Calmodulin (CaM) is a ubiquitous Ca2+-binding receptor that modulates diverse target proteins and biological processes in all eukaryotes.1,6,7 CaM-binding proteins have been extensively studied in yeasts, plants and animals, but little is known regarding CaM target proteins in the filamentous fungi.1,6,7 Recently, our studies have shown that CaM plays an important role in the secondary metabolism of Beauveria bassiana (a filamentous fungus) through the regulation of BbKIVR and BbPAL.3,4 In this way, the isolation and characterization of CaM-binding proteins involved in the fungal secondary metabolism will provide novel aspects of the CaM-mediated signaling pathway unique to filamentous fungi.
Binding of CaM to the target protein modulates structural changes in both the CaM and the target protein. In general, CaM binds to calcium ions with high affinity (Kd 10−5–10−6M), particularly by environmental stimuli.1,6,7 CaM can affect a variety of biological activities through the direct binding of many cellular proteins in Ca2+-dependent manner. As the intracellular Ca2+ concentration rises to 10−5M, the four Ca2+ ions bind to CaM and initiate various types of downstream signaling pathways. CaM has four EF-hand motifs that change shape during calcium ion binding.1,6,7 In the absence of calcium, the α-helix of EF-hand motif of CaM is nearly parallel to each other.1,6,7 In this case, CaM also regulates many biological activities through the direct binding with target proteins in a Ca2+-independent manner.
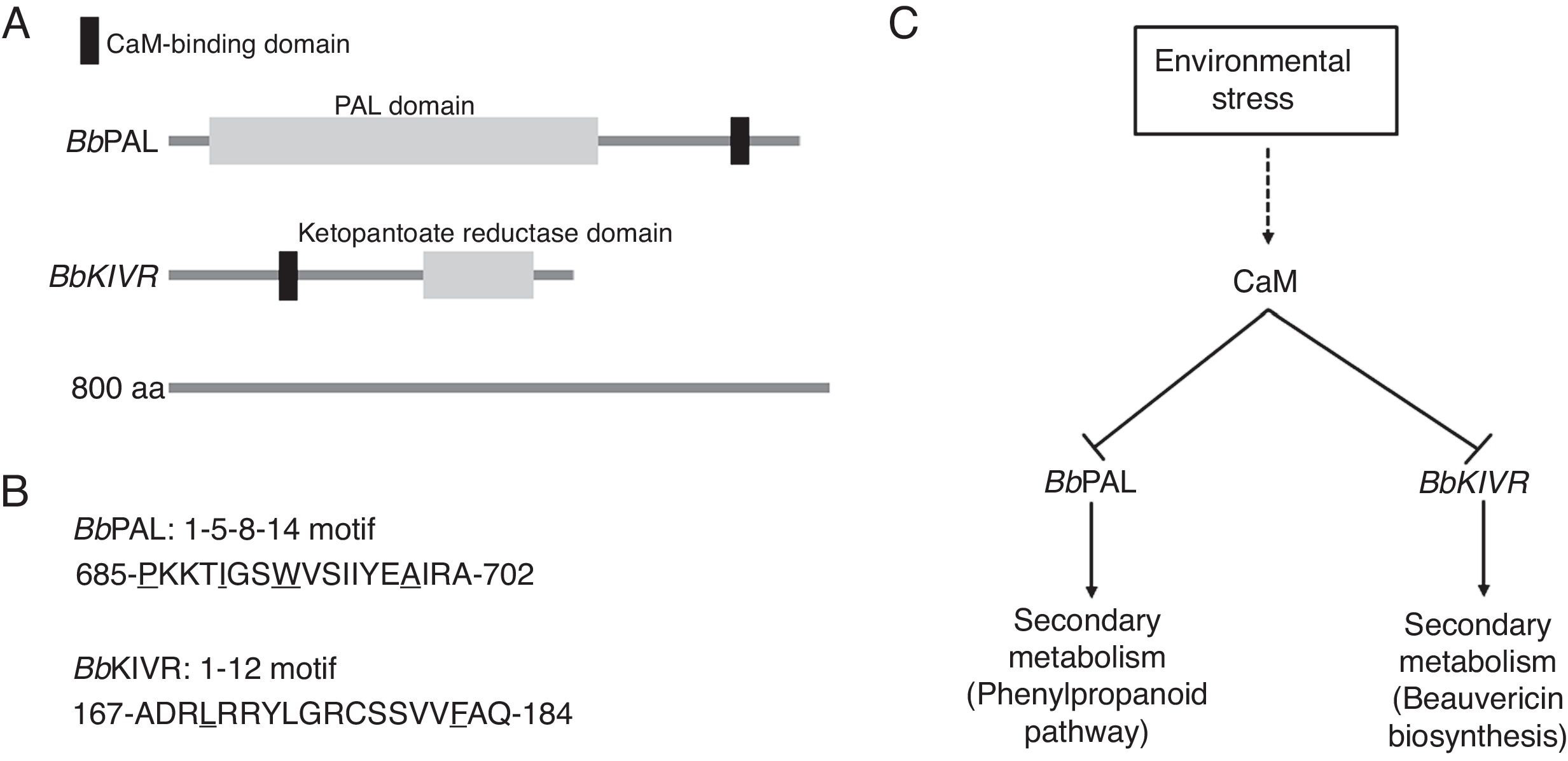
B. bassiana acts as a parasite of various arthropods, causing white muscardine disease. Thus, it belongs to the group of insect pathogens. Beauvericin is a cyclooligomer depsipeptide with many cellular effects.5 Non-ribosomal peptides, such as beauvericin, belong to a broad family of biologically active natural products. Hydroxyisovalerate (Hiv) is a common 2-hydroxycarboxylic acid constituent of depsipeptides such as beauvericin. In B. bassiana, Hiv is formed by the specific reduction of 2-ketoisovalerate (Kiv) by 2-ketoisovalerate reductase (KIVR). We found that BbKIVR interacts with CaM in vitro and in vivo.4 The functional role of CaM-binding to BbKIVR is to suppress BbKIVR activity. Environmental stimuli, such as light and salt stress, inhibit BbKIVR activity. This negative effect on BbKIVR activity caused by light and salt stress is partially reversed by CaM inhibitors (such as W-5 and W-7), suggesting that inhibition of BbKIVR by stress is mediated by the stimulation of CaM. Thus, the KIVR-mediated beauvericin biosynthesis is highly regulated by molecular crosstalk between beauvericin biosynthesis and CaM signaling to respond to light or salt stress.4 Beauvericin is a fungal product with a variety of biological activities such as insecticidal, antimicrobial, antiviral and cytotoxic activities. These useful bioactive activities make beauvericin a potential target for agricultural and pharmaceutical applications. Through the mutation of the CaM binding motif of BbKIVR, using an in vitro mutagenesis method, it will be possible to develop a B. bassiana strain that increases the biosynthesis of beauvericin by preventing the inhibitory effect of CaM. Fungi produce many secondary metabolites that play an important role in a variety of cellular and molecular responses. Many secondary metabolites are being used for agricultural, pharmaceutical and industrial applications. Fungi directly synthesize phenylalanine in the shikimic acid pathway. Phenylalanine is either used directly for protein synthesis or metabolized through the phenylpropanoid pathway. The phenylpropanoid pathway is an essential biosynthetic pathway for the production of a variety of secondary metabolites.3 Phenylalanine ammonia lyase (PAL), the first key enzyme in the phenylpropanoid pathway, catalyzes the deamination of phenylalanine to cinnamic acid.2 There is much information about the structure, expression and function of PAL in plants, but the biological function of fungal PAL has not been established.2 Significantly, our study has showed that BbPAL interacts with CaM in vitro and in vivo, indicating that BbPAL is a novel CaM binding protein in B. bassiana.3 The functional role of CaM in BbPAL action is to inhibit BbPAL activity. High-performance liquid chromatography shows that phenylalanine is reduced and cinnamic acid is increased in response to the CaM inhibitor W-7.3 Darkness inhibits BbPAL activity compared to light. Heat or cold also inhibit BbPAL activity. This negative effect of BbPAL activity is partially reversed by W-7 treatment, suggesting that the stimulation of CaM leads to this inhibitory mechanism. In fact, this study presents a new discovery of the PAL-mediated phenylpropanoid pathway in that BbPAL responds rapidly to environmental stress by regulating crosstalk between the phenylpropanoid pathway and CaM signaling.3 Through the mutation of the CaM binding motif of BbPAL (that implies preventing the inhibitory effect by CaM), it would be possible to produce a B. bassiana strain with an increased production of valuable secondary metabolites (Fig. 1).
Regulation of secondary metabolism by calmodulin signaling in B. bassiana. (A) CaM-binding domains of BbPAL and BbKIVR in B. bassiana. (B) BbPAL contains a 1–5–8–14 motif in the C-terminal region for CaM-binding and BbKIVR has a 1–12 motif in the N-terminal region. (C) Suppression of phenylpropanoid pathway and beauvericin biosynthesis by calmodulin in B. bassiana.
To date, little research has been done on identifying novel CaM target proteins in filamentous fungi. Recently, we have identified and characterized some CaM target proteins in B. bassiana involved in fungal secondary metabolism.3,4 However, the information of CaM-mediated signal transduction pathway is still weak in filamentous fungi compared to other eukaryotes. In the near future, it would be of great help to establish an overall CaM-mediated signaling mechanism in the filamentous fungi.
Conflict of interestThe authors declare that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
This study was partially supported by the Individual Basic Research Support Project, the National Research Foundation of Korea (NRF-2018R1D1A1B07051052).