Pharmacological screening and usage of natural products for the treatment of human diseases has had a long history from traditional medicine to modern drugs. The majority of modern drugs are reported to be mostly from natural products.
ObjectiveThe aim of the present study was to evaluate the inhibitory activity of 5-(2,4-dimethylbenzyl) pyrrolidin-2-one (DMBPO) extracted from marine Streptomyces VITSVK5 spp. isolated from sediment samples collected at Marakkanam coast of Bay of Bengal, India.
MethodsThe lead compound was isolated by bioactive guided extraction and purified by silica gel column chromatography. Structural elucidation of the lead compound was carried out by using UV, FT-IR, 1H NMR, 13C NMR, DEPT and HR-MS spectral data.
ResultsSystematic screening of isolates for antimicrobial activity lead to identification of a potential strain, Streptomyces VITSVK5 spp. (GQ848482). Bioactivity guided extraction yielded a compound DMBPO and its inhibitory activity was tested against selected bacterial and fungal strains. DMBPO showed maximal activity against Escherichia coli with a MIC value of 187μg/ml, followed by Klebsiella pneumoniae (MIC of 220μg/ml and 10.3mm zone of inhibition), Staphylococcus aureus (MIC of >1000μg/ml and 4.4mm zone of inhibition) and Bacillus subtilis (MIC of 850μg/ml and 2.6mm zone of inhibition). Furthermore, DMBPO was found to be a potent inhibitor of opportunistic fungal pathogens too. It showed a maximum activity against Aspergillus niger with a MIC value of 1μg/ml and 28mm zone of inhibition.
ConclusionThe result of this study indicates that DMBPO possess antibiotic activity to selected bacterial and fungal pathogens and exhibited better activity against fungi than bacteria.
Antecedentes: El cribado farmacológico y el uso de productos naturales para el tratamiento de las enfermedades humanas tiene un largo historial que comienza en la medicina tradicional y se extiende hasta los fármacos modernos. La mayoría de los fármacos modernos proceden principalmente de productos naturales.
ObjetivoEl objetivo del presente estudio fue valorar la actividad inhibidora of 5-(2,4-dimetilbencil) pirrolidin-2-uno (DMBPO) extraído de Streptomyces VITSVK5 sp. marino aislado de muestras de sedimento recolectadas en la costa de Marakkanam de la bahía de Bengala, India.
MétodosEl compuesto principal se aisló mediante extracción bioactiva guiada y se purificó mediante cromatografía de columna de gel de sílice. La dilucidación estructural del compuesto principal se efectuó utilizando datos espectrales de las técnicas UV, FT-IR, 1H NMR, 13C NMR, DEPT y HR-MS.
ResultadosEl cribado sistemático de los aislamientos en busca de actividad antimicrobiana dio lugar a la identificación de una cepa potencial, Streptomyces VITSVK5 sp. (GQ848482). Con la extracción bioactiva guiada se obtuvo un compuesto DMBPO y su actividad inhibidora se examinó frente a cepas bacterianas y fúngicas seleccionadas. DMBPO mostró una actividad máxima frente a Escherichia coli con un valor de la concentración inhibitoria mínima (CIM) de 187μg/ml, seguida de Klebsiella pneumoniae (CIM de 220μg/ml y zona de inhibición de 10,3mm), Staphylococcus aureus (CIM>1.000μg/ml y zona de inhibición de 4,4mm) y Bacillus subtilis (CIM de 850μg/ml y zona de inhibición de 2,6mm). Además, se puso de relieve que DMBPO también fue un inhibidor potente de los patógenos fúngicos oportunistas. Se demostró una actividad máxima frente a Aspergillus niger con un valor de CIM de 1μg/ml y una zona de inhibición de 28mm.
ConclusiónEl resultado del presente estudio indica que DMBPO posee actividad antibiótica frente a patógenos bacterianos y fúngicos seleccionados y exhibió una mejor actividad frente a hongos que bacterias.
Natural products are chemical compounds derived from living organisms e.g., plants, animals and microorganisms. They can be defined as chemical compounds isolated or derived from primary or rather secondary metabolism of organisms concerned naturally. Nature acts as a prominent reservoir for new and novel therapeutics. By employing sophisticated techniques under various screening programs, the rate of discovery of natural compounds exceeded 1 million so far, out of which 22,500 biologically active compounds that have been extracted are from microbes; 45% are produced by actinobacteria, 38% by fungi and 17% by unicellular bacteria5. Over the past 75 years, natural product derived-compounds have led to the discovery of many drugs to treat numerous human diseases6. The oceans cover more than 70% of earth surface and little is known about the microbial diversity of marine sediments, which is an inexhaustible resource that has not been fully exploited. Marine extremophiles serves as valuable natural resource for novel products such as antibiotics, antitumor agents, and other therapeutic substances1. Microbial secondary metabolites have been known as one of the immense reservoir of natural chemical diversity with potent biological activity4,5. Most bacterial secondary metabolites are generated through a unique, multi-step biosynthetic process with specific enzymes for each complex structure formation. Their encoding genes are normally clustered within the genome of the organism and the precursors for the biosynthesis are derived from primary metabolites. Marine actinomycetes are potential provider of novel bioactive metabolites and have currently emerged as an important source for natural products with unique chemical diversity. Members of the class actinobacteria especially Streptomyces spp. have long been recognized as prolific sources of useful bioactive metabolites, providing more than 85% of the naturally occurring antibiotics discovered to date and continuing as a rich source of new bioactive metabolites3. Actinomycetes are the most abundant group in antibiotic production, and a good number of antibiotics available in the market are extracted from marine actinomycetes17.
Actinobacteria or actinomycetes are a group of Gram-positive bacteria with high G+C ratio. Actinobacteria are widely distributed in terrestrial and aquatic ecosystems, especially in soil, where they play a crucial role in the recycling of refractory biomaterials by decomposing complex mixtures of polymers in dead plant, animal and fungal materials. Marine actinomycetes exhibit very different 16S rRNA sequences compared to their terrestrial counterparts. Marine and terrestrial actinomycetes produce many different metabolites due to their taxonomic distance and difference between them. Marine actinomycetes produced many secondary metabolites and served as a potential source for new anti-infective drugs13. These represent one of the most studied and exploited classes of bacteria for their ability to make a wide range of biologically active metabolites8. The actinobacteria play an important role among the marine bacterial communities, because of its diversity and ability to produce novel chemical compounds of high commercial value1,7. Studies on marine actinomycetes are very limited in the Indian sub-continent and most of the actinomycetes isolated are yet to be screened for bioactive secondary metabolites.
In this study we have reported the antibiotic activity of the compound 5-(2,4-dimethylbenzyl) pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. against selected bacterial and fungal pathogens.
Materials and methodsIsolation of actinomycetesThe strain Streptomyces VITSVK5 spp. was isolated from the salt marsh of Marakkanam coast of Bay of Bengal, southern India. The strain was selectively isolated on starch casein agar, ISP No.1 medium and the culturing conditions were optimized.
Extraction and purification of compoundWell grown slant culture of the Streptomyces VITSVK5 spp. was used for preparation of seed culture. The seed culture was inoculated in 50ml medium containing the optimized production medium prepared with sea water 50%, distilled water 50%, pH 8.2 and incubated for 2 days in rotary shaker (200rpm) at 30°C. The inocula (10%) were then transferred into 200ml production medium in 1l Erlenmeyer flasks and kept for shake flask growth for a week. After fermentation, the broth was centrifuged at 4000rpm for 10min at 10°C and the supernatant was separated by using 0.2μm membrane filter. The supernatant was extracted twice with n-butanol (400ml) and washed with 500ml water. Then the culture was harvested by centrifugation for 10min at 4000rpm at 10°C and the filtrate was separated by centrifugation (Remi High speed centrifuge). After separation, the organic phase was dried over Na2SO4 (anhydrous). The extract was then concentrated in rotary vacuum and lyophilized using a freeze drier (Thermo, USA) at 5°C for 5h. The crude extracts were stored at −20°C. The butanol layer was concentrated and the residual suspension (750mg) was chromatographed over silica gel column and eluted with chloroform:methanol (10:0, 9.5:0.5, 9:1, 8.5:1.5, 8:2, 7.5:2.5, 7:3). The active fractions were collected, concentrated and further separated by preparative thin layer chromatography (TLC) on silica gel with chloroform:methanol (8:2) and the purity of the compound was analyzed.
Structure elucidationThe UV spectra of the compound were measured using UV–visible spectrophotometer (Techcomp, Hong Kong). The sample was lyophilized and mixed with KBr (1:20; 0.02g of sample with KBr at a final weight of 0.4g) and the pure compound was then grounded, desorbed at 60°C for 24h and pressed to obtain IR-transparent KBr-pellets. Infrared spectra of the compound were obtained using a Fourier Transform Infrared Spectrometer (FT-IR-AVATAR 330). The spectra were collected within a scanning range of 400–4000cm−1. The FT-IR was first calibrated for background signal scanning with a control sample of pure KBr, and then the experimental sample was scanned. The spectrum obtained was analyzed for various functional groups.
The proton NMR (1H NMR) and carbon NMR (13C NMR, V Bruker Avance III 500 MHz–AV 500) spectra of the compound were obtained by using a dimethyl sulfoxide d6 (DMSO-d6) as solvent. It was further evaluated with DEPT-135, and further confirmed by mass spectroscopy (HRMS, Jeol GCMATE II). The structure of the compound was established with the help of spectral data obtained from various spectroscopic techniques. The 3D structure of the compound was obtained by using Chemdraw software (Ultra 8.0).
Bacterial and fungal pathogensThe following bacterial strains Staphylococcus aureus (ATCC 25923), Bacillus subtilis (ATCC 6633), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 10273), Bacillus cereus (ATCC 14579), Streptococcus pneumoniae (ATCC 6301), Proteus mirabilis (ATCC 8259) and fungal strains Candida albicans (ATCC 10231) Aspergillus fumigatus (ATCC 6645), Aspergillus niger (ATCC 6404), Cryptococcus albidus (ATCC 60109) and Trichophyton rubrum (ATCC 14001) were used in this study.
Assay of antibacterial activityThe antibacterial activity of lead compound was tested by agar diffusion assay2. The plates were incubated at 37°C for 24h during which activity was evidenced by the presence of a zone of inhibition surrounding the well. Each test was repeated three times and the antibacterial activity was expressed as the mean of diameter of the inhibition zones (mm) produced by the compound when compared to positive controls (chloramphenicol). Agar well diffusion method was further evaluated by determining the minimum inhibitory concentration (MIC) by the broth two-fold macro dilution method. The compound was serially diluted at varying concentration of 1500–1μg/ml in Mueller Hinton broth. Culture broth (0.1ml) was added to the each tube containing the compound. The tubes were incubated aerobically at 37.8°C for 24h. Positive controls were prepared separately with respective organisms in the same culture media without the compound. After incubation, the tube with the least concentration of the compound showing no growth was taken as the MIC value for the respective organism.
Assay of antifungal activityThe antifungal activity of lead compound was tested by agar diffusion assay. C. albicans inocula was prepared by picking five distinct colonies of approximately 1mm from 48h old culture grown on Sabouraud dextrose agar and incubated at 35±2°C. Colonies were suspended in 5ml of sterile 0.85% saline. Muller Hinton agar plates were prepared with 2% glucose and 0.5μg/ml methylene blue dye medium and further used for carrying out antifungal activity by agar diffusion method. The antifungal activity was expressed as the mean of diameter of the inhibition zones (mm) produced by the lead compound when compared to the control (amphotericin B). Agar well diffusion method was further evaluated by broth microdilution method using the standard protocol (CLSI M38-A). Fungal conidia were taken from well grown slant culture, adjusting a suspension to a concentration of 1×106conidia/ml using RPMI medium. It was further diluted with PBS/Tween-20 to have a suspension of 2×103conidia/ml. Dilutions were plated and spread over Sabouraud's dextrose agar plates. These plates are then incubated at 37°C for 48h. For each isolates add 100μl of inocula into all the wells in the appropriate row, which makes the final inoculum to 5×105conidia/ml. Microtitre plates were then incubated for 48h at 37°C in a moist chamber and the MIC was read visually.
ResultsOur systematic screening of marine actinomycetes isolates for antimicrobial activity resulted in the selection of the strain Streptomyces VITSVK5 spp. The strain was identified by molecular taxonomic characterization using 16S rRNA partial gene sequence analysis and 16S rDNA sequence analysis. The 16S rDNA sequence (1424 base pairs) of Streptomyces VITSVK5 spp. has been submitted to NCBI under the accession no. GQ848482. The crude extract obtained from the isolate showed significant antifungal activity against drug resistant strains of Aspergillus clinical isolates. Since the butanolic extract of Streptomyces VITSVK5 spp. showed the maximum inhibitory activity against drug resistant Aspergillus clinical isolates, it was subjected to bioactive guided extraction and purification of active principle. Extraction and purification of 10l of the culture broth yielded 112.3mg of pure compound.
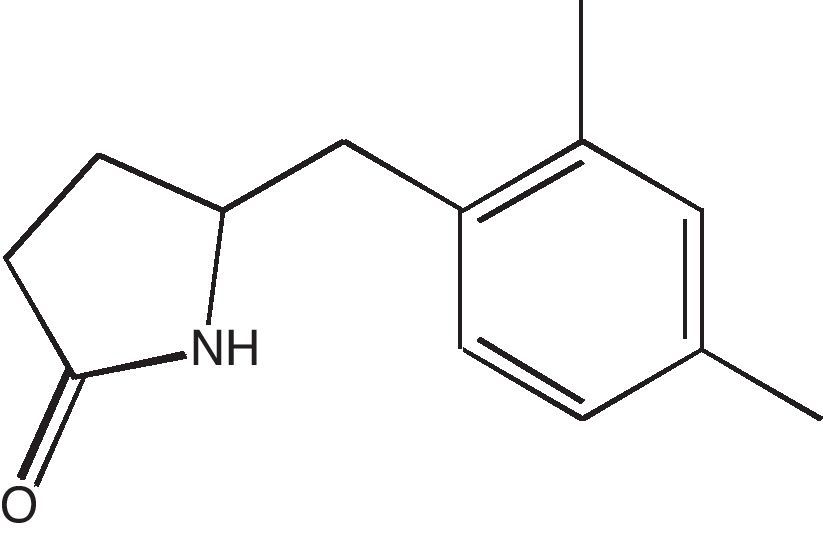
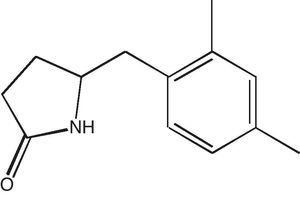
The purity of compound was checked by thin layer chromatography with Rf value of 0.43 (chloroform–methanol, 8:2) and single band obtained was visualized by iodine reagent and sulphuric acid. The spectral data (UV, FT-IR, 1H NMR, 13C NMR, DEPT, and HR-MS) obtained for the compound were used to establish the structure of the compound. UV/vis (MeOH) λmax 290; FT-IR cm−1 3436 (–NH), 2928, 1729 (CO); 1H NMR (DMSO-d6 500MHz): 0.871 (s, J=64Hz, –CH3), 1.23–1.39 (m, J=44Hz, 2×CH2), 3.37 (t, 1H), 4.29 (s, 2H), 7.25 (s, 1H), 7.65–7.69 (t, 2H), 8.17 (s, 1H); 13C NMR (DMSO-d6 500MHz): 13.80 (CH3), 18.60 (CH3), 34.65(2×CH2), 60.34(CH2), 64.98 (CH); 128.62, 131.4, 133.6, 134.3, 136.7, 144.7, 166.9. DEPT, HR-MS and m/z (found/cal.): 203.1325/203.1310.
Based on the spectral data the structure of the compound extracted from Streptomyces VITSVK5 spp. was identified as 5-(2,4-dimethylbenzyl) pyrrolidin-2-one (DMBPO) and the molecular formula was determined as C13H17NO. The structure of the compound is illustrated in Fig. 1.
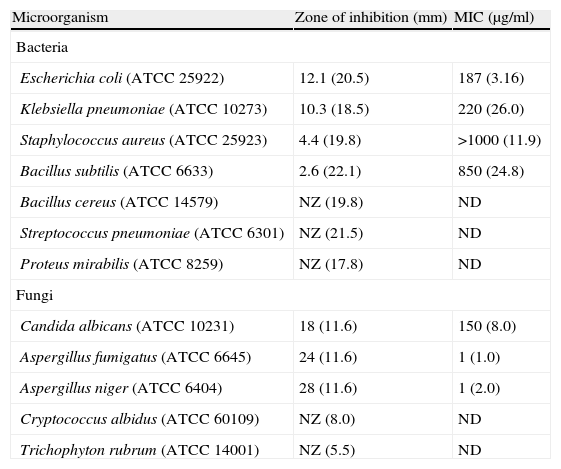
Antibacterial and antifungal activityAmong the microorganisms tested, Gram-negative bacteria were found to be more susceptible to DMBPO (Table 1). DMBPO showed maximal activity against E. coli with the MIC value of 187μg/ml when compared to chloramphenicol (3.16μg/ml). It also showed a significant activity against K. pneumoniae (MIC of 220μg/ml and 10.3mm zone of inhibition), S. aureus (MIC of >1000μg/ml and 4.4mm) and B. subtilis (MIC of 850μg/ml and 2.6mm) (Table 1). DMBPO was found to be a potent inhibitor for opportunistic fungal pathogens too. It showed significant zone of inhibition of 18mm (MIC – 150μg/ml), 24mm (MIC – 1μg/ml) and 28mm (MIC – 1μg/ml) against C. albicans, A. fumigatus and A. niger respectively. No zone of inhibition was observed against B. cereus, S. pneumoniae, P. mirabilis, C. albidus and T. rubrum.
Effect of DMBPO against tested bacterial and fungal pathogens.
| Microorganism | Zone of inhibition (mm) | MIC (μg/ml) |
| Bacteria | ||
| Escherichia coli (ATCC 25922) | 12.1 (20.5) | 187 (3.16) |
| Klebsiella pneumoniae (ATCC 10273) | 10.3 (18.5) | 220 (26.0) |
| Staphylococcus aureus (ATCC 25923) | 4.4 (19.8) | >1000 (11.9) |
| Bacillus subtilis (ATCC 6633) | 2.6 (22.1) | 850 (24.8) |
| Bacillus cereus (ATCC 14579) | NZ (19.8) | ND |
| Streptococcus pneumoniae (ATCC 6301) | NZ (21.5) | ND |
| Proteus mirabilis (ATCC 8259) | NZ (17.8) | ND |
| Fungi | ||
| Candida albicans (ATCC 10231) | 18 (11.6) | 150 (8.0) |
| Aspergillus fumigatus (ATCC 6645) | 24 (11.6) | 1 (1.0) |
| Aspergillus niger (ATCC 6404) | 28 (11.6) | 1 (2.0) |
| Cryptococcus albidus (ATCC 60109) | NZ (8.0) | ND |
| Trichophyton rubrum (ATCC 14001) | NZ (5.5) | ND |
The zones of inhibition and the MIC value for the standard antibiotic (μg/ml) are given within parenthesis. Chloramphenicol and amphotericin B were used as a positive control.
NZ – No zone of inhibition; ND – Not done.
The DMBPO extracted from Streptomyces VITSVK5 spp. exhibited antimicrobial activity against all the bacterial and fungal pathogens with the MIC value ranging from 187 to 1000μg/ml for bacteria and 1–150μg/ml for fungi. For antibacterial activity the concentration of DMBPO required to exhibit minimal inhibitory activity was comparatively higher than chloramphenicol MIC value. DMBPO exhibit better antifungal activity against A. niger and A. fumigatus with the MIC value equivalent or less than the MIC value of amphotericin B (Table 1). However, DMBPO exhibits comparatively higher MIC value against C. albicans when compared to amphotericin B. The results of our study show that DMBPO is very selective to inhibit the growth of A. fumigatus and in the case of A. niger DMBPO exhibits better antifungal activity with lesser MIC value when compared to amphotericin B. Our findings demonstrated the broad spectrum of antimicrobial activity of the compound DMBPO against selected human pathogens and have shown better antifungal activity than antibacterial activity. It was found to have potent inhibitory activity against opportunistic fungi, which usually infect pre-infected patients with less immunity. Invasive aspergillosis occurs predominantly in highly immunocompromissed patients and it can cause up to 100% mortality if untreated. A. fumigatus is the most common causative species when compared to other Aspergillus species.
Microorganisms from extreme environments have gained considerable attention in recent years because of its diversity and biological activities, mainly due to its ability to produce novel chemical compounds of high commercial value. Recent investigations using enrichment techniques, new selection methods and media have led to the isolation of novel actinomycetes from sediment samples. Similarly, Streptomyces VITSVK5 spp. growth has been optimized with different process parameters10–12. Several earlier studies reported on the antagonistic activity of several novel marine natural products isolated from marine algae, fungi, bacteria, sponges and sea stars14–17. One important reason for discovering novel secondary metabolites is to circumvent the problem of resistant pathogens, which are no longer susceptible to the currently used drugs. The number of deaths due to these pathogenic organisms is on the rise, which needs to be controlled. Immuno-compromised patients are in higher risk of infection and they require a new class of antibacterial and antifungal drugs with less toxicity. In vitro antimicrobial activity of different secondary metabolites extracted from Streptomyces species isolated from soil samples have been reported earlier18. Kokare et al.9 reported the isolation of halophilic Actinopolyspora species AHI from the sediments of Alibag coast of Maharashtra India. The cell free supernatant extracted from the strain exhibited good antagonistic activity under in vitro conditions against Gram-positive bacteria viz. S. aureus, Staphylococcus epidermidis, B. subtilis and fungi such as A. niger, A. fumigatus, Aspergillus flavus, Fusarium oxysporum, Penicillum sp. and Trichoderma sp. It did not show any antibacterial activity against Gram-negative bacteria such as E. coli, Pseudomonas aeruginosa, Serratia marcescens, Enterobacter aerogenes and against the fungi, C. albicans and Cryptococcus sp. Although the exploitation of marine actinomycetes as a source for discovery of novel secondary metabolites is at an early stage, numerous novel metabolites have been isolated in the past.
Streptopyrrolidine, a benzyl pyrrolidine derivative isolated from the fermentation broth of a marine Streptomyces sp. KORDI-3973 has been shown to exhibit significant anti-angiogenesis activity toward capillary tube formation of human umbilical vein endothelial cells.20 Romanenko et al.19 have isolated Pseudomonas (strain KMM 3042), a Gram-negative Gammaproteobacteria dwelling on lithosphere as well as in a marine environment capable of producing diverse secondary metabolites including pyrrolidinedione capable of acting as antimicrobial agent. Ours is the first report on isolation DMBPO from marine Streptomyces VITSVK5 spp. In the present study, the efficacy of the bioactive compound DMBPO against A. fumigatus and A. niger clinical isolates was found to be equivalent to that of amphotericin B. It is apparent that the bioactive compound DMBPO from Streptomyces VITSVK5 spp. has antimicrobial potential against selected bacterial and fungal pathogens. However, in vivo animal model studies are required to elucidate bio-distribution, toxicity and serum levels of DMBPO to authenticate its antimicrobial potential.
Conflict of interestThe authors have no conflict of interest to declare.
Authors thank the management of VIT University for providing facilities to carry out this research.