The presence of melanin in the fungal cell is a major virulence factor of the genus Sporothrix since it protects the fungal cells against the defense systems.
AimsThe present study aimed to investigate the interference of melanin in the susceptibility of Sporothrix brasiliensis and Sporothrix schenckii sensu stricto to amphotericin B and itraconazole, drugs recommended as therapy for disseminated and subcutaneous sporotrichosis, respectively.
MethodsYeast cells were cultivated in minimal medium with or without l-DOPA in order to induce the production of melanin. Microdilution and killing assay methods were used to determine the antifungal activity against yeast cells with different amounts of melanin.
ResultsThe killing assay showed that melanization protected isolates within the S. schenckii complex from amphotericin B, particularly in the lower concentrations tested.
ConclusionsStudies combining amphotericin B and inhibitors of melanin are required in order to avoid this effect.
La presencia de melanina en la célula fúngica es un importante factor de virulencia del género Sporothrix en el proceso de infección, pues protege al hongo de la acción del sistema inmunitario.
ObjetivosEl objetivo del presente estudio fue investigar la interferencia de la melanina en la sensibilidad de Sporothrix brasiliensis y Sporothrix schenckii sensu stricto a la anfotericina B y el itraconazol, antifúngicos recomendados como terapia para la esporotricosis diseminada y la subcutánea, respectivamente.
MétodosSe cultivaron células en la fase levaduriforme en medio mínimo con o sin l-DOPA con el fin de inducir la producción de melanina. La valoración de la actividad antifúngica sobre células de Sporothrix con diferente contenido en melanina se realizó mediante microdilución en caldo y curvas de mortalidad.
ResultadosEl ensayo mostró que la melanización protege de la acción de la anfotericina B a las especies del complejo S. schenckii, particularmente en las concentraciones más bajas ensayadas.
ConclusionesSe requieren estudios de combinación entre diferentes clases de antifúngicos o de anfotericina B con inhibidores de la melanina, con el fin de disminuir o evitar este efecto.
Sporotrichosis is a fungal disease caused by dimorphic and thermotolerant fungi belonging to the Sporothrix schenckii complex that occurs in both subacute and chronic forms. This fungus primarily affects the skin and subcutaneous tissue and most frequently manifests as a lymphocutaneous infection.17 Most cases occur due to traumatic implantation of the fungus through the skin by handling plants, soil and infected animals.35 Disseminated sporotrichosis has been described in immunocompromised patients, especially in those under corticosteroid treatment, patients with AIDS, diabetes and alcoholics,6,8–11 and, although rare, dissemination has also been reported in immunocompetent patients.31,38 The S. schenckii complex encompasses six species capable of causing sporotrichosis.20 These species show different in vitro susceptibility profiles to antifungal agents,21 as well as different virulence in vivo.4
Itraconazole is the treatment of choice for lymphocutaneous sporotrichosis16; however, failure due to the in vitro resistance to this azole and cross-resistance with other azoles has been observed.21,39 In cases of disseminated sporotrichosis, amphotericin B is the advised antifungal, followed by a maintenance treatment with itraconazole.6,10,16
Melanin is a requirement for fungal cells to persist when facing up to host defense systems3,15,26,33 and is also considered a factor of fungal drug resistance, mainly for polyenes and echinocandins. Both morphological stages of Sporothrix are able to synthesize melanin via the polyketide pathway to produce dihydroxynaphthalene (DHN) melanin, which is present in the conidia of the fungus. It has also been shown that Sporothrix can produce melanin through phenolic substrates such as 3,4-dihydroxy-l-phenylalanine (l-DOPA),1 and some species can produce pyomelanin in the presence of l-tyrosine.2 Almeida-Paes2 demonstrated with one strain of Sporothrix brasiliensis that cells became more resistant to amphotericin B after melanization in medium with l-DOPA or l-tyrosine. Amphotericin B is the treatment of choice for systemic sporotrichosis.24 Thus, it may be possible that therapeutic failures occur in cases of sporotrichosis as a result of drug resistance driven by melanization.
This study approaches the interference of fungal melanin in the in vitro susceptibility of the most common and virulent Sporothrix species – S. schenckii sensu stricto and S. brasiliensis – to both amphotericin B and itraconazole, the antifungals for the treatment of disseminated and lymphocutaneous forms, respectively, of sporotrichosis. Recognition of such interference may be essential in redefining doses and treatments for cases of sporotrichosis.
Material and methodsChemicalsAmphotericin B (Sigma Chemical Co., St. Louis, MO) and itraconazole (Janssen-Cilag, São Paulo, SP) were obtained as standard powders and prepared according to the Clinical and Laboratory Standards Institute guidelines.7 RPMI 1640 medium (with l-glutamine and without sodium bicarbonate), morpholinepropanesulfonic acid (MOPS), and l-DOPA were purchased from Sigma Chemical Co. (Cleveland, OH).
Fungal strains and mediaThree isolates of S. schenckii sensu stricto and three isolates of S. brasiliensis isolated from cases of lymphocutaneous sporotrichosis were studied. One strain of Candida glabrata (ATCC) and one strain of Cryptococcus neoformans (ATCC) were included as negative and positive control of melanization, respectively. The fungal yeast cells were grown at 37°C for 7 days in a rotary shaker (150rpm) in brain heart infusion (BHI) broth (Himedia). Controls were obtained by growing yeast cells on minimal medium (MM) agar plates (15mM glucose, 10mM MgSO4, 29.4mM KH2PO4, 13mM glycine, 3.0μM vitamin B1, 2% agar; Difco); melanization was induced by growing yeast cells on minimal medium supplemented with l-DOPA (MML) for 10 days.34 Cells were then harvested and washed with sterile phosphate-buffered saline (PBS), pH 7.2.
MIC determinationMinimal inhibitory concentrations (MIC) were determined by the standardized protocol (M27-A3) according to CLSI,7 adapted to the yeast phase of Sporothrix. Final drug concentrations ranged from 0.06 to 4μg/ml for amphotericin B and itraconazole. After incubation at 37°C for 48h, MIC for amphotericin B was defined as the lowest concentration at which there was an absence of growth, and for itraconazole as the lowest drug concentration which achieved 50% growth inhibition when compared to the growth of the drug-free control.
Killing assayFor the killing assay, yeast cells were suspended in sterile normal saline at a density of 2.2×103cells/ml. Cell counts were determined with a hemocytometer. Microcentrifuge tubes containing 0.1ml aliquots of an antifungal at 10 times the final concentration were inoculated with 0.9ml of the yeast suspensions. Final drug concentrations ranged from 2 to 8μg/ml for amphotericin B, and from 1 to 4μg/ml for itraconazole. After incubation at 37°C for 2h, aliquots were plated on brain heart infusion agar (BHI) to determine their viability, as measured by the number of CFU. Before that, a screening with a time kill curve with the samples collected at 2, 4, 8, and 24h was performed. However, at the point of 4h onwards there was no significant difference in mortality. Thus, it was determined the point of 2h for the evaluation of mortality. The rate of survival was compared to that of fungal cells incubated in phosphate-buffered saline (PBS).36
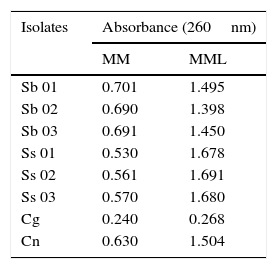
Spectrophotometry of yeast cell extractsExtraction of melanin was performed in order to quantify differences in the amount of melanin in the Sporothrix isolates grown in different media. The method consisted of an evaluation of the crude extract of the yeast mass obtained by autoclaving it for 90min at 120°C in 0.02mol/l citrate buffer pH 7 as previously described.18 Then, the suspension was centrifuged and the supernatant collected for spectrophotometric analysis. The extracts were analyzed at 260nm.34
StatisticsStatistical significance (p values) was determined using Wilcoxon test to compare MICs results with and without melanin interference. Student's t-test was used in the killing assay.
Results and discussionAll isolates produced higher amount of melanin within 10 days in medium supplemented with l-DOPA (MML), while isolates cultured in medium without l-DOPA (MM) remained with their standard melanization. The concentration of melanin was measured using spectrophotometry (Table 1). The absorbance of the melanized group was approximately twice the absorbance of the group not exposed to l-DOPA, showing the greater production of melanin when l-DOPA is present in the medium.
Variation in melanin content, expressed as absorbance, after subculture on minimal medium and minimal medium with l-DOPA.
| Isolates | Absorbance (260nm) | |
|---|---|---|
| MM | MML | |
| Sb 01 | 0.701 | 1.495 |
| Sb 02 | 0.690 | 1.398 |
| Sb 03 | 0.691 | 1.450 |
| Ss 01 | 0.530 | 1.678 |
| Ss 02 | 0.561 | 1.691 |
| Ss 03 | 0.570 | 1.680 |
| Cg | 0.240 | 0.268 |
| Cn | 0.630 | 1.504 |
MM, minimal medium; MML, minimal medium with l-DOPA; Sb, Sporothrix brasiliensis; Ss, Sporothrix schenckii sensu stricto; Cg, Candida glabrata; Cn, Cryptococcus neoformans.
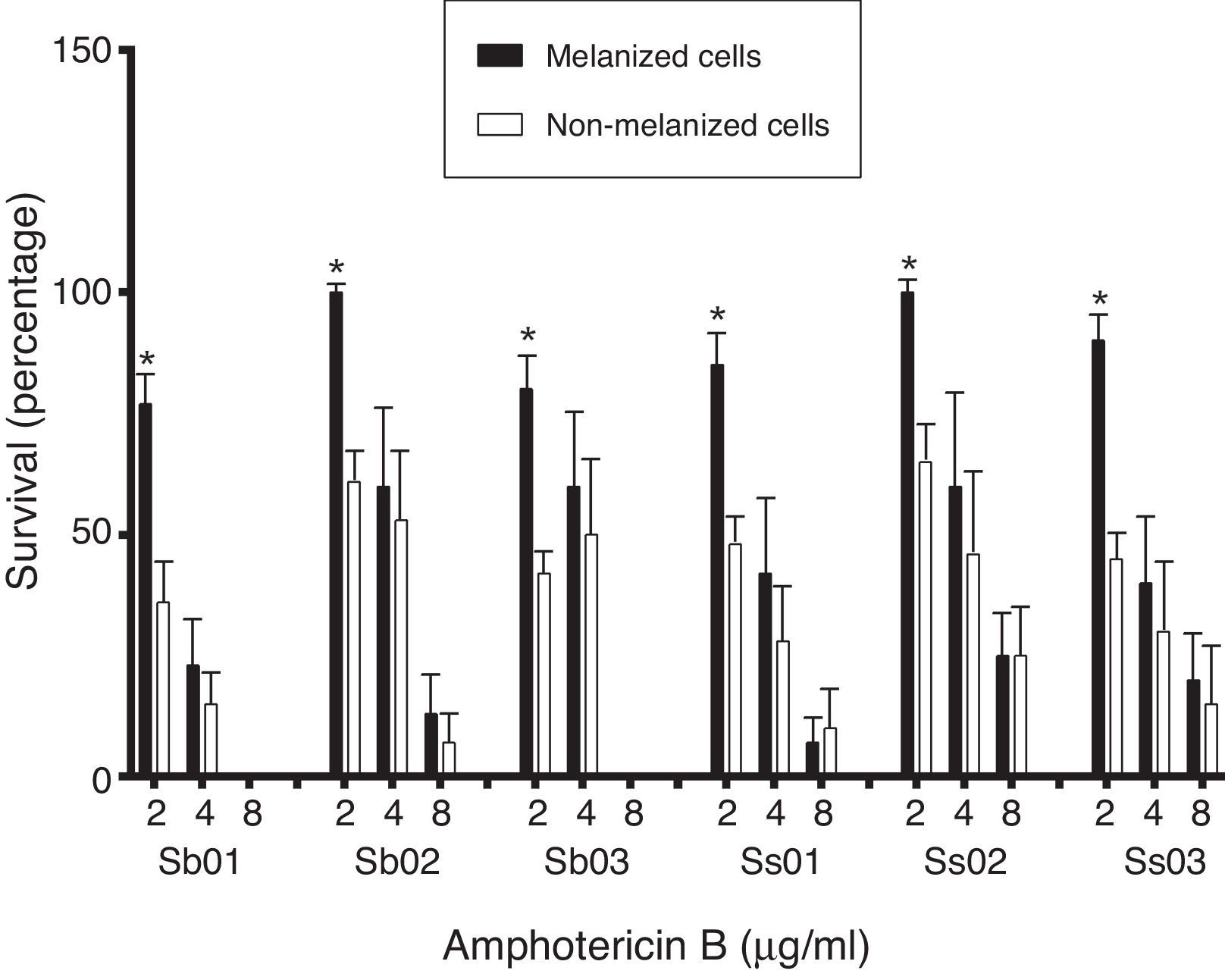
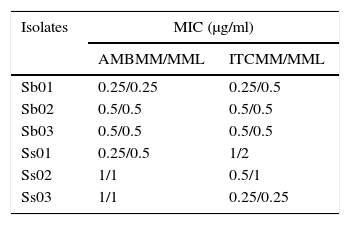
The geometric mean of MICs for amphotericin B and itraconazole for the isolates subcultured in the different media were compared. There was no significant difference among the MICs of the group without increasing melanin content (MM), and the group with melanin inducing conditions (MML) (Table 2) (p>0.05). In the killing assay, melanization protected the cells against amphotericin B. The differences were statistically significant at a concentration of 2μg/ml (p<0.001) (Fig. 1). The results obtained with itraconazole showed no differences between the groups tested (Fig. 1).
Minimal inhibitory concentrations of amphotericin B and itraconazole against S. brasiliensis (Sb) and S. schenckii sensu stricto (Ss) isolates cultivated in different media.
| Isolates | MIC (μg/ml) | |
|---|---|---|
| AMBMM/MML | ITCMM/MML | |
| Sb01 | 0.25/0.25 | 0.25/0.5 |
| Sb02 | 0.5/0.5 | 0.5/0.5 |
| Sb03 | 0.5/0.5 | 0.5/0.5 |
| Ss01 | 0.25/0.5 | 1/2 |
| Ss02 | 1/1 | 0.5/1 |
| Ss03 | 1/1 | 0.25/0.25 |
MIC, minimal inhibitory concentrations; AMB, amphotericin B; ITC, itraconazole; MM, minimal medium; MML, minimal medium with l-DOPA.
Amphotericin B and itraconazole killing assay. The rates of survival of yeast cells (S. brasiliensis [Sb 01, Sb 02, Sb 03] and S. schenckii sensu stricto [Ss 01, Ss 02, Ss 03]) grown in minimal medium (MM), and minimal medium with l-DOPA (MML) after exposure to various concentrations of amphotericin B and itraconazole for 2h were compared to those of fungal cells incubated in PBS. p-Values were calculated by comparing melanized groups with their respective non-melanized group using the Student's t-test. *p<0.001, **p<0.05. Similar results were obtained in two separate experiments.
Amphotericin B is the treatment of choice for disseminated and extracutaneous sporotrichosis.16 A previous study showed that both fungal melanin and synthetic melanin are capable of binding to amphotericin B, reducing the effectiveness of this agent. In contrast, fluconazole, itraconazole and 5-fluorocytosine activities were not modified by the presence of melanin, also observed in the study presented here. Tests with azole antifungal agents did not result in significant death of the fungus, which is consistent with the fungistatic properties of these drugs.36 However, the non-interference of melanin in the itraconazole activity, presented here for the first time, makes itraconazole a potential therapeutic option for sporotrichosis.
In this study, the broth microdilution technique7 was unable to demonstrate the interference of melanization in the antifungal susceptibility of Sporothrix strains. This finding is in agreement with studies performed with C. neoformans and Histoplasma capsulatum.13,36,37 It was reported that this could be due to the absence, in the culture medium, of substrates for melanization. In the same study the authors attempted to minimize this problem by adding l-DOPA to RPMI, but this resulted in the formation of a black precipitate which precluded the use of this modification to the assay.36
Here, the killing assay showed that melanization protected S. schenckii sensu stricto and S. brasiliensis from amphotericin B, particularly in the lower concentration tested, as has already been demonstrated in one strain of S. brasiliensis.2 At higher concentrations, although there was in general a greater survival of melanized cells, the differences were not statistically significant. Previous studies using the same technique have also demonstrated that melanization was able to protect C. neoformans, H. capsulatum and Wangiella dermatitidis against amphotericin B.13,29,36 These authors suggest that fungal melanin in its antioxidant role binds to amphotericin B and reduces its activity. In addition to its antifungal activity, amphotericin B is also a potent immunomodulator.25 Thus, its binding to fungal melanin can also reduce the effect on host factors, such as activation of T cells and macrophages. Furthermore, melanin is also an immunologically active molecule that suppresses inflammation.5,22In vitro studies have demonstrated that albino conidia are two-fold more highly phagocytized than the respective pigmented spores, what indicates that melanin contributes to the survival of the fungal cells in the host.32 The melanization of the fungal cells also affects the pathogenesis of cutaneous sporotrichosis because pigmented isolates showed greater ability to invade tissues than albino mutant strains in an experimental model of sporotrichosis.19
S. brasiliensis, the most virulent species of the complex,4 has frequently been reported in infections in Brazil, often affecting immunocompetent patients. Case reports of lung injury in HIV negative patients,28 a familial outbreak in the southeastern of Brazil,27 and even resistance to itraconazole treatment in animals have been reported.12Sporothrix species are also the most frequent pathogens in cases of osteoarticular infection in immunocompetent patients.30
In a recent study, Rodrigues et al.31 confirmed that S. brasiliensis has a lower genetic diversity and less variability in the in vitro susceptibility than S. schenckii sensu stricto (both species are phylogenetically related), whose high genetic variation couples with a high fluctuation in the antifungal susceptibility and virulence profile.4 Despite the slight number of strains studied here, higher MICs for amphotericin B and greater variability in the itraconazole MICs for the strains of S. schenckii sensu stricto were observed. Regarding the melanin content assessed in this study, there was no apparent variation among strains of the same species. However, if a large number of strains of the same population were evaluated, it is likely that differences in the production of this molecule would be found.
Disseminated sporotrichosis is a problem especially for immunocompromised patients. These individuals require higher doses of amphotericin B for some months and often require secondary prophylaxis for an extended period of time. Melanization is essential for the virulence of Sporothrix, and it is evident that melanin can be synthesized in a human host and in mammalian tissues during infection by Sporothrix species.23 The combination of different classes of antifungal agents has been shown to be promising,14 and can reduce the effect of melanin, since certain classes of drugs does not seem to be affected by it. In addition to this, our findings reinforce the importance of combination studies of amphotericin B with potent inhibitors of melanin synthesis for sporotrichosis infection.
FinancingMario DN thanks the Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for grant support, and Alves SH thanks the financial support provided by CNPq (Grant Proc. 470229/2012-8).
Conflict of interestThe authors have no conflict of interest to declare.






![Amphotericin B and itraconazole killing assay. The rates of survival of yeast cells (S. brasiliensis [Sb 01, Sb 02, Sb 03] and S. schenckii sensu stricto [Ss 01, Ss 02, Ss 03]) grown in minimal medium (MM), and minimal medium with l-DOPA (MML) after exposure to various concentrations of amphotericin B and itraconazole for 2h were compared to those of fungal cells incubated in PBS. p-Values were calculated by comparing melanized groups with their respective non-melanized group using the Student Amphotericin B and itraconazole killing assay. The rates of survival of yeast cells (S. brasiliensis [Sb 01, Sb 02, Sb 03] and S. schenckii sensu stricto [Ss 01, Ss 02, Ss 03]) grown in minimal medium (MM), and minimal medium with l-DOPA (MML) after exposure to various concentrations of amphotericin B and itraconazole for 2h were compared to those of fungal cells incubated in PBS. p-Values were calculated by comparing melanized groups with their respective non-melanized group using the Student](https://static.elsevier.es/multimedia/11301406/0000003300000001/v1_201601290205/S1130140615000376/v1_201601290205/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Amphotericin B and itraconazole killing assay. The rates of survival of yeast cells (S. brasiliensis [Sb 01, Sb 02, Sb 03] and S. schenckii sensu stricto [Ss 01, Ss 02, Ss 03]) grown in minimal medium (MM), and minimal medium with l-DOPA (MML) after exposure to various concentrations of amphotericin B and itraconazole for 2h were compared to those of fungal cells incubated in PBS. p-Values were calculated by comparing melanized groups with their respective non-melanized group using the Student Amphotericin B and itraconazole killing assay. The rates of survival of yeast cells (S. brasiliensis [Sb 01, Sb 02, Sb 03] and S. schenckii sensu stricto [Ss 01, Ss 02, Ss 03]) grown in minimal medium (MM), and minimal medium with l-DOPA (MML) after exposure to various concentrations of amphotericin B and itraconazole for 2h were compared to those of fungal cells incubated in PBS. p-Values were calculated by comparing melanized groups with their respective non-melanized group using the Student](https://static.elsevier.es/multimedia/11301406/0000003300000001/v1_201601290205/S1130140615000376/v1_201601290205/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

