Pityriasis versicolor (PV) is an infection caused by various species of Malassezia yeast. There is no agreement in the literature concerning the species of Malassezia and the demographic, clinical, and mycological data.
AimsTo prospectively identify Malassezia species isolated from lesions of patients with extensive, long standing and recurrent forms of PV and to estimate the relationship between Malassezia species and the demographic and clinical data of the patients.
MethodsAll patients with PV were enrolled over a four-year period. Malassezia species were isolated in cultures and identified by morphological features and physiological tests. In the last 2 years a PCR-based technique was used to confirm the species’ identification.
ResultsA total of 74 patients (43 males and 31 females, mean age 39.5 years) were enrolled. Only one species was isolated in 45 patients, and more than one species were identified in the remaining 28 patients (38%). M. globosa was the most frequently isolated (60.3%) species. There was a significant association between the isolation of 2 or more species and the presence of at least one predisposing factor. In the last 29 cases, which were subjected to PCR, there were no differences in the identification of isolated species as compared to traditional methods.
ConclusionsThe isolation of more than one species in a single lesion is not infrequent in PV and is related to the presence of one predisposing factor. The isolated species isolated were not influenced by demographic and clinical features. The traditional and more recent (PCR) procedures gave the same results in the isolated species.
La pitiriasis versicolor (PV) es una infección causada por varias especies de Malassezia. No hay acuerdo en la literatura sobre la relación entre las especies de Malassezia y los datos demográficos, clínicos y micológicos.
ObjetivosIdentificar prospectivamente las especies de Malassezia aisladas de las lesiones de pacientes con formas extensas, crónicas y recurrentes de pitiriasis, y valorar también la posible relación entre las especies y los datos demográficos y clínicos de los enfermos.
MétodosTodos los pacientes del estudio con PV fueron examinados a lo largo de un periodo de 4 años. Las especies fueron siempre identificadas en cultivo por medio de los aspectos morfológicos y de los exámenes bioquímicos. En los últimos 2 años las cepas fueron identicadas también por PCR.
ResultadosFueron estudiados 74 pacientes (43 varones, 31 mujeres, edad media 39,5 años). En 45 enfermos se aisló una sola especie; en 28, más de una (38%). Malassezia globosa fue la más frecuentemente aislada (60,3%). Existe una asociación significativa entre el aislamiento de 2 o más especies y la presencia de por lo menos un factor predisponente. En los últimos 29 casos estudiados, en los que también se utilizó PCR para la identificación, no se encontraron diferencias en cuanto a la identificación de las especies por ambos métodos.
ConclusionesEl aislamiento de una o más especies de una sola lesión no es infrequente en la PV y está relacionada con la presencia de por lo menos un factor predisponente. Las especies aisladas no parecían tener relación con los datos demográficos y clínicos de los enfermos.
Pityriasis versicolor (PV) is a superficial mycosis caused by Malassezia, a member of cutaneous microflora commonly found on humans and various warm-blooded animals. Following taxonomic revision, the Malassezia genus currently includes 14 species, of which Malassezia furfur, Malassezia globosa, Malassezia sympodialis, Malassezia slooffiae, Malassezia restricta, and Malassezia obtusa cause human disease.13M. pachydermatis, an animal-associated specie, and M. dermatis were rarely isolated from human lesion.12,33
Diagnosis is based on the clinical appearance of lesions (hypopigmented or hyperpigmented scaly macules) and on direct microscopic examination of scales, demonstrating typical round spores and short curved hyphae (“spaghetti and meatballs” appearance). The yeast is identified in fungal cultures on modified Dixon's agar or Leeming–Notman agar and the species are differentiated according to morphological features and by using physiological tests. The limits of these methods are their slowness and the difficulty of properly differentiating the newly identified species (Malassezia dermatis, Malassezia japonica, Malassezia nana, Malassezia equi, Malassezia yamatoensis, Malassezia caprae, and Malassezia cuniculi). The use of more sensitive and rapid molecular methods overcomes these disadvantages.1,7,9–12,15,16,21,35 A review on molecular methods for typing Malassezia was recently reported.8 The aim of this study was to prospectively identify the Malassezia species in a sequential sampling of extensive, long standing and recurrent forms of PV and to detect any relation between the Malassezia species and demographic and clinical findings.
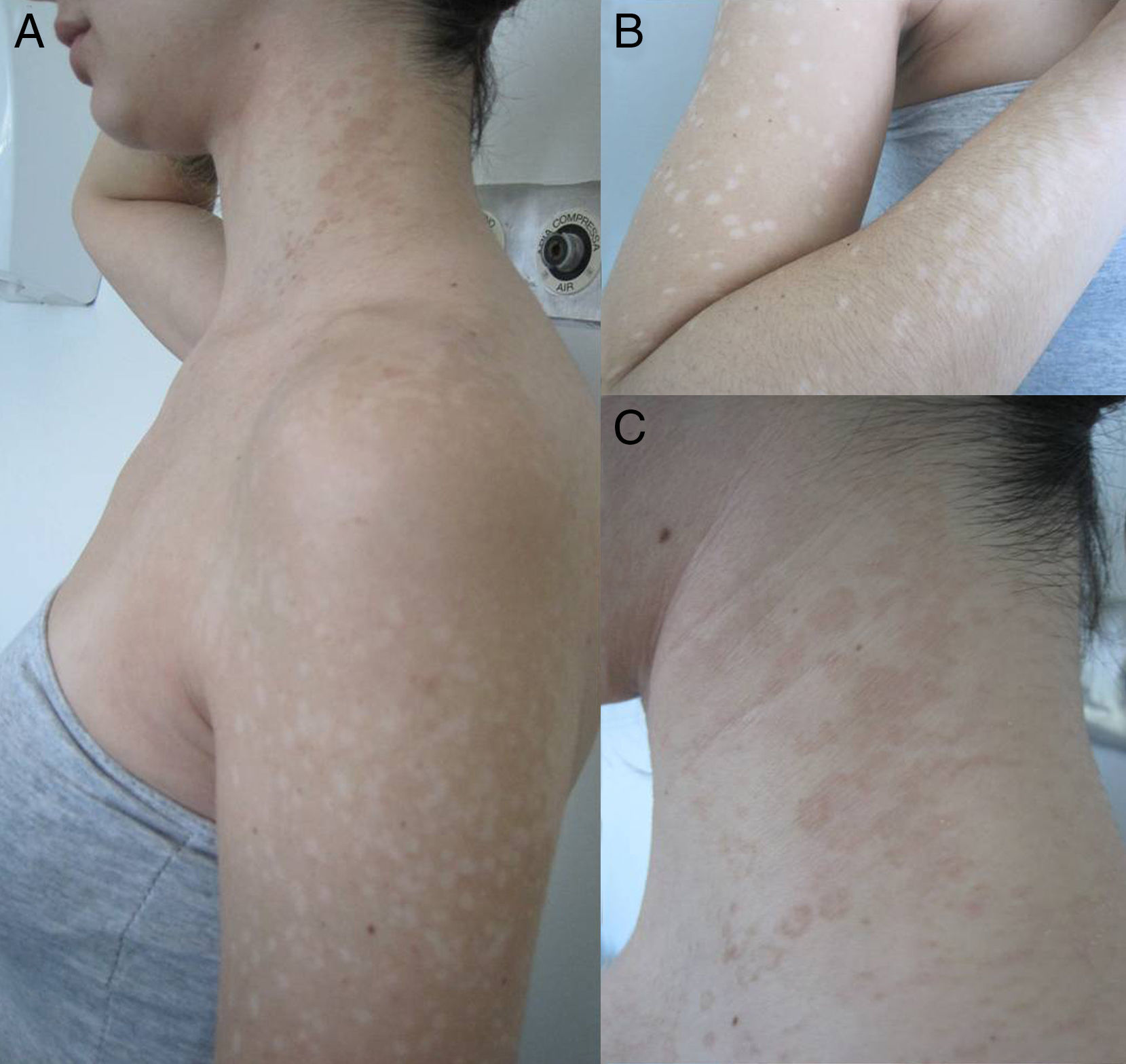
Materials and methodsThe study concerned 74 outpatients observed in the Dermatology Clinic of Siena University Hospital with extensive, recurrent and long-standing forms of PV during four consecutive years. Forms were considered extensive if they involved at least 4 out of 9 body sites (back, chest, neck, arm, face, abdomen, buttocks, genital area, and axilla) and each site presented more than 20 lesions (achromic and/or hyperchromic). The long-standing forms were defined as those lasting at least 1 year. The following details were recorded for every patient: age, sex, clinical appearance of the macules (hypochromic, hyperchromic, or mixed) (Fig. 1), body area, duration of the disease, possible predisposing factors of the mycosis (immunosuppressive drugs, oral contraceptives, hyperhidrosis and HIV).14 Clinical diagnosis was confirmed by direct microscopic examination of the scales scraped from lesions, following maceration in 30% potassium hydroxide. The scales were obtained in the body site where most of the lesions were evident, and many samples were taken. When the lesions were hypochromic and the scales were very few the scraping was repeated many times after asking patients not to wash themselves. The amount of scales inoculated was not standardized. Specimens were placed in Sabouraud's dextrose Agar with 0.5% chloramphenicol and cycloheximide (Mycobiotic Agar®, DID, Milan, Italy) and modified Dixon's agar (mDixon's agar) (3.6% malt extract, 0.6% peptone, 2% desiccated ox bile, 1% Tween 40, 0.2% glycerol, 0.2% oleic acid, 1.2% agar, 0.5% chloramphenicol and 0.5% cycloheximide). Sabouraud's dextrose Agar was chosen to highlight the possible presence of lipid-independent species M. pachydermatis. The plates were incubated at 30°C for 7 days and processed as described below. Lipid-dependent species were identified morphologically and biochemically as described by Guého et al.13 The presence of M. furfur, M. sympodialis and M. slooffiae was demonstrated using the Tween diffusion test, on the basis of their ability to assimilate various polyoxyethylene sorbitan esters. The identification of M. furfur was confirmed using the Cremophor EL (Sigma, St. Louis, MO, USA) assimilation test as reported by Raabe et al.31 The splitting of esculin was performed as an additional key to identify both M. furfur and M. sympodialis.23 The lack of catalase activity, which is a specific feature of M. restricta, showed the presence of this species. Liophylized fungal cultures from the Pasteur institute were used as reference strains.
In the last 29 consecutive cases admitted in the last 2 years, morphological and biochemical identification was confirmed by means of a PCR-based technique using restriction enzyme digestion specific for the differentiation of 11 Malassezia species, as described by Mirhendi et al.25 In order to achieve pure cultures, for each positive sample 5 colonies were subcultured on mDixon's Agar and stored at 20°C until analysis. The cell walls were mechanically disrupted by freeze-thawing and genomic DNA was extracted and purified according to the DNeasy™ protocol for animal tissue (Qiagen Inc., Valencia, CA, USA). The primers selected for this protocol amplified the target part of 26S rDNA, providing a single PCR product of an expected size of 580bp. The PCR products were subjected to REA using CfoI and BstF51, separately, according to the manufacturer's instructions (Fermentas International Inc., Burlington, Ontario, Canada). Digested fragments were analyzed by electrophoresis on 2% agarose gels stained with ethidium bromide. Identification was carried out at the Department of Animal Pathology, Faculty of Veterinary Medicine, University of Pisa.
Statistical methodsThe patients were preliminarily separated in two groups: one in which a single species of Malassezia was isolated and the other in which more than one species were isolated. Possible differences between these two groups were searched concerning age, gender, duration and body sites of the mycosis, and presence/absence of at least one predisposing factor. In order to facilitate statistical analysis, the patients were separated into 3 classes according to age (1.o: 17–30 years, 2.o: 31–45, 3.o: >45), the duration of the mycosis (1.°: <2 years, 2.o: between 2 and 5 years, 3.o: >5 years) and the body area (1.o: back, chest, neck and face 2.o: back, chest, neck and arm, 3.o: all the other possible combinations). Furthermore, on the basis of the frequency of the species identified, the group in which a single species was isolated was divided in two subgroups (patients in whom M. globosa was isolated vs. patients in which another species was isolated). Possible differences in the demographic and clinical parameters reported above were also investigated in this subgroup. The differences were analyzed using the χ2 test with Yates correction. The significance level was <0.05.
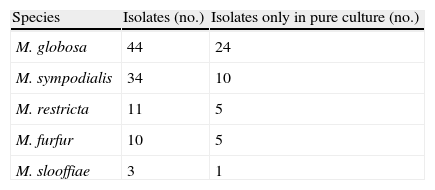
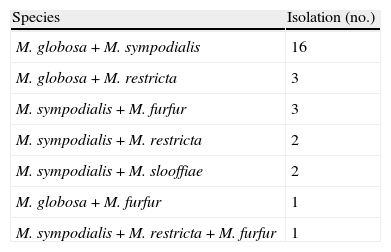
ResultsSeventy-four patients were affected by PV (diagnosis based on clinical appearance and direct microscopic examination): 43 males and 31 females, mean age 39.5±15.2 years (range 17–76, median 38 years). The age with highest frequency was between 30 and 39 years. The patients were not relatives. In all cases direct microscopic examination showed “spaghetti and meatball” appearance. Malassezia was isolated in culture and identified using morphological and biochemical methods in 73 cases. In the last 29 consecutive cases identification of the species was confirmed using a PCR-based technique. The culture was negative in one case (a 45-year-old woman with an achromic form). The clinically achromic form was found in 36 cases, the lesions being hyperpigmented in 30 cases and combined (hypopigmented and hyperpigmented) in 8 cases. The mean duration of the lesions was 4.4±3.2 years (range 1–15 years). The lesions had been present for <2 years in 38 cases (38.4%), between 3 and 5 years in 24 cases (32.9%), and over 5 years in 21 cases (28.8%). A single species was isolated in pure culture in 45 patients: mean age 38.9±16 years, 27 men (60%), mean duration of the mycosis 4.6±3.4 years. The patients in whom more than one species were isolated were 28: mean age 40.3±14.1 years, 15 men (53.6%), mean duration of the mycosis 4±3 years. Table 1 shows the frequency of the species isolated in pure culture or in combination with other species. Table 2 shows different combinations when more than one species of Malassezia were isolated. The species most frequently isolated was M. globosa in 44 patients (60.3%), of which 24 were in pure culture. The body sites involved were the back in 72 cases, the chest in 68, the neck in 57, the arm in 32, the abdomen in 30, the face in 27, the buttock 9, the genitals in 7, and the axilla in 6. The contemporary involvement of 4 sites was present in 60 cases (81%), of 5 sites in 10 cases, of 6 sites in 2 cases, and of 7 sites in 2 cases. The most frequent combination in the same patient was the involvement of the back, chest and neck, which in 21 patients was also associated with the involvement of the face, in 17 with the arm and in 11 with the abdomen. Twenty-eight patients had predisposing factors: 12 were taking immunosuppressive drugs (corticosteroids, cyclosporine, mycophenolate mofetil, adalimumab, cyclophosphamide, vincristine) for various diseases (asthma, pemphigus, renal or cardiac transplant, psoriasis, leukaemia, non-Hodgkin's lymphoma, rheumatoid arthritis), 3 were HIV+, 7 reported hyperhidrosis, one woman had undergone a mastectomy for cancer and was taking anti-oestrogen drugs and 5 women were taking oral contraceptives. The only difference between the group with one species isolated and the group with more than one species was the more frequent presence of predisposing factors in the second group (27% vs. 57%, χ2 6.8, p=0.013). In the subgroup of patients with one species isolated, the only difference regarded the sex. Compared to the males, the female patients showed a greater frequency of isolation of M. globosa than other species (78% vs. 22%, χ2 with Yates correction=5.66, p=0.017).
Species of Malassezia isolated in pure culture and in combination with other species.
| Species | Isolates (no.) | Isolates only in pure culture (no.) |
| M. globosa | 44 | 24 |
| M. sympodialis | 34 | 10 |
| M. restricta | 11 | 5 |
| M. furfur | 10 | 5 |
| M. slooffiae | 3 | 1 |
The overall number of Malassezia isolates in combination with other species is 28.
Different combinations when more than one species of Malassezia were isolated.
| Species | Isolation (no.) |
| M. globosa+M. sympodialis | 16 |
| M. globosa+M. restricta | 3 |
| M. sympodialis+M. furfur | 3 |
| M. sympodialis+M. restricta | 2 |
| M. sympodialis+M. slooffiae | 2 |
| M. globosa+M. furfur | 1 |
| M. sympodialis+M. restricta+M. furfur | 1 |
This survey reported the identification of species of Malassezia responsible of extensive, recurrent and long-standing forms of PV, which are the most difficult to treat. Therefore, as compared to the data in the literature, the differences and similarities found in this study may be affected by the restrictive case recruitment criteria applied. The most frequently isolated species was M. globosa, followed by M. sympodialis, and this agrees with many literature reports. The variation in isolation percentages is probably related to climatic differences, the sampling method (scraping, swabbing) and the composition of the culture medium. M. globosa, whose pathogenicity has been attributed to high lipophilic activity due to the levels of lipases and esterases,2 is the predominant species mainly in temperate climates and also in other areas.1,2,4,6,9,19,26,27,30,33–36M. furfur is reported as the main species in Indonesia20 and Brazil.24M. sympodialis is the most frequently isolated species in Canada,17 and M. sympodialis and M. globosa in Argentina.12 The isolation of M. slooffiae and M. restricta is less frequent.12,33,34 In our study the isolated Malassezia species were not influenced by the sites and number of the areas involved by the mycosis, or by the duration of the mycosis, patient age or gender, except in the subgroup of subjects in which a single species was isolated, where the women showed a greater frequency of isolation of M. globosa. This agrees with studies carried out in Bosnia,30 Spain4 and Argentina.12 An association between the species of Malassezia (M. globosa), the anatomical site of lesions (neck) and the age (20–45 years) has been shown in Iran (Tehran),33 but was not confirmed in the latest,35 nor in Argentina.12 In Turkey a difference was found in the distribution of the species isolated in relation to lesion duration, anatomical sites and clinical type. In our survey two species were isolated in the same lesion in 38% of the cases. In the literature the association of two species has been reported,2,4,6,12,34 but it has not been correlated with any predisposing factor. In our study the isolation of more than one species in a single lesion was more frequent in the cases with at least one predisposing factor.
Concerning the selection of predisposing factors the review of Gupta et al. was taken into consideration.14 There is an agreement in literature regarding iatrogenic immunosuppression and long term corticotherapy.5,22,29,37 The role of hyperhidrosis and HIV is uncertain. An experimental study on the use of glycine as a regulator of the synthesis of the tryptophan-dependent pigment showed that the addition of glycine into fluid medium resulted in an exponential increase in biomass of M. furfur. The yeast first utilizes the preferred amino acids on the skin surface, e.g. glycine, and higher concentrations of glycine are demonstrated in the sweat present in hyperhidrosis. The authors considered “hyperhidrosis as a prerequisite of pityriasis versicolor”.3 The association between recurrence and hyperhidrosis has been found to be statistically significant.19 PV is common and more extensive in HIV patients.28,32 Our HIV patients had PV in unusual sites and were immunosuppressed with low values in lymphocyte CD4. Therefore hyperhidrosis and HIV were considered as predisposing factors in this study. On the contrary we failed to consider genetic factors that may play a role because the disease occurs more frequently in first-degree family members.18
Finally, in the last 29 cases, which were subjected to PCR, we found no differences in the identification of isolated species in relation to the traditional (morphological and biochemical) methods, in contrast to previous studies carried out with different molecular techniques.1,15 Gupta et al. compared 3 molecular techniques such as amplified fragment length polymorphism (AFLP analysis), sequencing of the internal transcribed spacer and sequencing of the D1 and D2 domains of the large-subunit ribosomal DNA region with a misidentification rate of 13.8% probably due to phenotypic misidentification,15 while the simple PCR described by Affes et al. identified four Malassezia species.1 The occurrence of further yeast species (i.e. M. obtusa and M. slooffiae) could lead to higher rate of misidentification. Such biomolecular methods even if more expensive, appear to be less time-consuming and more accurate.
Conflicts of interestThe authors state that there are no conflicts of interest and they did not receive any grant support.