In Spain, data of candidemia are limited to surveys conducted in specific areas or tertiary care centers. Also, in recent years, attention has shifted toward episodes of candidemia in non-ICU wards.
AimsWe reviewed the cases of Candida isolates recovered from the blood of patients admitted to the Emergency Room (ER) in our tertiary care hospital.
MethodsThe patients selected for this study had an isolation of Candida in the blood culture. All data were collected retrospectively from the clinical records of a 11-year period.
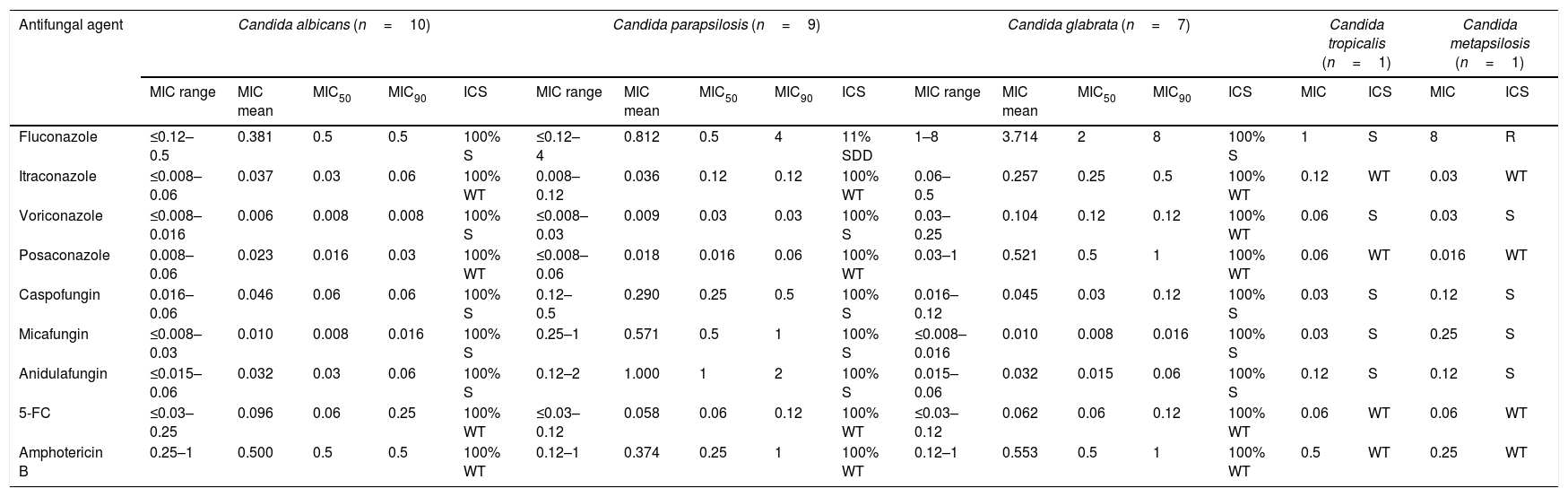
ResultsCandida albicans and other species of the genus were present in 10 and 18 patients, respectively. The patients did not present different clinical features in comparison with other reports of hospitalized patients. All patients had several risk factors for candidemia. Only two patients had received previous antifungal therapy before admission. All the isolates of C. albicans, Candida glabrata and the only isolate of Candida tropicalis were susceptible to all the antifungal agents tested. Only one isolate of Candida parapsilosis was susceptible dose-dependent to fluconazole, and the only isolate of Candida metapsilosis was resistant to fluconazole.
ConclusionsIt is essential to evaluate the risk factors, underlying conditions and clinical features in non-hospitalized patients in order to determine whether an empirical treatment for candidemia is appropriate.
En España, los datos sobre candidemia están limitados a áreas específicas u hospitales de tercer nivel. En los últimos años el foco de atención sobre esta infección se ha desplazado a áreas de pacientes que no son críticos.
ObjetivosSe revisaron los aislamientos de Candida obtenidos de hemocultivos de pacientes que ingresaron en el Servicio de Urgencias de nuestro hospital de tercer nivel.
MétodosLos pacientes se seleccionaron por presentar un aislamiento de Candida en hemocultivo. Todos los datos fueron recogidos retrospectivamente de las historias clínicas de los pacientes atendidos en un período de 11 años.
ResultadosCandida albicans y otras especies del género se aislaron en 10 y 18 pacientes, respectivamente. Las características clínicas de los pacientes no mostraban diferencias en comparación con los datos publicados en la bibliografía sobre pacientes hospitalizados. Todos los pacientes presentaban factores de riesgo para desarrollar candidemia. Únicamente dos pacientes habían recibido tratamiento antimicótico antes del ingreso. Todas los aislamientos de C. albicans,Candida glabrata y el único de Candida tropicalis fueron sensibles a todos los antimicóticos probados. Solo un aislamiento de Candida parapsilosis fue sensible al fluconazol en función de la dosis y el único aislamiento de Candida metapsilosis fue resistente al fluconazol.
ConclusionesEs fundamental evaluar los factores de riesgo, las enfermedades de base y otras características clínicas de los pacientes no hospitalizados con el fin de establecer la instauración de un tratamiento empírico para la candidemia.
Candidemia is the seventh most common nosocomial bloodstream infection in Europe.11 In Spain, however, data are limited to surveys conducted in specific areas or tertiary care centers.7 Intensive Care Units (ICU) and surgical wards were traditionally recognized as wards with a high incidence of candidemia.9 However, in recent years, attention has shifted toward episodes of candidemia in non-ICU wards. In Spain, CANDIPOP study of candidemia in non-ICU surgical and medical wards showed Candida albicans (43–50%) as the predominant species in our geographical area, with a low frequency of non-susceptibility to fluconazole (4.6%).10
The aim of this retrospective study was to review the Candida isolates from blood cultures of patients admitted to the Emergency Room (ER) during 11 years in our tertiary care hospital. We describe the clinical and demographic features of these patients and the antifungal susceptibility profiles of the isolates.
This study was performed at University Hospital La Paz, a tertiary care hospital in Madrid, Spain. Our ER department had 31 episodes of candidemia from 2007 to 2017.
The blood cultures were incubated in the BD BACTEC FX (Becton Dickinson, Franklin Lakes, NJ) or BACT/ALERT (bioMérieux, Marcy-l’Étoile, France). If yeast morphology was observed in the Gram stain, a subculture on BBL CHROMagar Candida Medium (Becton Dickinson) at 37°C was performed. Catheters were cultured in conventional media by the semiquantitative roll-plate method,4 and other clinical samples were cultured in conventional solid media, following the protocols of the Clinical Microbiology Department. Candida isolates were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS – Bruker Daltonics, Billerica, MA) and/or Vitek 1 YST ID card (bioMérieux) and API 20C Aux (bioMérieux). Antifungal susceptibility testing was performed using a semi-automated broth microdilution analysis (Sensititre Yeast One panel – Trek Diagnostic Systems, Cleveland, OH) according to the manufacturer. Minimal inhibitory concentration (MIC) values (MIC range, MIC mean, MIC50, MIC90) with each antifungal drug were calculated, and interpretive categories of susceptibility (S – susceptible, SSD – susceptible dose-dependent, R – resistant and WT – wild-type strains) were established using breakpoints and epidemiological cut-off values (ECV) according to the Clinical and Laboratory Standards Institute (CLSI) methods.1,6 The statistical data analysis was performed using the software SPSS v.22. Quantitative data are shown as the median and the interquartile (Q3–Q1) range, and qualitative variables are expressed as absolute and relative frequencies (Table 1). Categorical variables were compared using the χ2 test or Fisher's exact test, whereas Student's T test or U Mann Whitney test were applied for continuous variables, as appropriate. All the significance tests were two-tailed. This retrospective study had the approval of the Clinic Research Ethics Committee of University Hospital La Paz with the code PI-3175.
Demographic and clinical characteristics of the patients from the Emergency Room with Candida isolates in blood cultures.
| Characteristics of the patients | All cases, n=28 (%) | C. albicans, n=10 (%) | Other Candida species, n=18 (%) | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Male | 16 (57.1) | 8 (80) | 8 (44.4) | 0.11 |
| Median age, years | 65.5 (46.7-79)* | 59 (41-79.5)* | 66.5 (48.3-79.3)* | 0.72 |
| Median days of previous hospital stays | 17 (4.7-48.5)* | 19.5 (11.5-54)* | 15 (0-51.5)* | 0.30 |
| Median number of hospital stays in the previous year | 1.75 (0–8)* | 1.72 (1-3)* | 1.80 (0-8)* | 0.38 |
| Days after last discharge before current admission | 138.79 (3–>365) | 60.4 (5–165) | 182.33 (3–>365) | 0.02 |
| Underlying disease | ||||
| Oncological disease | 13 (46.4) | 5 (50) | 8 (44.4) | 1.00 |
| Hematological disease | 4 (14.3) | – | 4 (22.2) | 0.26 |
| Kidney disease | 3 (10.7) | 1 (10) | 2 (11.1) | 1.00 |
| Cardiovascular disease | 5 (17.9) | 2 (20) | 3 (16.7) | 1.00 |
| Others | 7 (25) | 1 (10) | 6 (33.3) | 0.36 |
| Comorbidities | ||||
| None | 4 (14.3) | 4 (40) | – | 0.01 |
| Cardiovascular disease | 20 (71.4) | 6 (60) | 14 (77.8) | 0.40 |
| Metabolic disorders | 13 (46.4) | 4 (40) | 9 (50) | 0.70 |
| Autoimmune disease | 6 (21.4) | – | 6 (33.3) | 0.06 |
| Kidney disease | 4 (14.3) | 2 (20) | 2 (11.1) | 0.60 |
| Respiratory disease | 5 (17.9) | 2 (20) | 3 (16.7) | 1.00 |
| Risk factors for candidemia | ||||
| Corticoids therapy | 5 (21.73) | 3 (30.0) | 2 (11.1) | 0.31 |
| Central venous catheter | 13 (46.4) | 5 (50) | 8 (44.4) | 1.00 |
| Urinary catheter | 7 (25) | 2 (20) | 5 (27.8) | 1.00 |
| Prior surgery (3 months) | 13 (46.4) | 6 (60) | 7 (38.9) | 0.43 |
| Total parenteral nutrition | 6 (21.4) | 2 (20) | 4 (22.2) | 1.00 |
| Hemodialysis | 1 (3.6) | 1 (10) | – | 0.35 |
| Immunosuppressive therapy | 13 (46.4) | 7 (70) | 6 (33.3) | |
| Neutropenia | 2 (7.1) | 2 (20) | – | 0.12 |
| Thrombocytopenia | 6 (21.4) | 4 (40) | 2 (11.1) | 0.14 |
| Previous therapy with azoles | 3 (10.7) | 2 (20) | 1 (5.6) | 0.28 |
| Previous therapy with echinocandins | – | – | – | – |
| Previous broad spectrum antibiotherapy | 18 (64.3) | 7 (70) | 11 (61.1) | 0.70 |
| Median hours passed until the beginning of the treatment | 38.5 (17.5-59.5)* | 40.5 (19-62.2)* | 34.5 (15-59.5)* | 0.75 |
| Clinical manifestations | ||||
| Fever (38–40°C)+/−shiver | 24 (85.7) | 9 (90) | 15 (83.3) | 1.00 |
| Urinary | 4 (14.3) | 2 (20) | 2 (11.1) | 0.60 |
| Cardiovascular | 2 (7.1) | – | 2 (11.1) | 0.52 |
| Respiratory | 7 (25) | 2 (20) | 5 (27.8) | 1.00 |
| Abdominal | 6 (21.4) | 1 (10) | 5 (27.8) | 0.37 |
| Source of infection | 0.62 | |||
| Catheter-related | 11(39.3) | 5 (50) | 6 (33.3) | – |
| Urological | 7 (25) | 2 (20) | 5 (27.8) | – |
| Cardiovascular | 4 (14.3) | 2 (20) | 2 (11.1) | – |
| Abdominal | 3 (10.7) | – | 3 (16.7) | – |
| Respiratory | 3 (10.7) | 1 (10) | 2 (11.1) | – |
| Therapeutic measures | ||||
| Antifungal therapy at admission | 2 (7.1) | – | 2 (11.1) | 0.52 |
| Antifungal therapy after positive blood culture | 26 (92.8) | 9 (90) | 17 (94.4) | 0.92 |
| Therapy with azoles | 14 (50) | 5 (50) | 9 (50) | – |
| Therapy with echinocandins | 10 (35.7) | 3 (30) | 7 (38.9) | – |
| Therapy with azoles+echinocandins | 2 (7.1) | 1 (10) | 1 (5.6) | – |
| Central venous catheter removal | 11 (39.3) | 5 (50) | 6 (33.3) | – |
| Clinical outcome | 0.06 | |||
| Discharge | 17 (60.7) | 4 (40) | 13 (72.2) | – |
| Transfer to Palliative Care Unit | 5 (17.9) | 2 (20) | 3 (16.7) | – |
| Exitus | 6 (21.4) | 4 (40) | 2 (11.1) | – |
*: interval values.
The blood cultures from thirty-one patients admitted to the ER from 2007 to 2017 rendered the isolation of Candida; three of those isolates were considered contaminants. C. albicans and other species of the genus were isolated in 10 and 18 patients, respectively (9 Candida parapsilosis, 7 Candida glabrata, 1 Candida tropicalis and 1 Candida metapsilosis). Table 1 shows the demographic and clinical features of these 28 cases with candidemia. Oncological and hematological diseases were the main underlying diseases in 17 patients (60.7%), and most of them had comorbidities. All patients had several risk factors for candidemia: antimicrobial broad-spectrum therapy (64.3%), central venous catheters, immunosuppressive therapy, surgery in the last 3 months (46.4%), thrombocytopenia (21.4%) and neutropenia (7.1%). Fever (38–40°C) was the most frequent clinical manifestation. Catheter-related infection was the source of infection in 11 patients (39.3%). Only 2 patients (7.1%) had received previous antifungal therapy with azoles due to previous fungal infections. After positive blood cultures (2–6 days post-admission) the remaining 26 patients (92.8%) received antifungal therapy with azoles (50% of the cases), echinocandins (35.7% of the cases) and both in combination therapy (7.1% of the cases). The average time elapsed until the beginning of the treatment was higher in C. albicans candidemias when compared with the rest of the cases (40.5 and 34.5h, respectively). All of the 11 central venous catheters were removed or replaced and cultured. Candida grew in eight of these catheters (72.7%). In other clinical samples, Candida was also isolated in six cases (4 urine cultures and 2 vegetations of infective endocarditis). Finally, candidemia episode was resolved in 17 patients (60.7%). Five patients (17.9%) were transferred to Palliative Care Unit due to their underlying disease, and 6 patients (21.4%) died (5 of them within 10 days post-admission). The susceptibility profiles of the isolates are shown in Table 2, highlighting one strain of C. parapsilosis which was susceptible dose-dependent (SDD) to fluconazole (MIC 4μg/ml), and the C. metapsilosis isolate, which was resistant to fluconazole (MIC 8μg/ml).
In vitro antifungal susceptibility profiles of Candida isolates in blood cultures. MIC data are expressed in μg/ml.
| Antifungal agent | Candida albicans (n=10) | Candida parapsilosis (n=9) | Candida glabrata (n=7) | Candida tropicalis (n=1) | Candida metapsilosis (n=1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC range | MIC mean | MIC50 | MIC90 | ICS | MIC range | MIC mean | MIC50 | MIC90 | ICS | MIC range | MIC mean | MIC50 | MIC90 | ICS | MIC | ICS | MIC | ICS | |
| Fluconazole | ≤0.12–0.5 | 0.381 | 0.5 | 0.5 | 100% S | ≤0.12–4 | 0.812 | 0.5 | 4 | 11% SDD | 1–8 | 3.714 | 2 | 8 | 100% S | 1 | S | 8 | R |
| Itraconazole | ≤0.008–0.06 | 0.037 | 0.03 | 0.06 | 100% WT | 0.008–0.12 | 0.036 | 0.12 | 0.12 | 100% WT | 0.06–0.5 | 0.257 | 0.25 | 0.5 | 100% WT | 0.12 | WT | 0.03 | WT |
| Voriconazole | ≤0.008–0.016 | 0.006 | 0.008 | 0.008 | 100% S | ≤0.008–0.03 | 0.009 | 0.03 | 0.03 | 100% S | 0.03–0.25 | 0.104 | 0.12 | 0.12 | 100% WT | 0.06 | S | 0.03 | S |
| Posaconazole | 0.008–0.06 | 0.023 | 0.016 | 0.03 | 100% WT | ≤0.008–0.06 | 0.018 | 0.016 | 0.06 | 100% WT | 0.03–1 | 0.521 | 0.5 | 1 | 100% WT | 0.06 | WT | 0.016 | WT |
| Caspofungin | 0.016–0.06 | 0.046 | 0.06 | 0.06 | 100% S | 0.12–0.5 | 0.290 | 0.25 | 0.5 | 100% S | 0.016–0.12 | 0.045 | 0.03 | 0.12 | 100% S | 0.03 | S | 0.12 | S |
| Micafungin | ≤0.008–0.03 | 0.010 | 0.008 | 0.016 | 100% S | 0.25–1 | 0.571 | 0.5 | 1 | 100% S | ≤0.008–0.016 | 0.010 | 0.008 | 0.016 | 100% S | 0.03 | S | 0.25 | S |
| Anidulafungin | ≤0.015–0.06 | 0.032 | 0.03 | 0.06 | 100% S | 0.12–2 | 1.000 | 1 | 2 | 100% S | 0.015–0.06 | 0.032 | 0.015 | 0.06 | 100% S | 0.12 | S | 0.12 | S |
| 5-FC | ≤0.03–0.25 | 0.096 | 0.06 | 0.25 | 100% WT | ≤0.03–0.12 | 0.058 | 0.06 | 0.12 | 100% WT | ≤0.03–0.12 | 0.062 | 0.06 | 0.12 | 100% WT | 0.06 | WT | 0.06 | WT |
| Amphotericin B | 0.25–1 | 0.500 | 0.5 | 0.5 | 100% WT | 0.12–1 | 0.374 | 0.25 | 1 | 100% WT | 0.12–1 | 0.553 | 0.5 | 1 | 100% WT | 0.5 | WT | 0.25 | WT |
5-FC: 5-Fluorocytosine; MIC50: minimal concentration of the antifungal that inhibits 50% of the isolates; MIC90: minimal concentration of the antifungal that inhibits 90% of the isolates; ICS: interpretive categories of susceptibility in percentages; S: susceptible strains; SDD: susceptible dose-dependent strains; R: resistant strains; WT: wild-type strains.
There is a lack of data concerning the epidemiology of candidemia and the incidence of this entity in patients admitted to the ER. In this study, Candida species distribution does not differ from other distributions in different groups of patients in our media, as Ramos-Martinez et al. describes.8 However, the epidemiology of Candida species and the differences in resistance to antifungal agents may change depending on the geographical area. Puig-Asensio et al. reported that fluconazole susceptibility has decreased in Spain, mainly due to an increase of C. glabrata strains in elderly patients, and also to a widespread use of azoles. In our study, C. parapsilosis and the cryptic species C. metapsilosis showed low susceptibility to fluconazole, as previously described.3
In this study, the patients do not present different clinical or demographic features in comparison with other reports of hospitalized patients. Central catheter was the most important primary source of candidemia, according to the medical history of the patients. We must emphasize that the majority of our patients had not received empirical antifungal therapy at admission (26 out of 28, 92.85%). After the microbiological diagnosis, 26 out of 28 patients received antifungal-targeted therapy. In addition, all central catheters were removed according to clinical recommendations.2,5 In patients with C. albicans candidemia, we observed an earlier time of admission to the ER in comparison with non-C. albicans Candida related infections after the last discharge of the hospital. Most of the cases of non-C. albicans candidemia had a good clinical outcome and the patients were discharged, whereas the mortality was higher among patients with C. albicans candidemia. This may be related to a slight increase in the time elapsed until the beginning of the treatment in those cases caused by C. albicans.11 In addition, although we found few cases, this could have been related to lower virulence of the non-C. albicans Candida isolates. A prompt and adequate antifungal treatment and source control are critical therapeutic measures in order to decrease early mortality.7 Clinicians should consider to treat with empirical antifungal therapy those patients who meet criteria and risk factors for candidemia, although not being admitted to hospital recently. Nevertheless, this study has a main limitation: the low number of cases reported. Other limitations are the retrospective design and local epidemiology of a tertiary care center.
In conclusion, although candidemia is a very rare infection in the ER, it is essential to evaluate the clinical features in each patient in order to determine whether an empirical treatment is appropriate. More studies focused on the incidence of patients with candidemia in the ER should be carried out to properly diagnose this clinical entity; medical records may help to soon treat the patients with antifungal therapy at admission, and follow an optimal clinical management.
FundingNone.
ContributionAll authors contributed significantly carrying out this study.
Conflicts of interestThe authors declare that there are no conflicts of interest.
We thank the Biostatistics Department of the University Hospital la Paz.