Hemodialysis has been described as an important risk factor for the development of candidemia in patients suffering from chronic renal failure.
AimsThe aim of this study was to evaluate the epidemiology of candidemia in outpatients with renal replacement therapy (RRT) by hemodialysis where the fungemia clearly represents a healthcare-associated infection.
MethodsWe retrospectively collected clinical and laboratory data from patients undergoing at least 3 months of RRT by hemodialysis who developed candidemia within 48h of hospital admission.
ResultsWe identified 14 patients with candidemia with central venous catheters (CVC) in place for 11–277 days before developing fungemia. Deep-seated infection was documented in 6 out of 14 candidiasis cases (43%), including 5 cases of endocarditis (36%).
ConclusionsCVC in patients under RRT should be promptly replaced by fistulas and grafts to avoid bloodstream infections. Facing a case of candidemia, adequate source control and prompt initiation of antifungal therapy are mandatory to avoid morbidity and mortality.
La hemodiálisis se ha descrito como un importante factor de riesgo para el desarrollo de candidemia en pacientes con insuficiencia renal crónica.
ObjetivosEl objetivo de este estudio fue evaluar la epidemiología de la candidemia en pacientes en hemodiálisis con terapia renal sustitutiva (TRS), en la que la fungemia representa claramente una infección asociada a los cuidados hospitalarios.
MétodosSe recogieron retrospectivamente datos clínicos y microbiológicos de pacientes con, al menos, 3 meses de hemodiálisis con TRS que desarrollaron candidemia dentro de las primeras 48 horas tras la admisión hospitalaria.
ResultadosIdentificamos a 14 pacientes con candidemia asociada con el uso de catéter venoso central (CVC) durante períodos de 11 a 277 días previos al desarrollo de la fungemia. En 6 de los 14 casos de candidemia, el diagnóstico fue de candidiasis invasiva (43%), incluidos 5 casos de endocarditis (36%).
ConclusionesLos CVC en pacientes con TRS deberían ser sustituidos inmediatamente por fístulas o injertos arteriovenosos para evitar infecciones del torrente sanguíneo. Ante los casos de candidemia, un control adecuado de las posibles fuentes de infección y el comienzo inmediato de la terapia antifúngica deberían ser imperativos para reducir tanto la morbilidad como la mortalidad.
Nosocomial bloodstream infections due to Candida species represent a frequent complication in patients submitted to invasive medical procedures, including major surgeries, the use of central venous catheters (CVCs) and hemodialysis.8,23 Indeed, invasive fungal infections represent a substantial cause of morbidity and mortality in patients with chronic renal failure.7,25
Chronic renal failure is a medical condition with an increasing incidence worldwide.16,29 It is estimated that by 2020, two thirds of end-stage renal disease (ESRD) patients in the United States will require hemodialysis, which makes this population highly susceptible to bloodstream infections.7 Brazil, United States, Germany, Italy and Japan are home to 12% of the world's population, and more than half of all patients with ESRD are now being treated in these countries.18 Arteriovenous fistulas are the ideal permanent vascular access method for patients under renal replacement therapy (RRT) by hemodialysis to avoid bloodstream infections.2,3 However, in Brazil, there is a delay in constructing the fistula, which leads to the prolonged use of central venous catheters for hemodialysis and an increased risk of infections.4
Several studies have reported the occurrence of candidemia in chronic renal failure patients during their hospitalization, where hemodialysis represents one among several other risk conditions and comorbidities that may contribute to patient outcomes.25,27,28 The focus of our study was to evaluate the epidemiology of candidemia in outpatients with RRT by hemodialysis where the fungemia clearly represents a healthcare-associated infection.
Materials and methodsStudy settingWe retrospectively collected the clinical and laboratory data of patients undergoing at least 3 months of RRT by hemodialysis who developed candidemia within 48h of hospital admission during a four-year period. This study was conducted in the Hospital do Rim e Hipertensão, a center of excellence and world leader in kidney transplantation with approximately 150 beds located in São Paulo (Brazil).
DefinitionsAn episode of candidemia was defined as the incident isolation of Candida from one or more blood cultures (BACTEC System) of a patient under RRT by hemodialysis. Candidemia occurring more than 30 days after the incident isolation was defined as a new episode. Patients were followed for 90 days after the diagnosis of candidemia. Endocarditis was defined according to the DUKE criteria.11 The diagnosis of endophthalmitis was based on clinical features observed on dilated fundoscopy performed by an ophthalmologist.
For all episodes of candidemia, we collected clinical and laboratorial data using a standard clinical form, including the following variables: age, gender, date of candidemia, date of admission, exposure to invasive medical procedures, use of antibiotics or corticosteroids, clinical management of candidemia (antifungal treatment and time of CVC removal), and outcome (mortality). The presence of comorbidities, including diabetes mellitus, arterial hypertension, autoimmune diseases, hepatitis (B and C), cardiac, pulmonary, and neurologic diseases documented within the previous 3 months, was also recorded. The presence of vascular devices was recorded and classified as tunneled catheters, short-term catheters and fistulas with grafts for dialysis. The protocol was approved by the local ethics committee.
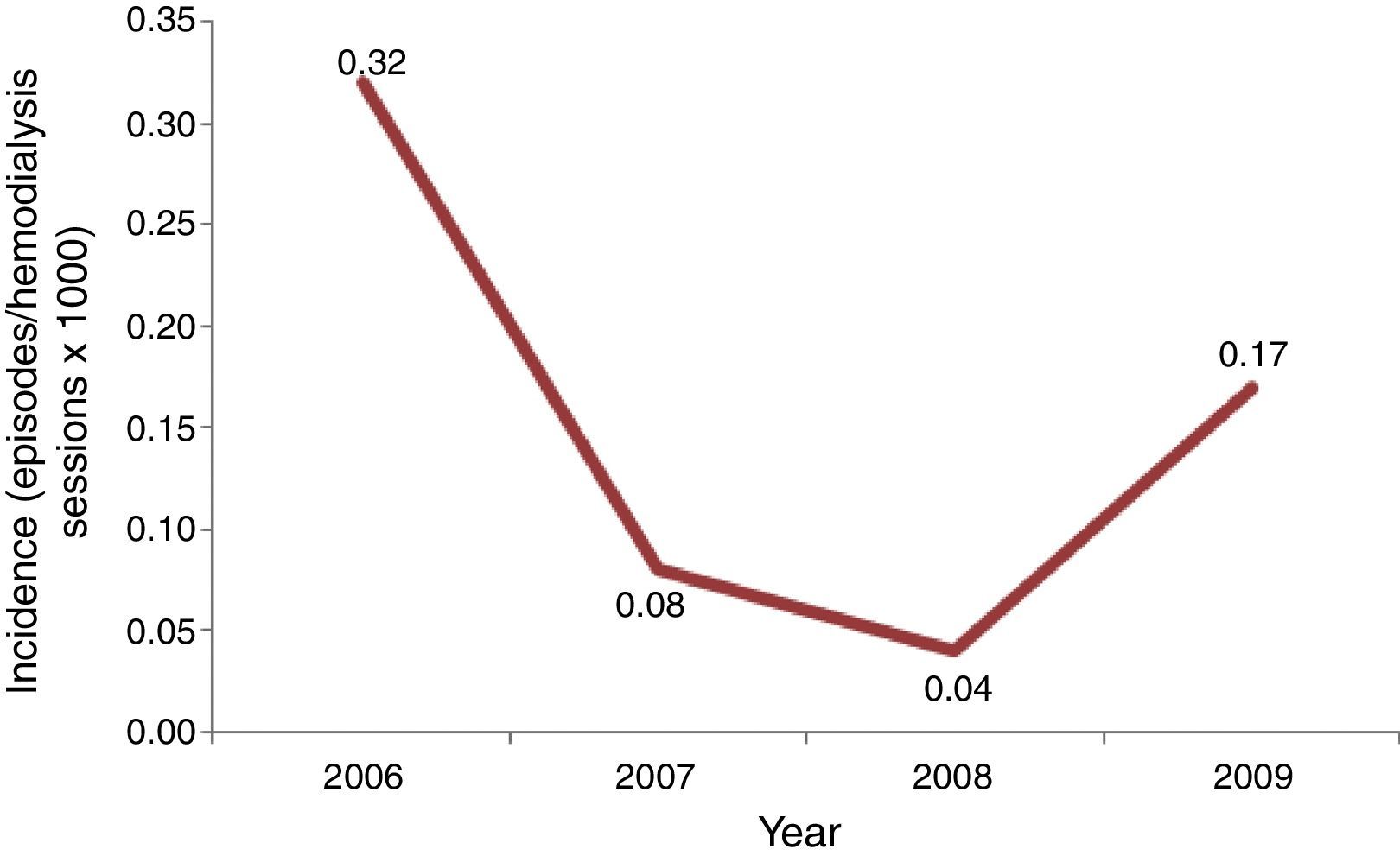
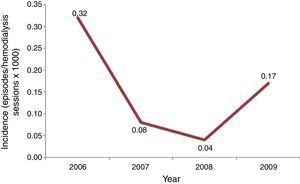
Incidence of candidemia was calculated using the number of episodes of candidemia as numerator, and total of hemodialysis sessions per year as denominator, multiplied per 1000.
Yeast identificationBlood samples were aseptically collected and processed for culture by the BACTEC automatic system. All the species of Candida recovered from the blood cultures were initially identified by conventional methods (ID32C®, bioMérieux, France) at the routine laboratory of Hospital São Paulo (Escola Paulista de Medicina, Universidade Federal de São Paulo). Isolates stored in the routine laboratory were further sent to the Laboratório Especial de Micologia, Universidade Federal de São Paulo, São Paulo, Brazil, for further molecular identification. Sequencing of the internal transcribed spacer (ITS) region of ribosomal DNA was used for the molecular identification of Candida species.13 The nucleotide sequences generated in this study were deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank) [GenBank: JQ585710, GenBank: JQ989500, GenBank: JQ585713, GenBank: KP659256, GenBank: KP659257, GenBank: KP659258, GenBank: KP659259 and GenBank: KP659260].
Results and discussionInitially, we screened 29 patients with candidemia undergoing hemodialysis along the period of our study. A total of 15 patients were excluded because they developed candidemia before a 3 month-period of RRT was completed or were hospitalized for more than 48h. Consequently, our casuistic consisted of 14 outpatients with candidemia and RRT by hemodialysis. The incidence of candidemia ranged from 0.04 to 0.32 cases per 1000 hemodialysis sessions per year, and the highest rate was recorded in 2006, suggesting the occurrence of a possible outbreak (Fig. 1).
Demographics, clinical characteristics, underlying conditions and outcomes are summarized in Table 1. Most patients (57%) were female, and the median age was 45.5 years, ranging from 22 to 79 years. We noted the occurrence of previous episodes of bacteremia in 5 patients (35.7%), diabetes mellitus in 4 patients (28.6%) and kidney engraftment failure in 4 patients (28.6%). Other predisposing conditions for candidemia included the presence of central venous catheters in 13 cases (92.9%), previous exposure to antibiotics in 7 cases (50%) and corticosteroids in 6 cases (43%).
Demographics, clinical characteristics, risk factors and outcomes of 14 candidemia patients under renal replacement therapy.
| Variables | Outpatient n=14 (%) |
|---|---|
| Gender | |
| Female/male | 8 (57.1)/6 (42.9) |
| Age (year) | |
| Median/range | 45.5/22–79 |
| Underlying conditions | |
| Diabetes mellitus | 4 (28.6) |
| Neoplasia | 3 (21.4) |
| Autoimmune disease | 1 (7.1) |
| Heart failure | 1 (7.1) |
| Brain stroke | 3 (21.4) |
| Dementia | 1 (7.1) |
| Kidney engraftment failure | 4 (28.6) |
| Previous bacteremia (≤14 days) | 5 (35.7) |
| Antibiotics use | 7 (50) |
| Gastric protector use | 4 (28.6) |
| Central venous catheter | 13 (92.9) |
| Previous corticosteroids | 6 (42.9) |
| Vascular device used at the onset of candidemia | |
| Tunneled catheter | 10 (71.4) |
| Short-term catheter | 3 (21.4) |
| PTFE grafts | 1 (7.1) |
| Deep-seated infections | |
| Endocarditis | 5 (35.7) |
| Endophthalmitis | 1 (7.1) |
| Mortality at 30 days | 1 (7.1) |
| Species | |
| C. parapsilosis complex | 14 (100) |
| C. orthopsilosis | 8 (57.1) |
CVCs are a major risk factor for candidemia in patients under chronic hemodialysis.6,27 In our study, 13 out of 14 patients with candidemia were under hemodialysis through CVC, included tunneled catheters in 10 cases (71.4%) and short-term catheters in 3 cases (21.4%) (see Table 1). It is relevant to mention that those patients had been under the use of vascular devices for periods ranging from 11 to 277 days before developing candidemia. Indeed, even after the diagnosis of candidemia, catheters were removed only after a median of 10 days. The role of catheter use for dialysis (vs. native graft or fistula) as an independent risk factor for candidemia was illustrated by Pyrgos et al.25
It is well established that arteriovenous fistulas (AVFs) are the best means of access for hemodialysis therapy due to their lower complications rates than with CVC.2,12 In countries such as Japan, Italy, Germany, France, Spain and England, the use of AVF ranges from 67% to 91% in hemodialysis patients. However, in the United States, this rate is significantly lower than in other countries (approximately 47%) due to the high incidence of chronic renal diseases in patients with diabetes, myocardial ischemic disease and peripheral vascular disease, which are known to lead to early failure of this type of access to hemodialysis.14,17
Our findings contrast with the data provided by the Brazilian Society of Nephrology (BSN) regarding the rate of patients dialyzed without fistula.21 In our study, all patients with candidemia were under hemodialysis using a CVC, whereas the BSN 2013 reported that approximately 15% of patients were dialyzed by catheter. As previously described, vascular catheters should be utilized for short periods of time, ideally only until a permanent vascular access has matured, to reduce the risks of bloodstream infections.2,7
Candida parapsilosis complex is frequently identified as a skin colonizer among patients and healthcare workers and is strongly associated with catheter-related candidemia.6,20 As expected, all community candidemia episodes from our series were caused by C. parapsilosis complex, with C. orthopsilosis identified in 8 out of 14 patients. In light of the sharp increase in the number of candidemia cases per 1000 hemodialysis sessions per year and the high prevalence of C. orthopsilosis, we suggest that we faced an outbreak of candidemia in our division. Indeed, the prevalence of C. orthopsilosis among Brazilian patients with C. parapsilosis complex candidemia is usually less than 10%.5,13,26 A relevant aspect to be considered in the epidemiology of catheter-related candidemia in patients under RRT is that C. orthopsilosis and other microorganisms may contaminate and form biofilms in tanks, dead spaces and tubing within the hemodialysis equipment.19,22,24
Unlike our series, other authors addressing candidemia in patients under hemodialysis have reported a large spectrum of non-C. albicans Candida species causing fungemia. However, the authors did not attempt to evaluate only community-acquired infections as we did, including in their study patients who developed candidemia after 48h of hospitalization and patients who were exposed to broader spectra of risk conditions.25,27,28
Deep-seated infection was documented in 6 out of 14 cases (43%), including 5 cases of endocarditis (36%) and 1 case of endophthalmitis. All cases of endocarditis were caused by C. orthopsilosis. Most series published in the literature report lower rates of endocarditis and endophthalmitis in patients with candidemia, especially without a previous history of heart surgery.9,15,28 The high rates of endocarditis found in our series may be justified by the late removal of CVCs; in these cases, the vascular devices remained in place a median of 8 days (3–180) after the diagnosis of candidemia. Indeed, the failure to quickly control the candidemia and thus avoid deep-seated infections is likely related to the delay in removing the CVC followed by starting primary therapy with fluconazole, a fungistatic antifungal drug without any activity against Candida biofilm.10,30 Survival rates were calculated for 14 patients at 30 days after candidemia, and only one report of death was observed in a patient who developed endocarditis due to C. orthopsilosis. A low rate of 30-day mortality has been documented in patients with catheter-related C. parapsilosis candidemia, especially among young adult non-neutropenic patients without deep-seated infections.1 There is a lack of data concerning the morbidity and mortality of invasive infections due to C. orthopsilosis. Although some publications suggest that C. orthopsilosis may be less virulent than C. parapsilosis sensu stricto, we documented 5 cases of endocarditis related to this cryptic species. Furthermore, after 6 months of follow up (information available for only 4 out of 5 cases), 2 patients died, representing an accumulate mortality rate associated with C. orthopsilosis endocarditis of nearly 50%. The single case of C. parapsilosis complex endophthalmitis exhibited a good outcome and the patient was alive after 6 months of candidemia.
The use of central venous catheters in patients under RRT should be promptly replaced by fistulas and grafts to avoid bloodstream infections. Facing a case of candidemia, adequate source control and the prompt initiation of antifungal therapy are mandatory to avoid morbidity and mortality. Finally, C. orthopsilosis is able to cause fungemia and deep-seated infections in patients under prolonged use of vascular access and RRT.
Conflicts of interestALC has received educational funds from Pfizer, MSD and Gilead, funding for research from Pfizer and Astellas and funds for advisory board membership from MSD and United Medical. All other authors have nothing to declare.
This study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Grant (2012/04767-1) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Grant 308011/2010-4). ACRS received a doctoral fellowship from FAPESP (2012/04769-4). SSG received a postdoctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (PNPD 23038.007393/2011-11). ALC received grants from FAPESP and CNPq.