/5) fueron productores de fosfolipasa. Se describe por primera vez en la literatura científica la producción de proteasas por cepas de C. haemulonii y C. ciferri.
ConclusionesNuestros resultados muestran el potencial que tienen los aislamientos de Candida provenientes de lesiones cutáneas para producir proteasas y fosfolipasas.
Superficial mycoses of the skin and its appendages are amongst the most prevalent human infectious diseases observed in clinical practice. The etiological agents comprise the dermatophytes and yeasts responsible for infections including dermatophytosis, pityriasis versicolor and candidiasis. The prevalence of these fungi tends to follow geographic variations, cultural habits and migration that can change with time.11 Cases of superficial and invasive diseases related to species belonging to the Candida genus have been progressively reported in the last years, involving isolates of Candida albicans, Candida ciferri, Candida dubliniensis, Candida famata, Candida glabrata, Candida guilliermondii, Candida haemulonii, Candida kefyr, Candida krusei, Candida lipolytica, Candida lusitaniae, Candida parapsilosis, and Candida tropicalis, among others.19
Crucial steps during pathogenesis of superficial candidiasis comprise fungal adhesion, colonization and subsequent penetration of tissues. In all these phases, the production of hydrolytic enzymes is a fundamental event.13,14 In this sense, two major classes of enzymes have prominent and central roles: proteases and phospholipases, since proteins and phospholipids represent the major chemical constituents of the host cell membrane, which must be destroyed in order to obtain nutrients for fungal growth/development as well as to facilitate the dissemination inside the host.10,20
The aim of the present study was to investigate the in vitro production of phospholipase and protease activities in different Candida species isolated from human cases of cutaneous candidiasis.
Materials and methodsCandida isolatesThe clinical isolates (n=58) were obtained from patients presenting cutaneous candidiasis, who were attended during the period of November 2008 until August 2009 at the Sector of Human and Veterinary Diagnostic Mycology, Microbiology and Parasitology Department of Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brazil. The fungal isolates were recovered from the following body location: toe nail (n=24), finger nail (n=20), sole of the foot (n=4) and skin (n=10). The study was approved by the Bioethics Committee of Hospital Universitário Antônio Pedro (CEP CMM/HUAP number 188/08 and CAAE number 0147.0.258.000-08).
Identification testsClinical isolates were initially tested on CHROMagar Candida medium (CHROMagar Candida Company, Paris, France). Plates were incubated for 24–72h at 37°C under aerobic conditions. Metabolic properties, such as assimilation of carbon compounds and enzymatic reactions, were analyzed by VITEK 2 yeast identification system (bioMérieux, Marcy l’Etoile, France). In addition, germ-tube tests as well as the capacity of yeasts to grow at different temperatures and salt concentrations were performed.30 Growth was estimated by counting the fungal cells in a Neubauer chamber.
Protease assayIsolates were tested for protease production by using yeast carbon base (YCB) medium (1.17%) supplemented with 0.2% bovine serum albumin (BSA). The YCB-BSA medium was adjusted to pH 5.0, sterilized by filtration and added to previously autoclaved agar (2%).23 Aliquots (10μl) of 48h-old cultured fungal cells (106 yeasts) were spotted onto the surface of the YCB-BSA medium and incubated at 37°C for seven days. The proteolytic activity results in a clear zone around the colony, which corresponds to the hydrolysis of the BSA present in the medium. The colony diameter (a) and the diameter of colony plus the precipitation zone (b) were measured by a digital paquimeter.
Phospholipase assayPhospholipase production was assayed using the egg-yolk agar plate containing 1M NaCl, 5mM CaCl2 and 8% sterile egg-yolk emulsion.21 Suspensions of approximately 106 yeasts (10μl) of each isolate, previously cultivated on Sabouraud during two days, were inoculated onto the surface of the egg-yolk agar plate medium and incubated at 37°C for seven days. Hydrolysis of lipid substrates present in the egg-yolk results in the formation of a calcium complex with fatty acids released by the action of the secreted enzymes, resulting in a precipitation zone around the colony. The colony diameter (a) and the diameter of the colony plus the precipitation zone (b) were measured by a digital paquimeter.
Enzymatic score and statistical analysisThe enzyme activities were expressed as Pz value (a/b) as described by Price and co-workers.21 According to this definition, low Pz values mean high protease or phospholipase production and, inversely, high Pz values indicate low enzymatic production. The enzymatic activity was scored into four categories: a Pz of 1.0 indicated no enzymatic activity; a Pz between 0.999 and 0.700 indicated low enzymatic activity; Pz between 0.699 and 0.400 corresponded to moderate activity; and low Pz values between 0.399 and 0.100 meant high enzymatic activity. All the experiments were repeated at least three times and all assays were carried out in triplicate. The data were analyzed statistically using Student's t test. P values of 0.05 or less were considered statistically significant.
Results and discussionThe prevalence of superficial mycoses has tremendously risen in the last years, affecting around 20–25% of the world's population. In this scenario, skin mycoses are the most frequent forms of infection and, consequently, constitute a major public health problem worldwide.15,25 Our results revealed that from the 58 Candida isolates recovered from cutaneous infections, C. parapsilosis was the most frequently isolated species (n=16; 27.6%), followed by C. famata (n=11; 19%), C. albicans (n=9; 15.5%), C. haemulonii (n=7; 12%), C. ciferri (n=5; 8.6%), C. guilliermondii (n=4; 6.9%), C. tropicalis (n=3; 5.2%) and C. lipolytica (n=3; 5.2%). Considering the site of the cutaneous infections, C. parapsilosis (20.5%) was the most prevalent Candida species isolated from finger followed by C. albicans (18.2%), C. famata (18.2%), C. haemulonii (13.6%), C. ciferri (11.4%), C. guilliermondii (9.1%), C. tropicalis (4.5%) and C. lipolytica (4.5%), while C. parapsilosis (70%) was again by far the most isolated species from skin followed by C. albicans (10%), C. famata (10%) and C. tropicalis (10%).
Epidemiological and etiological studies of superficial mycoses of the foot revealed that the major isolated causal agents were C. parapsilosis (25%) and Trichophyton rubrum (18.7%).5,6C. parapsilosis was the leading cause of onychomycosis in a group of patients from Malta followed by C. tropicalis and C. guilliermondii.27 Similarly, a study performed in Brazil reported that the leading pathogen detected in samples from infected nails (n=200) was C. parapsilosis (40.5%) followed by C. albicans (31.5%), C. tropicalis (26.0%) and C. guilliermondii (2.0%).7 Contrarily, C. albicans was the most common Candida species able to cause cutaneous/nail and vaginal candidiasis in Singapore,18 Jordan,1 Slovakia12 and the United States.8 Importantly, the increased tendency for non-C. albicans Candida infections poses a challenge in the disease management, since these species are often notorious to develop antifungal resistance that is correlated with routine fluconazole prophylaxis adopted in some patients and the intrinsic/acquired azole resistance of Candida spp.3
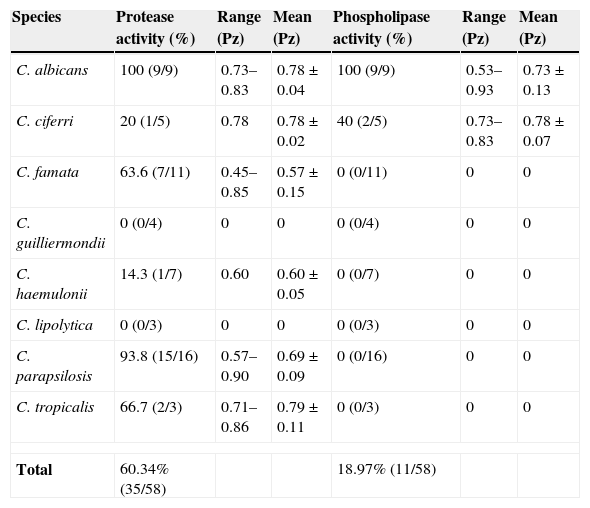
The production of proteases, especially secreted aspartic proteases (Saps), has been reported to be one of the most important determinants for pathogenicity of Candida.9 The culture of Candida yeasts in YCB-BSA at acidic pH is a recognized condition that induces the secretion of Saps.28 Under these conditions, we showed that protease activity was detected in 35 strains of all Candida species tested, among which all strains of C. albicans and almost all strains of C. parapsilosis were protease producers (Table 1). All C. albicans isolates exhibited high Pz values, which indicate low enzymatic production, whereas the majority (66.7%) of C. parapsilosis isolates displayed moderate Pz and the minority (33.3%) high Pz values. However, strains of C. famata showed lower Pz values when compared to the other Candida species (Table 1). In addition, the protease activity of C. famata was significantly higher than that of C. albicans (P<0.001) or C. parapsilosis (P<0.03). None of the C. lipolytica or C. guilliermondii isolates showed proteolytic activity (Table 1), under the employed experimental conditions. The analysis of the body location also revealed a significant difference (P<0.05) in the protease production: there was a 90% of producers between the skin isolates, 55% between the finger nails’ isolates, 54.2% between the toe nails’ isolates, and 50% in the case of the sole of feet isolates.
Distribution of protease and phospholipase activities from Candida spp. isolated from cutaneous infections.
| Species | Protease activity (%) | Range (Pz) | Mean (Pz) | Phospholipase activity (%) | Range (Pz) | Mean (Pz) |
|---|---|---|---|---|---|---|
| C. albicans | 100 (9/9) | 0.73–0.83 | 0.78±0.04 | 100 (9/9) | 0.53–0.93 | 0.73±0.13 |
| C. ciferri | 20 (1/5) | 0.78 | 0.78±0.02 | 40 (2/5) | 0.73–0.83 | 0.78±0.07 |
| C. famata | 63.6 (7/11) | 0.45–0.85 | 0.57±0.15 | 0 (0/11) | 0 | 0 |
| C. guilliermondii | 0 (0/4) | 0 | 0 | 0 (0/4) | 0 | 0 |
| C. haemulonii | 14.3 (1/7) | 0.60 | 0.60±0.05 | 0 (0/7) | 0 | 0 |
| C. lipolytica | 0 (0/3) | 0 | 0 | 0 (0/3) | 0 | 0 |
| C. parapsilosis | 93.8 (15/16) | 0.57–0.90 | 0.69±0.09 | 0 (0/16) | 0 | 0 |
| C. tropicalis | 66.7 (2/3) | 0.71–0.86 | 0.79±0.11 | 0 (0/3) | 0 | 0 |
| Total | 60.34% (35/58) | 18.97% (11/58) | ||||
Kantarcioglu and Yücel showed that 78.9% of all examined Candida strains (n=95) were protease-positive.16 Those authors also interpreted their data by different viewpoints. For example, 95% of C. albicans produced proteases. However, considering only the site of fungal isolation, a new profile of protease producers could be seen, as follows: oral cavity (17/22=77.3%), respiratory tract (37/46=80.4%), urogenital system (21/23=91.3%), and blood (0/4=0%).16 These discrepancies in percentages of positivity may be considered as being relevant due to the number of the different species tested as well as the site of isolation. Corroborating these findings, De Bernardis and co-workers reported that all cutaneous isolates of C. parapsilosis had uniformly elevated secreted protease activity, more than four times higher than the enzyme activity of the blood isolates.4 Additionally, the cutaneous isolates of C. parapsilosis were highly vaginopathic in a rat vaginitis model when compared to the blood isolates.4 Cassone also detected a higher proteolytic activity in vaginal C. parapsilosis isolates when compared with blood isolates.2 Different published works revealed that 65–75% of clinical strains of C. tropicalis were able to yield aspartic proteases,17,26,29 which are in accordance with our findings (≈67%). We reported herein that cutaneous clinical isolates of C. famata were the most potent protease producers. For the first time, we emphasize that some clinical strains of C. ciferri and C. haemulonii are able to secrete aspartic proteases.
Phospholipases play an active role in the invasion of host tissue by Candida by disrupting the epithelial cell membranes and allowing the hyphal tip to enter the cytoplasm.10 Regarding phospholipase activity, all strains of C. albicans and only few strains (2/5) of C. ciferri were capable of producing phospholipase, while the great majority (81%) of the Candida strains isolated from cutaneous lesions was unable to yield it (Table 1). The 2 strains of C. ciferri and 5 strains of C. albicans able to produce phospholipase activity exhibited high Pz values, while the other 4 remaining strains of C. albicans showed moderate Pz values. Samaranayake and co-workers screened 41 Candida isolates for phospholipase activity and found no strains of C. tropicalis, C. glabrata and C. parapsilosis producing extracellular phospholipases, whereas 73% of the C. albicans isolates screened were found to be positive.24 Kumar and co-workers showed that 100% of clinical isolates of C. albicans isolated from HIV positive and cancer patients produced a pronounced phospholipase activity.17 The absence of protease and/or phospholipase in clinical isolates of Candida must be interpreted with caution. In this context, hydrolytic enzymes with activities against other substrates of relevance to human cutaneous infections, distinct to those used in the present study, can help in the detection of these enzymatic classes.
The comparison between C. albicans and non-C. albicans Candida species revealed that the frequency of isolation (15.5% versus 82.5%), as well as the production of both protease (100% versus 53.1%) and phospholipase (100% versus 4.1%) activities, was significantly different (P<0.05). Only 17.2% (10/58) of the Candida isolates produced both enzyme classes (all strains of C. albicans and one strain of C. ciferri). Previous studies also reported that proteases and phospholipases are produced at high rates in C. albicans, whilst non-C. albicans Candida species usually present low rates of these enzymes.16,22 In this sense, the hydrolytic enzyme profiles provide some data about the potential virulence factors produced by Candida strains isolated from cutaneous infection, resulting in a great variability production in non-C. albicans Candida species (probably strain specific), in contrast to a homogeneous production of both protease and phospholipase by clinical isolates of C. albicans.
Conflict of interestThe authors report no conflict of interest.
This study was supported by grants from the following Brazilian Agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ). The authors thank Dr. Marta Helena Branquinha (UFRJ) for critical suggestions on the manuscript.