Candidemia is one of the most frequent opportunistic mycoses worldwide. Limited epidemiological studies in Latin America indicate that incidence rates are higher in this region than in the Northern Hemisphere. Diagnosis is often made late in the infection, affecting the initiation of antifungal therapy. A more scientific approach, based on specific parameters, for diagnosis and management of candidemia in Latin America is warranted.
‘Recommendations for the diagnosis and management of candidemia’ are a series of manuscripts that have been developed by members of the Latin America Invasive Mycosis Network. They aim to provide a set of best-evidence recommendations for the diagnosis and management of candidemia.
This publication, ‘Recommendations for the management of candidemia in children in Latin America’, was written to provide guidance to healthcare professionals on the management of children who have, or who are at risk of, candidemia.
Computerized searches of existing literature were performed by PubMed. The data were extensively reviewed and analyzed by members of the group. The group also met on two occasions to pose questions, discuss conflicting views, and deliberate on a series of management recommendations.
‘Recommendations for the management of candidemia in children in Latin America’ includes prophylaxis, empirical therapy, therapy for proven candidemia, patient work-up following diagnosis of candidemia, duration of candidemia treatment, and central venous catheter management in children with candidemia.
This manuscript is the third of this series that deals with diagnosis and treatment of invasive candidiasis. Other publications in this series include: ‘Recommendations for the diagnosis of candidemia in Latin America’, ‘Recommendations for the management of candidemia in adults in Latin America’, and ‘Recommendations for the management of candidemia in neonates in Latin America’.
This article is also published in Spanish in this issue. It can be found inhttp://dx.doi.org/10.1016/j.riam.2013.05.011
La candidemia es una de las micosis oportunistas más frecuentes en todo el mundo. El escaso número de estudios epidemiológicos llevados a cabo en América Latina indica que las tasas de incidencia en esta región son mayores que las descritas en el hemisferio norte. A menudo el diagnóstico de la infección se establece tardíamente, lo que afecta el inicio del tratamiento antimicótico. Por esta razón, para el diagnóstico y el manejo de la candidemia está justificada una estrategia más científica, basada en parámetros específicos.
Recomendaciones para el diagnóstico y manejo de la candidemia constituye una serie de artículos preparados por miembros del grupo Latin America Invasive Mycosis Network. Su objetivo es proporcionar las mejores evidencias disponibles para el diagnóstico y el manejo de la candidemia.
El presente artículo, Recomendaciones para el manejo de la candidemia en niños en América Latina, ha sido redactado con el objetivo de orientar a los profesionales de la salud en el manejo de los niños que padecen, o pueden padecer, candidemia.
Mediante la base de datos PubMed se emprendió una búsqueda informatizada de los estudios publicados. Los miembros del grupo revisaron y analizaron exhaustivamente los datos. El grupo también se reunió en dos ocasiones para proponer preguntas, abordar los puntos de vista conflictivos y deliberar sobre las recomendaciones terapéuticas.
Recomendaciones para el manejo de la candidemia en niños en América Latina está orientado al manejo de pacientes neutropénicos y no neutropénicos, e incluye aspectos sobre la profilaxis, la terapia empírica, el tratamiento de la candidemia confirmada, el seguimiento del paciente después del diagnóstico de la candidemia, la duración del tratamiento y el manejo del catéter venoso central.
Este manuscrito es el tercero de los artículos de esta serie dedicada al diagnóstico y tratamiento de las candidiasis invasoras. Otras publicaciones de esta serie son Recomendaciones para el diagnóstico de la candidemia en América Latina, Recomendaciones para el manejo de la candidemia en adultos en América Latina, y Recomendaciones para el manejo de la candidemia en neonatos en América Latina.
Este artículo está publicado en español en este mismo número. Puede encontrarlo enhttp://dx.doi.org/10.1016/j.riam.2013.05.011
There are some differences in the risk factors for candidemia and invasive fungal infections between children and adults. Age is a risk factor for candidemia, with neonates at increased risk of candidemia compared with children and adults. For children, the main risk factor for Candida infection is cancer.6 A higher risk of candidemia exists in children undergoing treatment regimens that result in severe mucositis and/or prolonged neutropenia, as well as in certain other pediatric groups.9,18 These include patients undergoing acute myeloid leukemia (AML) induction, patients with relapsed acute leukemia or non-Hodgkin's lymphoma, patients in the early post-transplant period for allogeneic bone marrow transplant with myeloablative regimen, and patients with advanced-stage or relapsing solid tumors.
The overall frequency of invasive candidiasis in children with high-risk leukemia and/or bone marrow transplantation is between 8% and 10%. One study from Chile reported an invasive fungal disease incidence of 5.8% in children with febrile neutropenia.54 Childhood cancers differ biologically from adult tumors and tend to be more susceptible to treatments including chemotherapy.20 Children receive more intense chemotherapy than adults.53 As a result, the following risk factors for candidemia are more prevalent in childhood cancer: neutropenia; monocyte deficits; vascular access device or central venous catheter (CVC) use – in an Australian report, candidemia was attributed to a vascular access device in 70% of infected children, compared with 44% of adults6 – chemotherapy-induced mucositis; longer duration of hospitalization and intensive care unit (ICU) stay before infection; extended courses of broad-spectrum antibiotics; and the therapeutic use of corticosteroids, particularly in acute leukemia.25
Additional risk factors for candidemia in childrenVarious congenital conditions may result in an increased risk for candidemia in children, such as inherited immune disorders.23 Cardiothoracic surgery in children is usually undertaken because of congenital heart disease, and several associated factors may render them more susceptible to candidemia, including co-morbid conditions, prior extensive surgery with cardiopulmonary bypass, increased postoperative morbidity, a postoperative open chest, and ongoing invasive mechanical ventilation.26,33 Total parenteral nutrition (TPN) also presents a risk for candidemia in children, as it does in adults. In one study, TPN was significantly associated with the development of candidemia in children.28 Length of ICU stay presents another risk factor for candidemia; most invasive fungal infections in children occur in the hospital setting.30
Burns, particularly extensive burns, are also a significant risk factor for candidemia in children, as they are thought to warrant hospitalization more often than burns in adults.5,14 In patients with burns, candidemia remains a significant cause of morbidity and a potential cause of death. In a 5-year retrospective study, 14.4% of pediatric patients with acute burns had Candida species isolated from one or more sites during their hospital stay, and 12.3% of these patients developed candidemia.52 In another study, factors independently associated with candidemia included presence of a CVC, use of vancomycin for >3 days in the prior 2 weeks, and receipt of agents with activity against anaerobic organisms for >3 days in the prior 2 weeks.64
Neutropenic childrenCandida prophylaxis in neutropenic childrenThere are no randomized controlled trials for the prophylaxis of candidemia in children. Prophylaxis should be considered only when local epidemiology data show a higher incidence of invasive fungal infection than usually reported in other centers or in the past.18 This further expounds the need for enhanced epidemiological information in Latin America on both a regional and a local level.
Fluconazole prophylaxis in childrenIn the absence of specific studies, information on Candida prophylaxis in adults can be extrapolated for use in children. In general, fluconazole is used in hematopoietic stem cell transplant patients,12 and this experience could be extrapolated to AML patients who are expected to develop severe mucositis and neutropenia. Therefore, the first choice for prophylaxis in neutropenic children should be fluconazole. A practical recommendation is to use 6mg/kg/day in children ≤30kg and an adult dose (400mg/day) in children >30kg. Immune status has no effect on the pharmacokinetics of fluconazole in either adults or children.19,36,48
Recommendations summary forCandidaprophylaxis in neutropenic children:
- 1.
Prophylaxis should be considered only in children who are at high risk of Candida infection when local epidemiology data demonstrate a high incidence of invasive fungal infection.
- 2.
Fluconazole is recommended as the first choice of prophylaxis, at a dose of 6mg/kg/day in children ≤30kg and at an adult dose (400mg/day) in children >30kg.
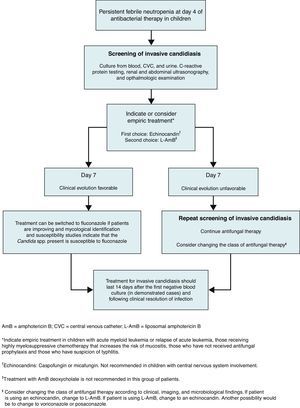
The decision on whether a neutropenic pediatric patient should receive empiric treatment for suspected invasive candidiasis should be made using a rational approach based on specific parameters (Fig. 1). Empiric therapy should be considered after day 4 of antibacterial therapy in children if fever and neutropenia continue and if the patient has not received antifungal prophylaxis, has received treatment that increases the risk of gastrointestinal tract mucositis, or has suspicion of typhlitis. It should be emphasized that in children with persistent fever and neutropenia, complete screening for invasive candidiasis is necessary; this involves: cultures from blood, CVC and urine; C-reactive protein testing; renal and abdominal ultrasonography; and ophthalmologic examination.
Pre-emptive antifungal therapy has been accepted as an alternative to empiric antifungal therapy in some adult neutropenic patients.17 Research describing the effectiveness and safety of this approach in children is needed. For empiric treatment in neutropenic children, the Working Group recommends an echinocandin (caspofungin or micafungin) as first choice, followed by liposomal amphotericin B (L-AmB) (Fig. 1). Empiric use of antifungal drugs has been investigated in prospective randomized controlled studies in neutropenic pediatric patients.29,44,57 In a randomized comparison study including adults (n=134) and children (n=204) with fever of unknown origin, L-AmB was more efficacious than amphotericin B deoxycholate (AmB-d).44 It was concluded that L-AmB at 3mg/kg/day was significantly safer than AmB-d in children and adults. In a double-blind, multicenter study of persistently febrile neutropenic pediatric patients (2–17 years old), caspofungin (n=56) was comparable with L-AmB (n=25) in tolerability, safety, and efficacy as an empiric antifungal therapy.29 In a randomized, multicenter study that included febrile neutropenic adults and children greater than 12 years of age, voriconazole was compared with L-AmB. This study demonstrated voriconazole to be a suitable alternative to L-AmB for empiric antifungal therapy.57
Recommendations summary for empiric treatment of invasive candidiasis in neutropenic children:
- 1.
Empiric therapy should be considered after day 4 of antibacterial therapy in children that continued with fever and neutropenia who have not received antifungal prophylaxis, have received treatment that increases the risk of gastrointestinal tract mucositis, or have suspected typhlitis.
- 2.
An echinocandin (caspofungin or micafungin) is recommended as first choice empiric treatment. If an echinocandin is not available, L-AmB is recommended.
There are very few comparative trials of antifungal agents in children, and those performed are underpowered.34,45 Therefore, pediatricians have to rely on data from adult clinical trials when managing invasive candidiasis.
Recommendations for the specific treatment of invasive candidiasis in neutropenic children are shown in Fig. 1. The Working Group recommends an echinocandin over fluconazole for the treatment of invasive candidiasis in neutropenic children. The reason for this is partly that echinocandins are fungicidal agents, as opposed to fluconazole which is fungistatic.61 Echinocandins that have been evaluated for the treatment of invasive candidiasis in neutropenic children include caspofungin and micafungin. A multicenter, prospective, open-label pediatric study reported an 81% overall response rate in caspofungin-treated children with invasive candidiasis.63 This result was similar to the response rate seen in adults (approximately 74–81%).11,35 A multicenter, randomized, double-blind study investigated the efficacy of caspofungin for empiric therapy in children with persistent fever and neutropenia, with L-AmB as the comparator. In this study, caspofungin was found to be as effective as L-AmB (46.4% vs. 32% success rate, respectively) in overall treatment and to have fewer side effects.29 For pediatric patients with febrile neutropenia and Candida spp. infection, caspofungin should be administered as a single 70mg/m2 loading dose on day 1 of treatment, followed by 50mg/m2 daily thereafter8 (Table 1). A dose based on body surface area is recommended, as this has been shown to result in consistent exposure in all pediatric age groups. A weight-based approach resulted in sub-optimal plasma concentrations in children in one study.55
Summary of drug and dose recommendations for the treatment of invasive candidiasis in children.
| Antifungal | Dose recommendation | Additional information |
| Caspofungin | Single 70mg/m2 IV loading dose on day 1 of treatment, followed by 50mg/m2 daily thereafter | Echinocandins are not indicated in meningitis due to Candida; if the 50mg/m2 daily dose does not provide an adequate clinical response, the daily dose can be increased to 70mg/m2 (do not exceed a daily dose of 70mg) |
| Micafungin | 2mg/kg/day IV if body weight is ≤40kg, or 100mg/day IV if body weight is >40kg | If the patient's response is inadequate, the dose may be increased to 4mg/kg/day in patients weighing ≤40kg, or 200mg/day in patients weighing >40kg3 |
| Anidulafungin | 1.5mg/kg/day IV | Dose based on a multicenter, ascending-dosage study of neutropenic pediatric patients4 |
| L-AmB | 3–5mg/kg/day IV | No dosage adjustment is required in this population |
| Voriconazole | 14mg/kg/day IV twice daily or 200mg oral twice daily | Use in pediatric patients aged 2 to <12 years with hepatic or renal insufficiency has not been studied; plasma levels are necessary |
| Fluconazole | 12mg/kg/day, IV or oral administration |
IV, intravenous; L-AmB, liposomal amphotericin B.
Results from a preliminary study suggest that micafungin is an alternative to caspofungin for treatment of invasive candidiasis in neutropenic children.49 In this study, doses of up to 4mg/kg/day were well tolerated, with no side effects in persistent febrile neutropenic children (2–17 years of age). In another study, micafungin was compared with L-AmB in pediatric patients (n=48 and n=50, respectively). Micafungin was found to be as effective and safe as L-AmB for the treatment of invasive candidiasis.45 The pharmacokinetic parameters of micafungin in febrile neutropenic pediatric patients are similar to those observed in adults.49 Micafungin should be administered at a dose of 2mg/kg daily for children with a body weight ≤40kg or 100mg/day for children with a body weight >40kg, with the option of dose escalation to 4mg/kg daily or 200mg/day, respectively16 (Table 1). An increased dosage may be needed in the very young, because clearance is higher in these patients.49
Little is known about the PK/PD and safety of anidulafungin in pediatric patients. One study has demonstrated that anidulafungin is well tolerated in neutropenic pediatric patients.4 A Phase III study is underway to assess the safety, tolerability, and efficacy of anidulafungin in children diagnosed with invasive candidiasis, with an estimated study completion date of June 2013.10
Treatment recommendation when echinocandins are not availableIf an echinocandin is not available, the Working Group recommends L-AmB, AmB lipid complex (ABLC), or voriconazole for the treatment of invasive candidiasis in neutropenic children. Treatment with AmB-d is not recommended in pediatric patients undergoing chemotherapy, considering the nephrotoxicity of both AmB-d and chemotherapy.21,37 Pharmacokinetic and safety data for lipid formulations of AmB in pediatric patients indicate no fundamental differences relative to adult populations.21 Studies support the use of ABLC as an effective antifungal agent in children. In an open-label pediatric study, therapeutic response (complete or partial) was seen in 81% (22/27) of patients who had candidiasis.58 In a retrospective study, ABLC treatment in children was shown to have resulted in an 89% (17/19) response rate against candidiasis.22 In a prospective study involving 56 centers, the safety and efficacy of L-AmB was evaluated in adults (n=260), children <15 years of age (n=242), and infants <2 months of age (n=43) with suspected fungal infections. In this study, infants tolerated the largest daily dose of L-AmB for the longest period of time (median dose, 2mg/kg/day; median duration, 16 days) followed by children (median dose, 1.5mg/kg/day; median duration 13 days). In comparison, adults tolerated a median dose of 1mg/kg/day for 12 days.1 As an alternative treatment, the Working Group recommends 3–5mg/kg daily for L-AmB.
A study on the safety, tolerability and plasma pharmacokinetics of voriconazole in immunocompromised pediatric patients found that a higher dosage is required in children than in adults to attain similar serum concentrations over time (Table 1).56
Duration of treatment in neutropenic childrenTreatment for invasive candidiasis in neutropenic children should continue for 14 days after the first negative blood culture and following clinical resolution of infection41 (Fig. 1). Treatment can be switched to a narrower-spectrum drug, such as fluconazole, when patients are improving, when patients experience neutrophil recovery, and when mycological identification and susceptibility studies indicate that the Candida species present is susceptible to fluconazole. The distribution volume and clearance of fluconazole are greater in children than in adults.7 Therefore, a relatively high mg/kg dose of fluconazole is necessary in young patients.31 To achieve comparable drug exposure to that achieved in adults, the daily fluconazole dose is 12mg/kg daily in children of all ages. However, it should be noted that some older children may have clearances similar to those of adults and, therefore, may require an adult dose (400mg/day).
Recommendations summary for treatment of invasive candidiasis in neutropenic children:
- 1.
An echinocandin (caspofungin or micafungin) is recommended as first-choice treatment for invasive candidiasis in neutropenic children.
- 2.
If an echinocandin is not available, the Working Group recommends L-AmB or voriconazole.
- 3.
Treatment with AmB-d is not recommended in this group of patients.
- 4.
Treatment for invasive candidiasis in neutropenic children should last 14 days after the first negative blood culture and following clinical resolution of infection.
- 5.
Treatment can be switched to a narrower-spectrum drug, such as fluconazole, when patients are improving, patients experience neutrophil recovery, and mycological identification and susceptibility studies indicated that the Candida spp. present is susceptible to fluconazole.
The Working Group recommends abdominal imaging, fundoscopy and echocardiography as baseline candidemia patient work-up to evaluate evidence of organ invasion. Although endocarditis is much less frequent in neutropenic than non-neutropenic patients, echocardiography should be considered in patients with persistent candidemia.40 Some patient work-up can be challenging in pediatric patients. For example, computed tomography (CT) scans may require sedation in younger children, and repeat exams increase radiation exposure.18
Catheter management in neutropenic childrenSource of infectionThe rationale for catheter removal is based on the hypothesis that skin colonization near the catheter insertion site can lead to Candida colonization of the catheter and subsequent fungal infection;60 however, in one study it was found that it was the gut and not the skin that appeared to be the primary source of infection.15 In this study, stool colonization was present in all patients with Candida parapsilosis infection and preceded skin colonization. A review of the literature found that the source of candidemia in neutropenic cancer patients tends to be primarily of gastrointestinal origin, rather than cutaneous.38
Early and late central venous catheter removalEvidence for early CVC removal in neutropenic pediatric patients is scarce. In a nested case-control study within a cohort of hospitalized children with candidemia already present, persistent candidemia (>3 days) with a CVC in place was an independent risk factor for disseminated disease.62 In a multivariate analysis, failure to remove the CVC was also an independent risk factor for early mortality among pediatric patients with candidemia. However, in this study, the time point at which catheters were removed was not defined.42 In the same study, it was shown that systematic removal of catheters from pediatric patients with candidemia did not reduce the occurrence of late death (death 8–30 days after candidemia was diagnosed). Based on these studies, the Working Group cannot make a strong recommendation for early CVC removal in all neutropenic children with candidemia. Early removal of CVC is recommended if there is evidence of infection at the catheter site. However, if the CVC is not removed early, later CVC removal is advised under one of the following conditions: if the patient is not improving after 3 days of treatment, if the patient has persistently positive blood cultures (especially if C. parapsilosis is detected), or if the patient has a tunnel or pocket infection.
Recommendations summary for catheter management in neutropenic children:
- 1.
Based on current evidence, the Working Group recommends early removal of CVCs if there is evidence of infection at the catheter site.
- 2.
If the CVC is not removed early, the Working Group recommends late CVC removal if the patient is not improving after 3 days of treatment, if the patient has persistently positive blood cultures, or if the patient has a tunnel or pocket infection.
The most frequent complications of candidemia seen in neutropenic children include secondary localization to the eyes, kidneys, spleen, liver, bones, and heart.9,54 For the management of complications, see the manuscript ‘Recommendations for the management of candidemia in neonates in Latin America’.47
Management of chronic disseminated candidiasis in neutropenic childrenRisk factors for chronic disseminated candidiasisChronic disseminated candidiasis (CDC) can be a complication of candidemia in neutropenic children despite antifungal therapy. In one study, risk factors for CDC in children were defined as persistent positive blood cultures for Candida (>3 days) with a CVC in place (odds ratio [OR] 3.0; 95% CI: 1.2–7.8; p=0.02), and immunosuppression (OR, 2.9; 95% CI: 1.2–7; p=0.02).62 Prolonged neutropenia is the most cited risk factor for CDC.32 Younger patients with acute leukemia are more likely to receive aggressive chemotherapy and, therefore, are more likely to have longer and deeper neutropenic periods, putting them at greater risk of developing CDC.32
Chronic disseminated candidiasis treatment recommendationThe Working Group recommends an echinocandin or L-AmB as initial treatment for CDC in neutropenic children, followed by extended treatment with fluconazole. The optimal duration of primary therapy is not defined. Several months of antifungal therapy are typically recommended for eradication of CDC.27,46,59 The Working Group recommends maintaining treatment as long as images show evidence of active infection. However, a drawback of imaging techniques in the diagnosis of CDC is the inability to visualize fungal lesions during the neutropenic phase owing to the absence of an inflammatory response essential to form the infiltrate.43,50 Repeating the studies after recovery of neutrophil count is recommended. Magnetic resonance imaging (MRI) appears to be superior to CT scanning and ultrasound for the identification of CDC.2,50,51 However, CT scanning and ultrasound are valid options when cost and availability limit the use of MRI.32
If a patient does not respond to treatment (e.g. continuing fever, weight loss, or loss of appetite), the Working Group recommends corticosteroid therapy (CST), prednisone, 1mg/kg/day, for 3 weeks. CST accelerates the recovery from CDC.24 In one study, CST in addition to antifungal therapy was beneficial for CDC-related clinical symptoms and inflammatory response in adults and children. CDC-related clinical symptoms disappeared as early as 1 day after initiation of CST in almost all patients, and inflammatory markers decreased within 1 week.24
Recommendations summary for management of CDC in neutropenic children:
- 1.
The Working Group recommends an echinocandin or L-AmB as initial treatment for CDC in neutropenic children, followed by extended treatment with fluconazole.
- 2.
Treatment should be maintained for as long as images show evidence of active infection.
- 3.
Repeating imaging studies after recovery of neutrophil count is recommended.
- 4.
If a patient does not respond to treatment, CST to accelerate the recovery from CDC is recommended.
There are no randomized controlled trials for the prophylaxis of candidemia in non-neutropenic children. Risk factors can be used as determinants of the need for prophylaxis. However, non-neutropenic children are a very heterogeneous population and there are no well-defined risk factors that can be used to help select a population at higher risk. Based on randomized controlled trials in adults, prophylaxis with fluconazole should be considered in high-risk liver transplant recipients and in those with recurrent gastrointestinal perforations or anastomotic leakages due to recent major abdominal surgery.13
Empiric treatment of invasive candidiasis in non-neutropenic childrenThere are no data regarding empiric treatment of invasive candidiasis in non-neutropenic children. As such, no treatment recommendations can be made. Owing to the lack of evidence, following adult recommendations is currently the only option (see the manuscript ‘Recommendations for the management of candidemia in adults in Latin America’).39
Treatment of acute invasive candidiasis in non-neutropenic childrenThere has been only one randomized clinical trial for the treatment of invasive candidiasis in non-neutropenic children. The study compared L-AmB (3mg/kg/day) with micafungin (2mg/kg/day), and the results showed that they had similar overall treatment success (76% vs. 72%, respectively). Although the incidence of serious adverse events (9.3% vs. 3.8%) and the rate of patients discontinuing therapy because of an adverse event (16.7% vs. 3.8%) were lower in micafungin-treated patients,45 these results indicate that either an echinocandin or L-AmB could be considered the first option for treatment in this setting.
As a second-choice treatment in this setting, the Working Group recommends other lipid formulations of AmB (i.e. ABLC, or AmB colloidal dispersion [ABCD]) or AmB-d and, as a third-choice treatment, fluconazole. De-escalation therapy from an echinocandin to an oral drug is a reasonable approach; however, it is not validated in children. Fluconazole is the first choice for de-escalation, especially in cases in which patients are improving and mycological identification and susceptibility studies indicate that the Candida species present is susceptible to fluconazole.
Recommendations summary for treatment of invasive candidiasis in non-neutropenic children:
- 1.
For the treatment of invasive candidiasis in non-neutropenic children, the following is recommended:
- –
First-choice treatment: an echinocandin or L-AmB.
- –
Second-choice treatment: lipid formulations of AmB or AmB-d.
- –
Third-choice treatment: fluconazole.
- –
- 2.
De-escalation therapy from an echinocandin to an oral drug, although not validated in children, is a reasonable approach.
- –
Fluconazole is recommended as first choice for de-escalation.
- –
The Working Group recommends abdominal imaging, fundoscopy, and echocardiography as patient work-up after candidemia diagnosis in non-neutropenic children.
Duration of treatment in non-neutropenic childrenTreatment in non-neutropenic children should continue for 14 days after the first negative blood culture and until clinical improvement of infection is evident.
Catheter management in non-neutropenic childrenThere is insufficient evidence to make recommendations. See recommendations for neutropenic children.
Management of complications in non-neutropenic childrenEnd-organ damage involving the central nervous system, eyes, heart, bones, kidneys, spleen, and liver can be a complication of candidemia. Treatment recommendations have been made according to the type of complication. See the manuscript ‘Recommendations for the management of candidemia in neonates in Latin America’.47
Conflict of interestsA.L. Colombo has received research grants from Pfizer, MSD, United Medical and Luminex, medical education grants from Pfizer, MSD, United Medical and Astellas. Moreover, he has also been a consultant for MSD, Pfizer and Gilead. J.A. Cortes has received research grants and support to attend educational meetings from Pfizer and MSD. M. Nucci has received research grants from Pfizer and MSD, and has acted as a consultant and speaker for Pfizer, MSD, Astellas and Gilead. F. de Queiroz-Telles has participated in Continuing Education activities in laboratories for Astellas, MSD, Pfizer and United Medical, and in research activities in laboratories for Astellas, MSD and Pfizer. I.N Tiraboschi has been a speaker for Pfizer and Gilead. J. Zurita has been advisory board member and consultant for Pfizer, and has received research grants from Wyeth and MSD for participating in the SMART study.
Editorial support in the form of assistance with the first draft, collating author comments and editorial suggestions to draft versions of this manuscript was provided by Brigitte Teissedre, PhD, of Choice Healthcare Solutions and funded by Pfizer. Responsibility for opinions, conclusions and recommendations lies with the authors.