In the last years, food grade antioxidants are used safely as an alternative to traditional fungicides to control fungal growth in several food and agricultural products.
AimsIn this work, the effect of butylated hydroxyanisole (BHA) and propyl paraben (PP) on two hydrolytic enzyme activity (β-d-glucosidase and α-d-galactosidase) by Aspergillus section Nigri species under different water activity conditions (aW; 0.98, 0.95 and 0.93) and incubation time intervals (24, 48, 72 and 96h) was evaluated on peanut-based medium.
MethodsThe activity of two glycosidases, β-d-glucosidase and α-d-galactosidase, was assayed using as substrates 4-nitrophenyl-β-d-glucopyranosido and 4-nitrophenyl-α-d-galactopyranosido, respectively. The enzyme activity was determined by the increase in optical density at 405nm caused by the liberation of p-nitrophenol by enzymatic hydrolysis of the substrate. Enzyme activity was expressed as micromoles of p-nitrophenol released per minute.
ResultsThe major inhibition in β-d-glucosidase activity of A. carbonarius and A. niger was found with 20mmoll−1 of BHA or PP at 0.98 and 0.95 aW, respectively, whereas for α-d-galactosidase activity a significant decrease in enzyme activity with respect to control was observed in A. carbonarius among 5 to 20mmoll−1 of BHA or PP in all conditions assayed. Regarding A. niger, the highest percentages of enzyme inhibition activity were found with 20mmoll−1 of BHA or PP at 0.95 aW and 96h.
ConclusionsThe results of this work provide information about the capacity of BHA and PP to inhibit in vitro conditions two of the most important hydrolytic enzymes produced by A. carbonarius and A. niger species.
En los últimos años, para controlar el crecimiento fúngico, en lugar de los fungicidas tradicionales, tanto en la industria alimentaria como en los productos agrícolas se utilizan antioxidantes como aditivos alimentarios bien tolerados y sin riesgos de efectos adversos.
ObjetivosEn el presente estudio, en un medio de cultivo con cacahuete, se examinó el efecto de hidroxianisol butilado (BHA) y propilparabeno (PP) sobre la actividad de 2 enzimas hidrolíticas (β-d-glucosidasa y α-d-galactosidasa) producidas por especies de Aspergillus sección Nigri, en función de diferentes valores de actividad de agua del sustrato (aW; 0,98, 0,95 y 0,93) y tiempos de incubación (24, 48, 72 y 96h).
MétodosLa actividad de las 2 glucosidasas (β-d-glucosidasa y α-d-galactosidasa) se evaluó usando como sustrato 4-nitrofenil-β-d-glucopiranósido y 4-nitrofenil-α-d-galactopiranósido, respectivamente. La actividad enzimática se determinó mediante el aumento de la densidad óptica a 405nm provocado por la liberación de p-nitrofenol, resultado de la hidrólisis enzimática del sustrato. La actividad enzimática se expresó como micromoles de p-nitrofenol liberado por minuto.
ResultadosLa mayor inhibición en la actividad de β-d-glucosidasa de Aspergillus carbonarius y Aspergillus niger se observó con 20mmoll−1 de BHA o PP a 0,98 y 0,95 aW, respectivamente. Comparado con el control, en A. carbonarius se detectó una disminución significativa de la actividad de α-d-galactosidasa con 5–20mmoll−1 de BHA o PP en todas las condiciones examinadas. Con respecto a A. niger, los porcentajes mas elevados de inhibición enzimática se observaron con 20mmoll−1 de BHA o PP a 0,95 aW y un tiempo de incubación de 96h.
ConclusionesLos resultados del presente estudio proporcionan información sobre la capacidad de BHA y PP para inhibir dos de las enzimas más importantes producidas por las especies A. carbonarius y A. niger.
Peanut (Arachis hypogaea L.) is an economically important seed in Argentina. Most of the production is exported to different regions of the world, including European Union countries, and the USA; the rest is consumed internally. Each year, an important percentage of the peanut total production is left outside the external market due to fungal contamination at the post-harvest stage and mycotoxin production.15 In previous works, aflatoxin and ochratoxin producer species belonging to Aspergillus sections Flavi and Nigri have been reported as the main toxigenic fungi associated with peanut grains in Argentina.20,26
In several studies Aspergillus carbonarius is reported as the main ochratoxin A (OTA) producer, followed by species belonging to Aspergillus niger aggregate in tropical and temperate countries.1 Ochratoxin A has been extensively informed in several agricultural commodities, among them cereals and oilseeds destined for human and animal consumption.4,20,25
Many scientists worldwide have studied the optimal conditions of water activity (aW) and temperature for growth and biosynthesis of OTA by ochratoxigenic species belonging to section Nigri.5,10,11,23,24,32 In previous works, Astoreca et al.5 evaluated the influence of ecophysiological factors on growth and OTA production by Aspergillus section Nigri Aspergillus species isolated from different substrates in Argentina. These authors observed that OTA concentration increased as aW level increased, with no significant production from 0.85 to 0.91 aW over a range of temperatures (15, 25 and 30°C); and optimum production occurred among 0.95–0.99 aW and 25 or 30°C depending on the species considered.
Food grade antioxidants, e.g., butylated hydroxyanisole (BHA), butylated hydroxyl toluene (BHT) and antimicrobial agents as propyl paraben (PP), are used safely as alternatives at traditional fungicides to control fungal growth in several food and agricultural products.2,30 These antioxidants and antimicrobial agents have showed capacity to limit Fusarium species growth and fumonisin production on natural substrates.12,36 Passone et al.26 have also shown that these compounds have antifungal effects against Aspergillus section Flavi species on peanuts. In previous works, BHA and PP single and combined have demonstrated ability to control growth of Aspergillus section Nigri species, and to inhibit OTA accumulation on peanut-based media under determined aW and temperature conditions.6–9
Kinetic studies with Penicillium verrucosum have shown a close correlation between hydrolytic enzyme production and OTA production.18,19 Quantitative enzyme assays for specific hydrolytic enzymes produced by spoilage fungi, have been shown to be good early indicators of growth prior to visible molding.13,16,17,22,29 In addition, Aspergillus spp. produced large amounts of hydrolytic enzymes such as N-acetyl-β-d-glucosaminidase, β-d-glucosidase, α-d-galactosidase, phosphatases, fucosidases, pectinases, lipases, amylases and sulphatases.33,34 However, less information is available in relation to environmental factors such as water availability on hydrolytic enzyme production by toxigenic fungi during colonization of grains.14,22
Previous studies have demonstrated that BHA and PP increase the lag-phase length before growth of Aspergillus section Nigri species on peanut based media and peanut kernels when aW and temperature decreased.6–8 However, no studies are available about the effects of phenolic antioxidants and antimicrobials on key hydrolytic enzymes produced during the early stages of infection by Aspergillus section Nigri species on cereals and oilseeds.
The aim of the present study was to continue the evaluation of the effect of antioxidant butylated hydroxyanisole and the antimicrobial propyl paraben now on the activity of two of the most important hydrolytic enzymes (β-d-glucosidase and α-d-galactosidase) produced by Aspergillus section Nigri species during the initial phases of growth, under different water activity conditions on peanut meal extract agar.
Materials and methodsFungal strains and identificationTwo Aspergillus section Nigri species, A. carbonarius (strain RCPG) and A. niger (strain RCP42), isolated from Argentinean peanuts, were used in this study. The morphology of these strains was examined according to Samson et al.31 The OTA production by RCPG and RCP42 on YES medium (yeast extract sucrose agar) and on peanut meal extract agar has previously been reported; and these strains were representative of the ones tested previously.6,7,20
A PCR-based method was used to analyze DNA of A. carbonarius and A. niger to confirm the morphological identification. Two pairs of primers (CARBO1/2 and NIG1/2) designed from the calmodulin gene, produced PCR products of 371 and 245bp for A. carbonarius and A. niger strains, respectively. The primers’ sequences read as follows: CARBO1: AAGCGAATCGATAGTCCACAAGAATAC, CARBO2: TCTGGCAGAAGTTAATATCCGGTT27; NIG1: GATTTCGACAGCATTT(CT/TC)CAGAA and NIG2: AAAGTCAATCACAATCCAGCCC.35 The amplifications were performed using the Termocicler MJ Research PTC-200 (MJ Research Inc., Watertown, MA) with the following cycling parameters: CARBO1/2: 94°C for 5min, followed by 35 cycles of 50s at 94°C, 50s at 58°C, and 1min at 72°C with final extension for 7min. NIG1/2: 94°C for 5min, followed by 30 cycles of 50s at 94°C, 50s at 60°C, and 1min at 72°C with final extension for 7min.
The strains are deposited in the culture collection of National University of Río Cuarto, Córdoba, Argentina (RC) (Aspergillus carbonarius RCPG, Aspergillus niger RCP42).
Culture mediumPeanut meal extract agar was prepared at 3% (w/v). Thirty grams of ground peanut per liter were boiled for 45min and the resultant mixture filtered through a double layer of muslin. The volume was made up to 1l and agar–agar at 2% (w/v) was added. The water activity of the basic medium was adjusted to 0.98, 0.95 and 0.93 with known amounts of glycerol.21 The basic medium was autoclaved at 120°C for 20min before cooling it at 50°C and poured into 90-mm sterile Petri dishes. Water activity of representative samples of each treatment was checked at the beginning of the experiment with an AquaLab Series 3 (Decagon Devices, Inc., WA, USA).
Antioxidant and antimicrobial agentThe antioxidant 2(3)-tert-butyl-4-hydroxyanisole (BHA) and antimicrobial agent n-propyl 4-hydroxybenzoate (PP) were used and obtained from Sigma–Aldrich Chemical (Dorset, UK). Stock solutions of BHA and PP (1M) were prepared by dissolving 18g in 100ml of 95% ethyl alcohol–water mixture (v/v). The antioxidant and antimicrobial agent were added to the basic medium at 50°C to obtain the required concentrations (1, 5, 10 and 20mmoll−1 of medium).
Inoculation and incubation conditionsPetri dishes with peanut meal extract agar and each antioxidant or antimicrobial treatments were inoculated with 200μl of spore suspension (1×104sporesml−1) from a 7-day-old culture on 2% malt extract agar (MEA) of each strain and spread-plate over the surface using a surface-sterilized bent Pasteur pipette. Inoculated Petri dishes of the same aW were sealed in polyethylene bags.5,6 Twelve replicate plates per treatment were used and incubated at 25°C for 24, 48, 72 and 96h; all the experiments were repeated twice. Twelve plates with peanut meal extract agar, without antioxidant or antimicrobial agent and conditioned at different levels of aW were used as controls at each sampling time.
Extraction of enzymes from basic mediumUsing a cork borer, three discs of agar (each 6mm diameter) were removed from both treatments and control plates at 24, 48, 72 and 96h, and placed in 4ml potassium extraction buffer (10mmoll−1, pH 7.2) for enzyme extraction. The glass bottles were shaken on a wrist-action shaker for 1h at 4°C. The washings were decanted into 1ml plastic microtubes and centrifuged in a bench microcentrifuge for 15min at 450×g at 4°C. The supernatant was removed and stored at −20°C until total enzyme activity determination.22 The completed assay was carried out twice.
Enzyme activity determinationThe activity of two glycosidases, β-d-glucosidase and α-d-galactosidase, was assayed using as substrates 4-nitrophenyl-β-d-glucopyranosido (4.0mmoll−1) and 4-nitrophenyl-α-d-galactopyranosido (2.0mmoll−1), respectively (Sigma–Aldrich). Enzyme extract (40μl), substrate solution (40μl) and 25mmoll−1 of acetate buffer (20μl) were placed into the wells of the microtitre plate along with the appropriate controls and incubated for 1h at 37°C. The reaction was stopped by the addition of 1M Na2CO3 solution (5μl). The enzyme activity was measured using a MRX multiscan plate reader (Dynex Technologies Ltd., Billinghurst, UK), by the increase in optical density at 405nm caused by the liberation of p-nitrophenol by enzymatic hydrolysis of the substrate. Enzyme activity was calculated from a calibration curve of absorbance against p-nitrophenol concentration and expressed as micromoles of p-nitrophenol released per minute.3
Number of enzymatic activity analysis=3aW×1 temperature×2 species×4 concentrations of 1 antioxidant and 1 antimicrobial×4 incubation time×3 replicates.
Statistical analysisAll assays were carried out in triplicates. Analysis of variance to evaluate the effect of antioxidant and antimicrobial treatments, aW, strain, and incubation time on total enzyme production was done. Effect of single, two and three-way interactions of treatments (aW×T, aW×C, T×C, aW×T×C) for enzyme production was done and the LSD test was performed to determine differences between pairs of treatments. The analysis was conducted using PROC GLM in SAS (SAS Institute, Cary, NC).28
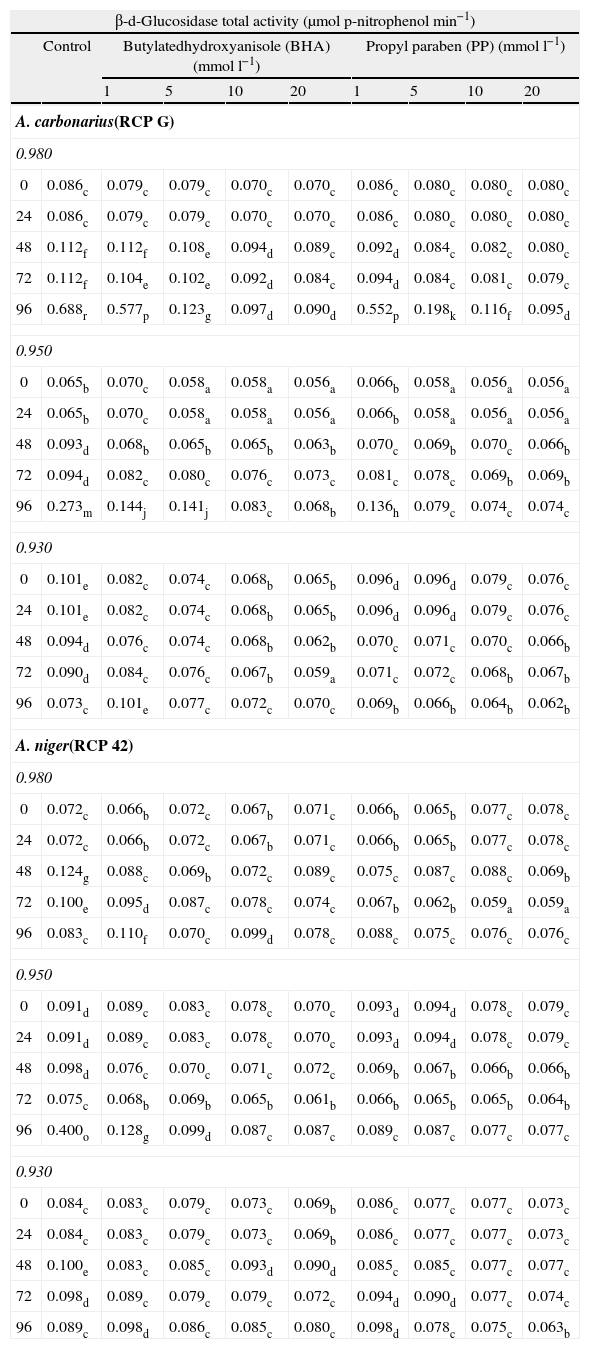
ResultsThis study investigated whether BHA and PP may inhibit the two most important hydrolytic enzymes production by Aspergillus section Nigri species growing on peanut based medium under different water availability conditions. In general, in control treatments (plates without antioxidant or antimicrobial agent), A. carbonarius produced more β-d-glucosidase than α-d-galactosidase at all incubation time, being the maximum production at 0.98 aW and 96h, whereas in A. niger no differences were observed in β-d-glucosidase and α-d-galactosidase production, being the maximum production at 0.95 aW and 96h (P<0.0001) (Tables 1 and 2).
Table 1 compares the effect of different levels of food grade antioxidant BHA and antimicrobial agent PP, aW and incubation time on the β-d-glucosidase total activity for A. carbonarius and A. niger. For A. carbonarius, in general, from 5mmoll−1 of BHA and PP, a significant reduction on enzyme activity with respect to control was observed in all conditions assayed, except at 0.98 aW, and 24h, whereas at 20mmoll−1 of BHA or PP and 0.98 aW the maximum reduction in enzyme activity was observed (P<0.0001). The β-d-glucosidase activity of A. niger was reduced when the concentration of BHA or PP was increased (P<0.0001). The highest percentages of enzyme inhibition activity were observed with 20mmoll−1 at 0.95 aW and 96h.
Effect of butylated hydroxyanisole and propyl paraben on β-d-glucosidase total activity of A. carbonarius (strain RCPG) and A. niger (strain RCP42) at different water activity conditions and incubation time.
| β-d-Glucosidase total activity (μmolp-nitrophenolmin−1) | |||||||||
| Control | Butylatedhydroxyanisole (BHA) (mmoll−1) | Propyl paraben (PP) (mmoll−1) | |||||||
| 1 | 5 | 10 | 20 | 1 | 5 | 10 | 20 | ||
| A. carbonarius(RCP G) | |||||||||
| 0.980 | |||||||||
| 0 | 0.086c | 0.079c | 0.079c | 0.070c | 0.070c | 0.086c | 0.080c | 0.080c | 0.080c |
| 24 | 0.086c | 0.079c | 0.079c | 0.070c | 0.070c | 0.086c | 0.080c | 0.080c | 0.080c |
| 48 | 0.112f | 0.112f | 0.108e | 0.094d | 0.089c | 0.092d | 0.084c | 0.082c | 0.080c |
| 72 | 0.112f | 0.104e | 0.102e | 0.092d | 0.084c | 0.094d | 0.084c | 0.081c | 0.079c |
| 96 | 0.688r | 0.577p | 0.123g | 0.097d | 0.090d | 0.552p | 0.198k | 0.116f | 0.095d |
| 0.950 | |||||||||
| 0 | 0.065b | 0.070c | 0.058a | 0.058a | 0.056a | 0.066b | 0.058a | 0.056a | 0.056a |
| 24 | 0.065b | 0.070c | 0.058a | 0.058a | 0.056a | 0.066b | 0.058a | 0.056a | 0.056a |
| 48 | 0.093d | 0.068b | 0.065b | 0.065b | 0.063b | 0.070c | 0.069b | 0.070c | 0.066b |
| 72 | 0.094d | 0.082c | 0.080c | 0.076c | 0.073c | 0.081c | 0.078c | 0.069b | 0.069b |
| 96 | 0.273m | 0.144j | 0.141j | 0.083c | 0.068b | 0.136h | 0.079c | 0.074c | 0.074c |
| 0.930 | |||||||||
| 0 | 0.101e | 0.082c | 0.074c | 0.068b | 0.065b | 0.096d | 0.096d | 0.079c | 0.076c |
| 24 | 0.101e | 0.082c | 0.074c | 0.068b | 0.065b | 0.096d | 0.096d | 0.079c | 0.076c |
| 48 | 0.094d | 0.076c | 0.074c | 0.068b | 0.062b | 0.070c | 0.071c | 0.070c | 0.066b |
| 72 | 0.090d | 0.084c | 0.076c | 0.067b | 0.059a | 0.071c | 0.072c | 0.068b | 0.067b |
| 96 | 0.073c | 0.101e | 0.077c | 0.072c | 0.070c | 0.069b | 0.066b | 0.064b | 0.062b |
| A. niger(RCP 42) | |||||||||
| 0.980 | |||||||||
| 0 | 0.072c | 0.066b | 0.072c | 0.067b | 0.071c | 0.066b | 0.065b | 0.077c | 0.078c |
| 24 | 0.072c | 0.066b | 0.072c | 0.067b | 0.071c | 0.066b | 0.065b | 0.077c | 0.078c |
| 48 | 0.124g | 0.088c | 0.069b | 0.072c | 0.089c | 0.075c | 0.087c | 0.088c | 0.069b |
| 72 | 0.100e | 0.095d | 0.087c | 0.078c | 0.074c | 0.067b | 0.062b | 0.059a | 0.059a |
| 96 | 0.083c | 0.110f | 0.070c | 0.099d | 0.078c | 0.088c | 0.075c | 0.076c | 0.076c |
| 0.950 | |||||||||
| 0 | 0.091d | 0.089c | 0.083c | 0.078c | 0.070c | 0.093d | 0.094d | 0.078c | 0.079c |
| 24 | 0.091d | 0.089c | 0.083c | 0.078c | 0.070c | 0.093d | 0.094d | 0.078c | 0.079c |
| 48 | 0.098d | 0.076c | 0.070c | 0.071c | 0.072c | 0.069b | 0.067b | 0.066b | 0.066b |
| 72 | 0.075c | 0.068b | 0.069b | 0.065b | 0.061b | 0.066b | 0.065b | 0.065b | 0.064b |
| 96 | 0.400o | 0.128g | 0.099d | 0.087c | 0.087c | 0.089c | 0.087c | 0.077c | 0.077c |
| 0.930 | |||||||||
| 0 | 0.084c | 0.083c | 0.079c | 0.073c | 0.069b | 0.086c | 0.077c | 0.077c | 0.073c |
| 24 | 0.084c | 0.083c | 0.079c | 0.073c | 0.069b | 0.086c | 0.077c | 0.077c | 0.073c |
| 48 | 0.100e | 0.083c | 0.085c | 0.093d | 0.090d | 0.085c | 0.085c | 0.077c | 0.077c |
| 72 | 0.098d | 0.089c | 0.079c | 0.079c | 0.072c | 0.094d | 0.090d | 0.077c | 0.074c |
| 96 | 0.089c | 0.098d | 0.086c | 0.085c | 0.080c | 0.098d | 0.078c | 0.075c | 0.063b |
Mean values based on triplicate data. Mean in a row with a letter in common are not significantly different according to LSD test (P<0.0001).
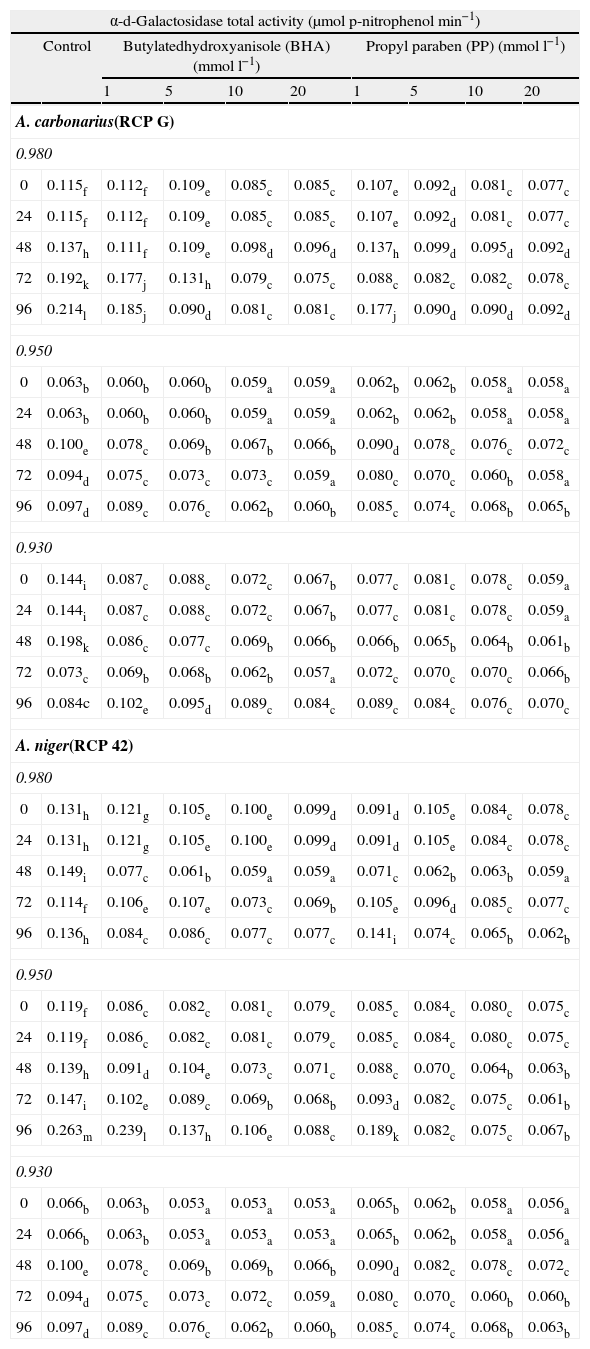
Table 2 shows the temporal changes in α-d-galactosidase activity of A. carbonarius and A. niger at different levels of BHA and PP, aW and incubation time. For A. carbonarius, among 5–20mmoll−1 of BHA or PP, a significant decrease in enzyme activity with respect to control was observed for both species in all conditions assayed. The highest percentage of enzyme inhibition was found at 20mmoll−1 of BHA and PP, 0.98 aW and 72h of incubation (P<0.0001). In addition, this behavior was also observed at 0.95 aW at the same antioxidant and antimicrobial agent concentration and incubation time. Regarding A. niger, in general, the activity of this enzyme diminished as BHA or PP concentration increased. The significant inhibition was observed from 1mmoll−1 of BHA or PP at 0.98 and 0.95 aW (P<0.0001). The highest percentages of enzyme inhibition activity were found with 20mmoll−1 of BHA or PP at 0.95 aW and 96h.
Effect of butylated hydroxyanisole and propyl paraben on α-d-galactosidase total activity of A. carbonarius (strain RCPG) and Aspergillus niger (strain RCP42) at different water activity conditions and incubation time.
| α-d-Galactosidase total activity (μmolp-nitrophenolmin−1) | |||||||||
| Control | Butylatedhydroxyanisole (BHA) (mmoll−1) | Propyl paraben (PP) (mmoll−1) | |||||||
| 1 | 5 | 10 | 20 | 1 | 5 | 10 | 20 | ||
| A. carbonarius(RCP G) | |||||||||
| 0.980 | |||||||||
| 0 | 0.115f | 0.112f | 0.109e | 0.085c | 0.085c | 0.107e | 0.092d | 0.081c | 0.077c |
| 24 | 0.115f | 0.112f | 0.109e | 0.085c | 0.085c | 0.107e | 0.092d | 0.081c | 0.077c |
| 48 | 0.137h | 0.111f | 0.109e | 0.098d | 0.096d | 0.137h | 0.099d | 0.095d | 0.092d |
| 72 | 0.192k | 0.177j | 0.131h | 0.079c | 0.075c | 0.088c | 0.082c | 0.082c | 0.078c |
| 96 | 0.214l | 0.185j | 0.090d | 0.081c | 0.081c | 0.177j | 0.090d | 0.090d | 0.092d |
| 0.950 | |||||||||
| 0 | 0.063b | 0.060b | 0.060b | 0.059a | 0.059a | 0.062b | 0.062b | 0.058a | 0.058a |
| 24 | 0.063b | 0.060b | 0.060b | 0.059a | 0.059a | 0.062b | 0.062b | 0.058a | 0.058a |
| 48 | 0.100e | 0.078c | 0.069b | 0.067b | 0.066b | 0.090d | 0.078c | 0.076c | 0.072c |
| 72 | 0.094d | 0.075c | 0.073c | 0.073c | 0.059a | 0.080c | 0.070c | 0.060b | 0.058a |
| 96 | 0.097d | 0.089c | 0.076c | 0.062b | 0.060b | 0.085c | 0.074c | 0.068b | 0.065b |
| 0.930 | |||||||||
| 0 | 0.144i | 0.087c | 0.088c | 0.072c | 0.067b | 0.077c | 0.081c | 0.078c | 0.059a |
| 24 | 0.144i | 0.087c | 0.088c | 0.072c | 0.067b | 0.077c | 0.081c | 0.078c | 0.059a |
| 48 | 0.198k | 0.086c | 0.077c | 0.069b | 0.066b | 0.066b | 0.065b | 0.064b | 0.061b |
| 72 | 0.073c | 0.069b | 0.068b | 0.062b | 0.057a | 0.072c | 0.070c | 0.070c | 0.066b |
| 96 | 0.084c | 0.102e | 0.095d | 0.089c | 0.084c | 0.089c | 0.084c | 0.076c | 0.070c |
| A. niger(RCP 42) | |||||||||
| 0.980 | |||||||||
| 0 | 0.131h | 0.121g | 0.105e | 0.100e | 0.099d | 0.091d | 0.105e | 0.084c | 0.078c |
| 24 | 0.131h | 0.121g | 0.105e | 0.100e | 0.099d | 0.091d | 0.105e | 0.084c | 0.078c |
| 48 | 0.149i | 0.077c | 0.061b | 0.059a | 0.059a | 0.071c | 0.062b | 0.063b | 0.059a |
| 72 | 0.114f | 0.106e | 0.107e | 0.073c | 0.069b | 0.105e | 0.096d | 0.085c | 0.077c |
| 96 | 0.136h | 0.084c | 0.086c | 0.077c | 0.077c | 0.141i | 0.074c | 0.065b | 0.062b |
| 0.950 | |||||||||
| 0 | 0.119f | 0.086c | 0.082c | 0.081c | 0.079c | 0.085c | 0.084c | 0.080c | 0.075c |
| 24 | 0.119f | 0.086c | 0.082c | 0.081c | 0.079c | 0.085c | 0.084c | 0.080c | 0.075c |
| 48 | 0.139h | 0.091d | 0.104e | 0.073c | 0.071c | 0.088c | 0.070c | 0.064b | 0.063b |
| 72 | 0.147i | 0.102e | 0.089c | 0.069b | 0.068b | 0.093d | 0.082c | 0.075c | 0.061b |
| 96 | 0.263m | 0.239l | 0.137h | 0.106e | 0.088c | 0.189k | 0.082c | 0.075c | 0.067b |
| 0.930 | |||||||||
| 0 | 0.066b | 0.063b | 0.053a | 0.053a | 0.053a | 0.065b | 0.062b | 0.058a | 0.056a |
| 24 | 0.066b | 0.063b | 0.053a | 0.053a | 0.053a | 0.065b | 0.062b | 0.058a | 0.056a |
| 48 | 0.100e | 0.078c | 0.069b | 0.069b | 0.066b | 0.090d | 0.082c | 0.078c | 0.072c |
| 72 | 0.094d | 0.075c | 0.073c | 0.072c | 0.059a | 0.080c | 0.070c | 0.060b | 0.060b |
| 96 | 0.097d | 0.089c | 0.076c | 0.062b | 0.060b | 0.085c | 0.074c | 0.068b | 0.063b |
Mean values based on triplicate data. Mean in a row with a letter in common are not significantly different according to LSD test (P<0.0001).
A significant stimulation in β-d-glucosidase activity of A. carbonarius with respect to control was observed in treatment with 1mmoll−1 of BHA at 0.93 aW and 96h. The same behavior was observed in A. niger species at major incubation time with 1mmoll−1 of BHA at 0.98 and 0.93 aW, and with 10mmoll−1 at 0.98 aW and 96h, whereas for PP this behavior was observed at the same incubation time and 0.93 aW only with the lowest antimicrobial agent concentration (Table 1). Regarding enzymatic activity of α-d-galactosidase in A. carbonarius a significant stimulation was found from 1 to 5mmoll−1 of BHA, 1mmoll−1 of PP at 0.93 aW and 96h (P<0.0001), whereas in A. niger a significant stimulation was observed with 1mmoll−1 of PP at 0.98 aW and 96h (P<0.0001) (Table 2).
The analysis of variance of the data showed that for α-d-galactosidase and β-d-glucosidase total activity, single factors (time – T, water activity – aW, and BHA and PP concentration – C) and most of two and three-way interactions were significant (aW×T, aW×C, T×C, aW×T×C) for enzymatic production (P<0.0001).
DiscussionIn control treatments the highest activities of α-d-galactosidase and β-d-glucosidase were found at 0.98 aW and 0.95 at 96 and 72h for A. carbonarius and A. niger species, respectively. Similar results were previously informed by Marin et al.,22 who evaluated the total and specific activities of α-d-galactosidase, β-d-glucosidase and N-acetyl-β-d-glucosaminidase by Fusarium verticillioides and F. proliferatum fumonisin producing species. These authors reported that the activity of these enzymes were important during early germination, and maximum growth was produced at 0.98 aW, with significantly less effect at 0.95 and 0.93 aW, being the exception the total activity of α-d-galactosidase, which was similar at both 0.95 and 0.93 aW. Likewise, other study (Alam et al.3) showed that the total activity of esterase, lipase, acid phosphatase, β-glucosidase and N-acetyl-β-d-glucosaminidase of A. flavus increased as aW and time increased when this species developed on Czapek Yeast Extract Agar (CYA) medium.
The analysis of variance on the effect of single (time, aW and antioxidant treatments), two- and three- way interaction showed that all factors alone and all interactions were statistically significant (P<0.0001). These results agree with those obtained by Reynoso et al.29, who evaluated the effect of BHA, PP and butylated hydroxytoluene (THBP) alone or in combination, on lag phase, growth rate, hydrolytic enzyme activity and fumonisins production by F. verticillioides and F. proliferatum species on maize based medium. Contrary to what was observed in the present study, those authors found in the control assay the highest α-d-galactosidase, β-d-glucosidase and N-acetyl-β-d-glucosaminidase activity at 0.995 aW after 96h. While all the antioxidant treatments (single or combined) produced a significant reduction in activity of these enzymes at all water availability assayed (0.995, 0.98 and 0.95) after 96h, an increase in β-d-glucosidase activity of F. verticillioides was observed in the treatment with 10mm THBP at all aW levels. In comparison with the present results, a significant stimulation in activity of β-d-glucosidase and α-d-galactosidase with respect to control in some treatments was observed. This stimulation was done at 96h and depended on the antimicrobial or antioxidant concentration, the species and aW condition assayed. In A. carbonarius species this behavior was observed only at the lowest aW condition (0.93), with all BHA levels, 1 and 5mmoll−1 of PP.
In the present study a significant inhibition in enzyme activity was observed from 5mmoll−1 of BHA or PP. These results partially agree with those obtained in previous ecophysiological studies6,7 with these species, where the inhibition of growth and OTA production in vitro conditions becomes significant from 10mmoll−1 of BHA and 5mmoll−1 of PP. In agreement with the present study the stimulation of both fungal growth and OTA production was also found, but only at the lowest concentration assayed (1mmoll−1). In another study on peanut kernels, Barberis et al.8 showed that the stimulation in OTA production was found at different levels of BHA or PP (5mmolg−1 PP and 5mmolg−1 BHA at 18°C, and 5, 10, and 20mmolg−1 PP at 25°C).
The data presented show that different levels of BHA or PP are able to inhibit two of the most important hydrolytic enzymes produced by A. carbonarius and A. niger species at 0.98, 0.95 and 0.93 aW and 25°C on peanut based medium. The effect of BHA or PP on the enzymes involved in early substrate colonization process suggests that the ochratoxigenic strains could be maintained in lag phase for a period of time by the use of certain concentrations of BHA and PP, delaying the spoilage and OTA contamination of grains in the storage.
In conclusion, further assays need to be carried out to test the effectiveness of PP in combination with BHA as inhibitors of hydrolytic enzyme activity and OTA production in ochratoxigenic Aspergillus section Nigri species on natural peanut seeds. From a health point of view it is known that BHA and PP are considered as safe additives for food (GRAS) by the US Food and Drug Administration (FDA). Our finding further emphasizes the antifungal impact of these chemical products against storage fungi on grains and strengthens the possibility to use them as an alternative to replace the traditional fungicides in controlling ochratoxigenic Aspergillus species and OTA occurrence.
Conflict of interestThe authors report no conflict of interest.
This work was carried out thanks to grants from the Consejo Nacional de Ciencia y Tecnología (CONICET-PIP), Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto (SECYT-UNRC) and Fondo para la Investigación Científica y Tecnológica (FONCYT-PICTO).