Cladophialophora bantiana is the most frequent cause of central nervous system phaeohyphomycosis.
AimsWe report a case of phaeohyphomycosis by C. bantiana in a patient with underlying lung disease on steroid therapy.
MethodsAn 81-year-old male was admitted in August 2011 with a history of difficulty speaking and deflection of the oral commeasure to the left side with a brain abscess. Brain tissue was cultured on Sabouraud media and sequence analysis of the internal transcribed spacer region of the ribosomal DNA was done for identification purposes. Susceptibility testing to various antifungal agents was performed using the microdilution test.
ResultsHistopathological examination of the brain tissue ruled out malignancy and the presence of dematiaceous hyphae was observed. Culture showed the presence of a single black fungus, identified as C. bantiana. It was susceptible to all antifungals, except to caspofungin. The patient was treated with voriconazole plus liposomal amphotericin B. Cerebral cranial computed tomography [CCT] scans demonstrated persistence of the intraparenchymal abscess collection. Despite surgical and medical treatment with antifungal drugs, the patient died 5 months after the first diagnosis of the cerebral occupying lesion was made.
ConclusionsPhaeohyphomycosis is an uncommon infection with severe limitations on the clinical clues that can help in early diagnosis. Fungal species identification is mandatory for epidemiological and therapeutic reasons. The MICs could be useful in selecting the appropriate antifungal agent. Avoiding the unnecessary exposure to soil or other media potentially contaminated with fungal spores should be recommended to any immunosuppressed patient.
Cladophialophora bantiana es la causa más frecuente de feohifomicosis del sistema nervioso central.
ObjetivosDescribimos un caso de feohifomicosis por C. bantiana en un paciente con una enfermedad pulmonar subyacente en tratamiento con corticosteroides.
MétodosEn agosto de 2011, ingresa un hombre de 81 años de edad con antecedentes de dificultad para hablar y desviación de la comisura bucal a la izquierda por un absceso cerebral. Se cultivó el aspirado del absceso cerebral en medio de Sabouraud y para la identificación definitiva del hongo se secuenció la región espaciadora transcrita interna del ADN ribosomal. Las pruebas de sensibilidad a los diferentes antifúngicos se efectuaron mediante microdilución.
ResultadosEl examen histopatológico de las muestras descartó la presencia de un tumor maligno y confirmó la existencia de hifas. El cultivo reveló la presencia de un hongo dematiáceo identificado como Cladophialophora bantiana, sensible a todos los antifúngicos excepto a la caspofungina. El paciente fue tratado con voriconazol combinado con anfotericina B liposomal. La tomografía computarizada craneal mostró la persistencia del absceso intraparenquimatoso. A pesar del tratamiento con antifúngicos y del procedimiento quirúrgico, el paciente falleció 5 meses después de que se estableciera el diagnóstico inicial.
ConclusionesLa feohifomicosis es una infección poco frecuente, con importantes limitaciones de los indicios clínicos que pueden contribuir a un diagnóstico precoz. Por razones tanto epidemiológicas como terapéuticas, es indispensable la identificación de la especie de hongo responsable. La determinación de la concentración inhibitoria mínima podría ser de utilidad en la selección del tratamiento antifúngico apropiado. Los pacientes inmunodeprimidos deben evitar la exposición al suelo u otros medios potencialmente contaminados por esporas de hongos.
Phaeohyphomycoses are infections caused by dematiaceous moulds that contain melanin pigments in their walls and spores. The natural habitat of dematiaceous fungi is the soil or vegetative matter. The Cladophialophora genus consists of dematiaceous fungi that have a worldwide distribution.1 Nevertheless, infections by these fungi are very rare and occur mainly in subtropical and non-arid climate zones.7 Several species are potentially able to cause human infections, and they have being increasingly recognised as a cause of serious disease in both immunocompetent and immunocompromised patients.2,15
Binford et al.8 described the first culture-proven case of cerebral phaeohyphomycosis due to Cladophialophora bantiana in 1952. To date, there have been few reports of dematiaceous fungal infections in immunocompetent hosts, and up to 40% of cases were reported in patients with predisposing factors, such as solid organ transplants, glucocorticoid treatment, diabetes mellitus, lymphoma, eye and skin trauma, and intravenous drug use.7,10 This fungus is the most frequent cause of central nervous system phaeohyphomycosis. Although clinical presentations of phaeohyphomycosis vary considerably, disseminated infection is rarely identified,8 and the majority of cases are related to the formation of cerebral abscesses and have a high mortality.6,9,15
The high degree of phenotypic similarity between new recently described Cladophialophora species makes identification a challenging matter and requires expert interpretation of microscopic morphology or the use of molecular identification methods.3 Otherwise, the infection is usually diagnosed after the agent is isolated in culture, which usually delays treatment.12 Antifungal therapy is mainly based on the experience of clinicians or the drugs used in the scarce case reports published.2 The optimal therapy is unknown.11
We report the case of a patient with underlying lung disease on steroid therapy. This is the third case reported in Spain.5,13 Because no standard therapy is available, the antifungal treatment was based on the available antifungal susceptibility data.
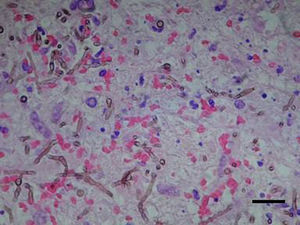
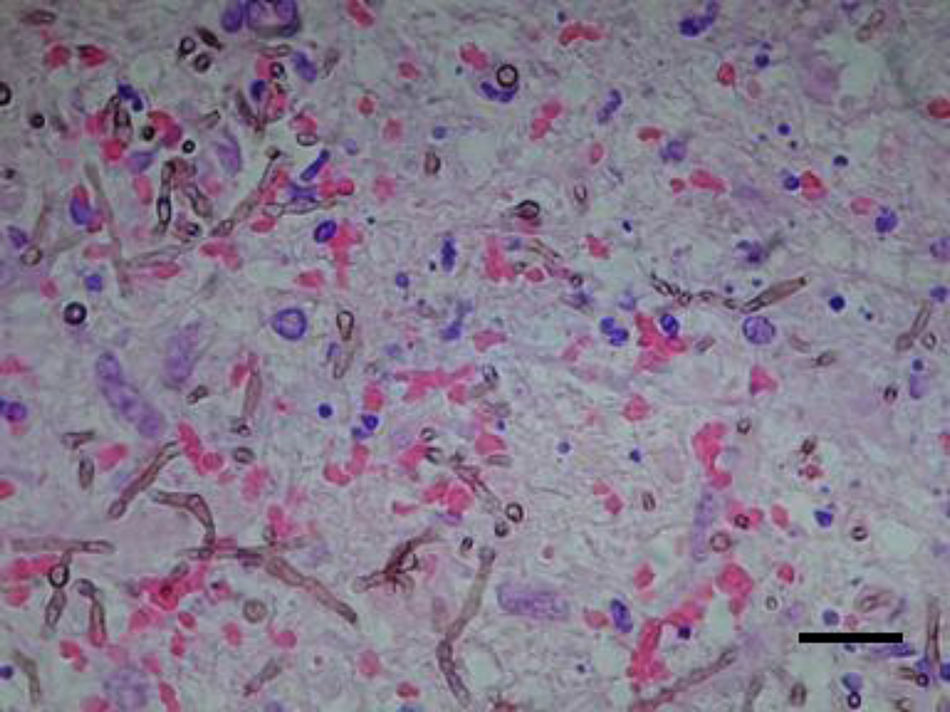
Case reportAn 81-year-old male former smelting iron worker who was diagnosed with extrinsic allergic alveolitis and treated with corticosteroid tapering (prednisone 40mg p.o. daily) was admitted in August 2011 with a history of difficulty in speaking and deflection of the mouth to the left side. Cerebral tomography revealed a space-occupying left frontal multinodular lesion with oedema, which was compatible with a brain abscess (Fig. 1). Thorax and cranial computed tomography ruled out any pulmonary lesion and sinusitis. A treatment with metronidazole, cefotaxime and linezolid was started before culture results were available. On day 2, a craniotomy was performed with a total resection of the abscess. Histopathological examination of the brain tissue ruled out malignancy, and the presence of dematiaceous hyphae was observed with a direct Gram stain and Gomori-methenamine-silver and hematoxylin–eosin stains (Fig. 2). Empiric antifungal treatment was started with voriconazole 4mg/kg/12h and caspofungin 50mg/d, while maintaining the broad-spectrum antibiotics. Prednisone was withdrawn after the diagnosis of a fungal infection was done.
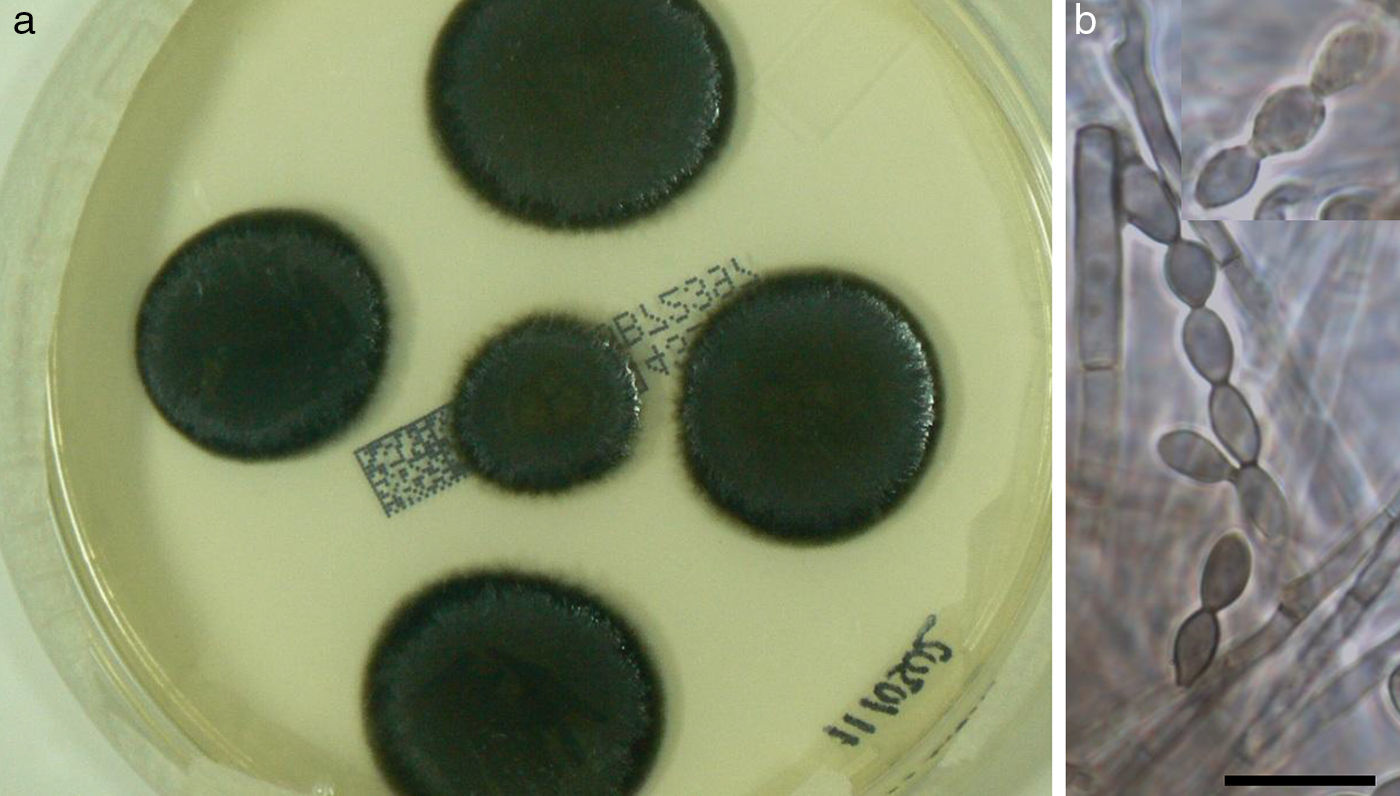
Culture on routine media for bacteria and fungi showed the presence of a single black fungus, which was isolated for identification purposes. C. bantiana was not isolated from other clinical samples, including lower respiratory tract specimens, nasal samples, and blood cultures. After 2 weeks of growth on Sabouraud media at 30°C, the fungus appeared as black velvety colonies (Fig. 3a) and microscopically produced long, sparsely branched, wavy chains of conidia (Fig. 3b) similar to those observed for C. bantiana. A culture of the isolate was sent to the Medical School at the University Rovira i Virgili from Reus (URV), Spain, for a sequence analysis and deposited as FRM 12155. Molecular studies included the amplification and sequencing of the internal transcribed spacer (ITS) region of nuclear rDNA.16 DNA was directly extracted from the colonies using the PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. The DNA was quantified using GeneQuant pro (Amersham Pharmacia Biotech, Cambridge, England). The ITS region was amplified and sequenced with the primer pair ITS5/ITS4 according to a previously described protocol.4 PCR products were purified and sequenced at Macrogen Inc. (Seoul, South Korea) with a 3730XL DNA analyser (Applied Biosystems). The program SeqMan (Lasergene, Madison, Wisconsin) was used to obtain a consensus sequences, and a BLAST identity search of the sequence (556bp) was then performed. The highest percentage identity in the BLAST search was 99% with sequences of reference strains from the CBS culture collection (CBS 444.96, CBS 102586, CBS 100436, CBS648.96, etc.) of C. bantiana, followed by C. emmonsii (CBS 979.96 and CBS640.96), with 90–91% identity.
Susceptibility testing of the isolate to various antifungal agents was performed using the microdilution test according to CLSI guidelines.17 Minimum inhibitory concentration (MIC) was defined as the lowest concentration of the antifungal agent that completely inhibited fungal growth. Minimal effective concentration (MEC) was defined as the lowest drug concentration resulting in aberrant hyphal growth through examination with an inverted microscope at 72–96h. The MICs were the following: terbinafine 0.03mg/l, voriconazole 0.25mg/l, posaconazole 0.25mg/l, amphotericin B 1mg/l, and 5-fluorocytosine 0.25mg/l. The MECs were the following: anidulafungin 0.25mg/l, caspofungin 4mg/l, and micafungin 2mg/l. Based on these findings, the treatment was changed, and caspofungin was substituted by liposomal amphotericin B.
In the following days, respiratory function worsened because of a respiratory infection caused by Klebsiella pneumoniae, which required ICU admission. During his stay in the ICU, the patient had several infectious complications: catheter-associated bacteraemia by Enterobacter cloacae, ventilator-associated pneumonia by multiresistant Acinetobacter baumannii, urinary tract infection by Klebsiella pneumoniae and Pseudomonas aeruginosa and a new episode of pneumonia by Klebsiella pneumoniae and Pseudomonas aeruginosa.
Cerebral cranial computed tomography (CCT) scans demonstrated persistence of the intraparenchymal abscess collection. A second craniotomy and evacuation of the abscess to identify margins free of invasion was performed. Again, C. bantiana grew on culture media. The recurrence of a new collection and the neurologic deterioration with right hemiparesis, arm paraplegia and facial involvement with motor aphasia made the family reject another surgical procedure. Follow-up care was performed with home hospitalisation with high doses of voriconazole. The patient died 5 months after the first diagnosis of the cerebral occupying lesion was made.
DiscussionPhaeohyphomycosis is an uncommon infection. Individual clinical case reports have been reported with limitations on the clinical clues that can help in early diagnosis. The data are not enough to make conclusive statements about therapy and outcomes, and it is hard to establish an incidence of this disease because it is not reportable and seems to occur sporadically.9,15
Pathogenicity could be due to the presence of melanin in the cell wall, which is thought to confer a protective advantage by scavenging free radicals produced by phagocytic cells, interfering with the oxidative burst that kills most organisms.3 The site of fungal entry is not well understood; haematogenous dissemination from a pulmonary source is one possibility, and exposure to soil or plant matter may be the source of respiratory infection.9 Multiple brain abscesses might also suggest dissemination through the bloodstream from a primary subclinical pulmonary focus.9,10 The patient presented in this report had various risk factors to acquire an infection and an identified contact with contaminated soil. He was diagnosed with allergic extrinsic alveolitis and treated with steroids. He had a history of exposure to soil through gardening, and the patient reported a recent cleaning of his small hen house. Clinical presentations vary considerably, although neurological signs and symptoms (headache followed by focal neurological deficit) are present in most cases.10 Typically, the abscess cavity has a thick wall filled with non-foul-smelling pus surrounded by hyperplastic tissue. When CSF samples are available, the laboratory usually reports an abnormal CSF, with hypoglycorrhachia, slight increases in protein concentration, and sometimes pleocytosis with eosinophils in addition to peripheral blood eosinophilia.10,15
Regarding C. bantiana identification, Cladophialophora is a polyphyletic genus that includes numerous species. By multilocus sequencing studies (LSU, SSU and RPB1) four different lineages have been identified. Molecular identification of the different species of the genus can be done by sequencing several loci, being the most discriminative the ITS region and the elongation factor 1−α. In our case, the percentages of similarity obtained with the ITS sequences, 99% similarity with C. bantiana and 91% similarity with C. emmonsii, allowed us to ensure that the identification to the species level was reliable, making unnecessary the sequencing of other loci.
Although the optimal antifungal therapy is not known, combining amphotericin B deoxycholate with flucytosine and either itraconazole or voriconazole is recommended. In the present case, before the fungus was identified the patient was treated with caspofungin plus voriconazole, being substituted by voriconazole plus liposomal amphotericin B when the fungus was identified as C. bantiana and the susceptibility report was available. Echinocandins do not appear to be active in vitro, and their role in the treatment of phaeohyphomycosis is unclear. Revankar et al.15 suggested that treatment with a combination of amphotericin B, 5-fluorocytosine, and itraconazole improved patient survival, but the number of patients was reduced. Animal studies suggest that amphotericin B and fluconazole have poor activity, and only 5-fluorocytosine at high doses showed some degree of efficacy.11 Broad-spectrum triazoles, especially posaconazole, have also showed efficacy in a murine model of cerebral infection by C. bantiana.11 Echinocandins do not appear to be active in vitro, and their role in the treatment of phaeohyphomycosis is unclear. The isolate of C. bantiana described in this case report was susceptible to all antifungals and most drugs tested, except caspofungin. Antifungal susceptibility should be monitored at the beginning and during the course of the treatment because of the risk of resistance and cross-resistance induced by exposure to antifungal drugs. Clinical and radiological improvement within a week or two of initiating treatment is the best sign of clinical efficacy.10 Lipid preparations of amphotericin B were infrequently used, and firm conclusions on their usefulness are not possible. Nevertheless, they have been suggested as a method to increase the dose while reducing its toxic effects.3,15 Combined therapies of posaconazole plus micafungin and 5-fluorocytosine showed high activity in a murine model of disseminated infection by C. bantiana.11 Nevertheless, susceptibility testing has not been completely evaluated.9 The MIC breakpoints for moulds have not been firmly established, but they are most likely useful in selecting the appropriate antifungal agents. It is necessary to understand the pharmacokinetic and pharmacodynamic properties of different antifungal drugs to establish the most effective treatment.14 It is generally accepted that drainage and surgical resection are the most important therapeutic interventions in prolonging patient survival.13,14 Complete surgical clearance should be associated with a higher rate of survival.15 Nevertheless, mortality is high: 65% with surgery and approximately 100% when surgery is not performed.3,7,10 In the reported patient, despite almost total resection of the abscess and appropriate antifungal therapy, the evolution was fatal.
This case is reported because of its rarity and to alert physicians to suspect phaeohyphomycosis when evaluating a brain abscess in both immunocompetent and immunosuppressed hosts. Mycological examination should be performed in any patient with cerebral abscess that require a surgical approach, especially if a ring-enhancing lesion is observed on CT scan images. Antifungal chemotherapy and surgical debridement should be performed as soon as possible. Only early diagnosis and aggressive therapy can offer a cure in patients with phaeohyphomycosis. Finally, unnecessary exposure to potentially highly contaminated material with fungal spores should be avoided in patients with underlying conditions that could favour fungal infections.






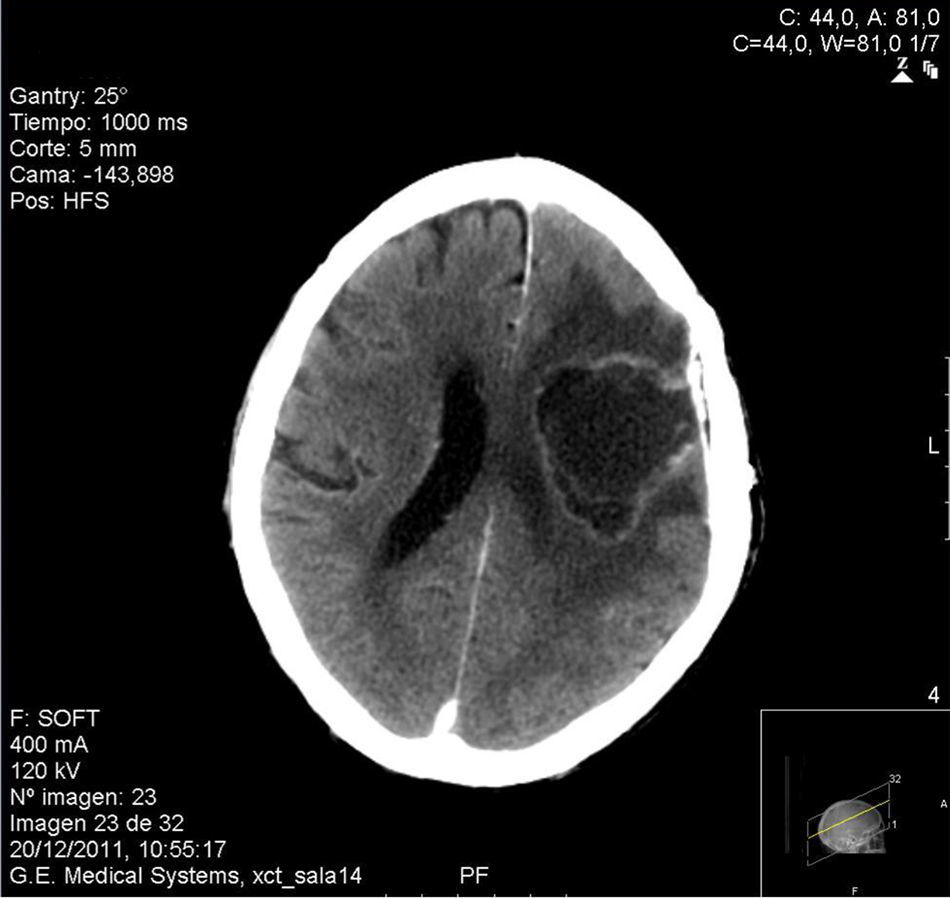
![Computerised tomography [CT] showing the left frontal occupying lesion. Computerised tomography [CT] showing the left frontal occupying lesion.](https://static.elsevier.es/multimedia/11301406/0000003100000003/v1_201409200309/S113014061300048X/v1_201409200309/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)