Mucormycosis is a fungal infection caused by species of the Mucorales order. These microorganisms are angioinvasive, with rapid disease progression and potentially lethal in its rhinocerebral form.
Case reportWe present the case of a 12-year-old female with trisomy 21, acute lymphoblastic leukemia and diabetes, with fever and neutropenia who developed rhinocerebral mucormicosis. After treatment with amphotericin B lipid complex and extensive surgery, disease progressed and posaconazole was added as salvage treatment with full remission of the infection. Four years after diagnosis the patient continues without relapse of mucormycosis or leukemia.
ConclusionsThis case highlights the use of posaconazole as either monotherapy or combined therapy. Although it is still debated, it can be considered an option for salvage treatment in children with non-responding mucormycosis, despite lack of standard dosage in pediatric patients.
La mucormicosis es una infección fúngica causada por especies del orden de los mucorales. Estos microorganismos se caracterizan por ser angioinvasivos, con progresión rápida de la enfermedad y potencialmente letales en la forma rinocerebral.
Caso clínicoPresentamos el caso de una paciente de 12 años de edad con trisomía 21, leucemia linfoblástica aguda, diabetes, fiebre y neutropenia, que desarrolló una mucormicosis rinocerebral. La enfermedad progresó a pesar de recibir tratamiento con anfotericina B complejo lipídico y ser sometida a cirugía extensa. Se añadió posaconazol al tratamiento como terapia de salvamento, lo que llevó a la remisión total del proceso infeccioso. Cuatro años después la paciente continúa sin recaída de la mucormicosis o la leucemia.
ConclusionesEste caso destaca el uso del posaconazol, ya sea como monoterapia o terapia combinada en el tratamiento de la mucormicosis. Si bien aún es debatido su uso, se puede considerar como una opción en el tratamiento de niños con mucormicosis que no responden al tratamiento convencional a pesar de no contar con una dosis pediátrica establecida.
Mucormycosis is a fungal infection caused by species of the Mucorales order, which comprises the genera Mucor, Rhizopus, Rhizomucor, Cunninghamella, Saksenaea, Lichtheimia (Absidia), among others. These microorganisms are angioinvasive, with rapid progression of the disease and potentially lethal in its rhinocerebral form. Treatment includes therapy with extensive surgery and the administration of antifungal drugs.26,27,34
Case presentationA twelve-year old female, with trisomy 21, acute lymphoblastic leukemia-L2 and diabetes secondary to steroids in June 2012, was admitted with febrile neutropenia and hypoglycemia (37mg/dl) six days after second chemotherapy cycle. At physical examination only white lesions on the palate and tongue were observed. The CBC reported neutropenia (20cells/mm3) and thrombocytopenia (40,000cells/mm3). The patient started a treatment with cefepime and deoxycholate amphotericin B. Response was unsatisfactory, and after a week the patient began with increased volume of the left eyelid and superonasal region, accompanied by epiphora with mild crepitus and pain on palpation. A computed axial tomography (CT) showed maxillary sinusitis and swelling of nasal tissue. A sinus-septum fibro endoscopy was performed to debride necrotic tissue. Hyaline, straight, non-septate hyphae were seen on direct exam of the palate lesions. Antifungal therapy was changed to lipid complex amphotericin B (ABCL). The culture reported Rhizopus oryzae, confirmed by molecular biology testing. ITS1-2 regions were amplified and sequenced using the universal primers ITS1/ITS4. The subsequent nucleotide sequence was submitted to GenBank and assigned the accession number MF379466.17,29
The patient presented intermittent hyperglycemia without ketoacidosis during the first 18 days. On day 18th a head CT scan showed frontal lobe lesions, so posaconazole 800mg/day (30mg/kg/day) was added to the treatment. Repeated nasal sinus debridements were made without any mutilating surgery according to the medical-team and the family decision. On day 39th a SPECT-CT of orbit and skull showed more destruction of the skull and frontal lobe. Given the progression and extension of the damage and the need of mutilating surgery, the case was discussed in a multidisciplinary Grand Round with the Bioethics Committee. In conclusion, and according to the medical team criteria and family will, the patient continued only with medical treatment. Patient was discharged with oral posaconazole as compassionate treatment, local lavage, no-mutilating debridement and low-intensity chemotherapy to prevent leukemia relapse.
Unexpectedly, after five months of treatment with posaconazole, a new lavage showed healthy nasal and maxillary sinus mucosa. Posaconazole was discontinued after completing 6 months of treatment and the oncology department reinitiated regular doses of chemotherapy. The patient continues without relapse of mucormycosis or leukemia after four years of diagnosis.
DiscussionMortality from invasive fungal infections has decreased considerably due to the development of new diagnostic and therapeutic tools. However, mortality from mucormycosis has remained unchanged over 40% despite aggressive surgical treatment and antifungal polyenes.27,34 Roden et al. reported a 3% survival without treatment and 70% with medical and surgical treatment; according to their findings survival may depend on comorbidities and the site of infection, being cancer and diabetic patients the most affected.27 Other determinants of survival are early diagnosis, immune status, adequate surgical treatment and early start of a suitable antifungal treatment. At present, the first-line of antifungal treatment is amphotericin B in its different formulations, with a survival rate of 61% for amphotericin B deoxycholate and 69% for lipid formulations,27 with the lipid formulations of amphotericin B being preferable due to the adverse events associated with the deoxycholate formulation. A delay in the treatment with amphotericin B for more than 6 days results in twice the mortality.25 However, the use of amphotericin B is limited by its adverse effects, particularly renal toxicity. Recently, the FDA approved isavuconazole as first line treatment for mucormycosis in adults, with response rates of 31.6% for primary therapy and 36.4% for salvage therapy.2,4
Posaconazole has been used as salvage therapy, either as monotherapy or combined therapy. It is a second-generation triazole for oral administration7,25 that has a broad spectrum activity against Candida, Aspergillus, and mucorales including Mucor and Rhizopus.11 Usually it is well-tolerated, with mild gastrointestinal adverse effects. Currently posaconazole is approved for the prophylaxis and treatment of some invasive fungal infections in adults and children older than 13 years.14 The role of combined therapy with posaconazole is still debated due to conflicting results for in vitro and animal model trials.16 In adults, effectiveness of posaconazole in refractory mucormycosis was 62–79%; in children, Lehrnbecher et al. reported a multicenter survey of salvage treatment with posaconazole: seven of the 15 cases reported suffered mucormycosis, of which 71% survived.13,20,39,41 The ECIL-6 guidelines suggest a combination of lipid amphotericin B and posaconazole, a BIII recommendation, as salvage therapy with a response rate of 56%.37 Our patient received a combined treatment during 4 weeks and completed 6 months of antifungal treatment with posaconazole, with excellent response and without side effects or relapse of the fungal infection after four years of continuous monitoring.
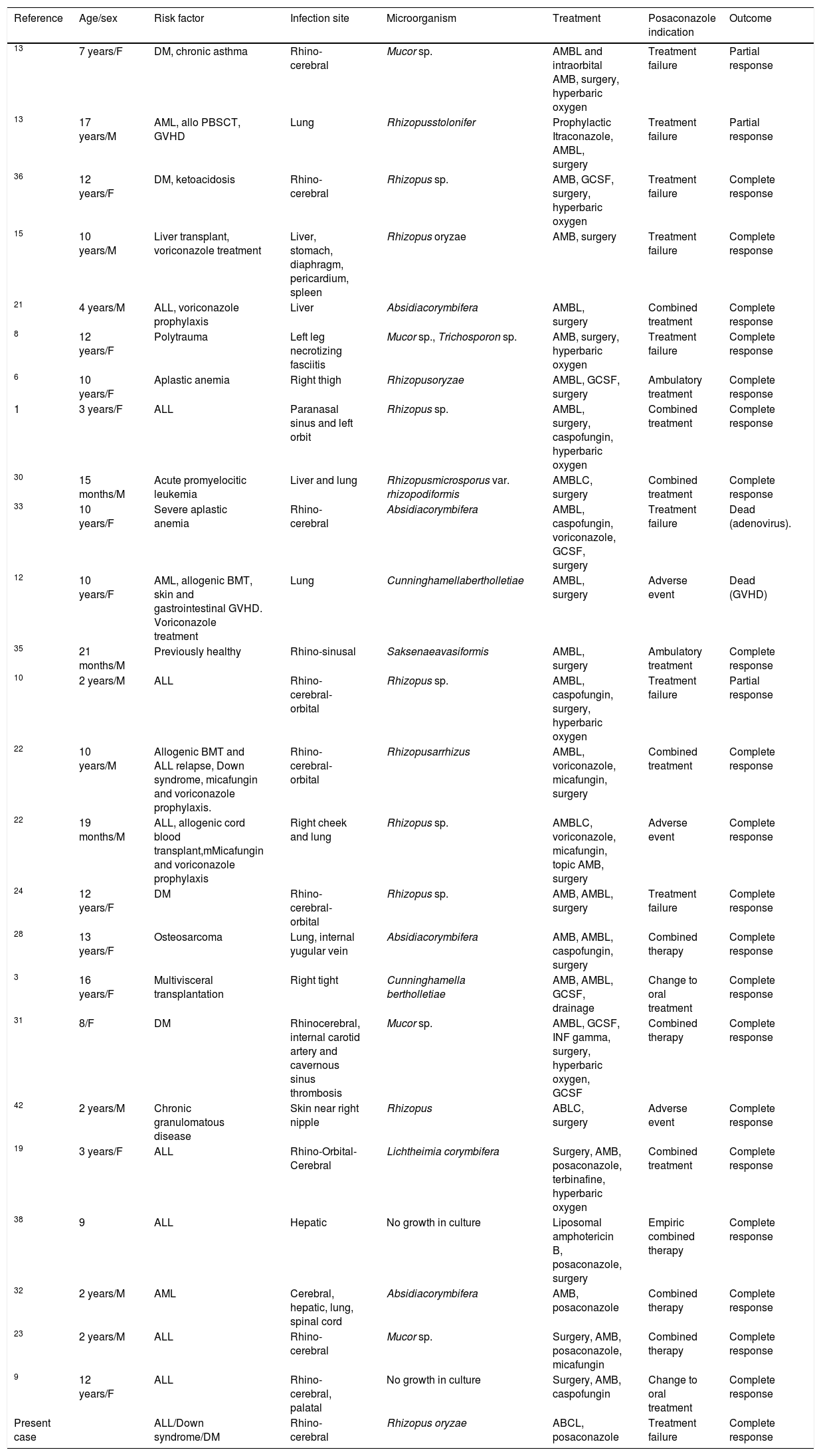
We found 23 reports with 26 children treated with posaconazole for mucormycosis, including this case.1,3,6,8–10,12,13,15,19,21–24,28,30–33,35,36,38,42 Nine of the 21 patients started treatment with posaconazole because of failure of the previous treatment, with partial response and/or cure in 100% of these cases. In 8 cases, posaconazole was added as combined therapy with complete response of all patients (Table 1). The main difficulty of using posaconazole in children is the limited drug information and the lack of reliable posology for children under 13 years. The recommended dose for severe infections in adults is 400mg twice a day. In children under 13 years, studies are still needed to determine the appropriate dosage. Bernardo et al. suggested a dose between 17 and 24mg/kg/day in order to achieve plasma concentrations of 0.7–1.25μg/ml.5 Vanstraelen et al. evaluated the PK of posaconazole in children, recommending in hematological patients younger than 13 years a prophylactic dose, based on the body surface area (BSA), of 120mg/m2 three times a day; this dose is the equivalent of the adult prohylactic dose (200mg three times a day) and an ideal BSA of 1.73m2. The minimum and average concentrations, as well as those in the 24h area under the curve, were similar to those in immunocompromised pediatric patients receiving a therapeutic dose of 400mg two times a day and higher than the adult hematological patients, but lower than healthy adult volunteers.40 Also Jancel et al. described doses between 10 and 49.2mg/kg/day in children with median age of 6.5 years, finding a significant association between normalized posaconazole concentration and the indication (prophylaxis or treatment) and age. An individual's average normalized concentration is expected to decrease by 0.23 (95% CI: 0.04–0.43) ng/ml/mg for every year increase in age (p=0.02). In this study posaconazole concentrations ranged from undetectable to 3620ng/ml, being the target concentration more than 1000ng/ml for a treatment and more than 700ng/ml for prophylaxis.18 However, as posaconazole concentrations may be affected by other factors (oral bioavailability, administration via feeding tube, concomitant medications, type of food), it is highly recommended to routinely monitor drug concentration to reach adequate therapeutic concentrations.5,18,40
Pediatric cases of zigomycosis treated with posaconazole.
| Reference | Age/sex | Risk factor | Infection site | Microorganism | Treatment | Posaconazole indication | Outcome |
|---|---|---|---|---|---|---|---|
| 13 | 7 years/F | DM, chronic asthma | Rhino-cerebral | Mucor sp. | AMBL and intraorbital AMB, surgery, hyperbaric oxygen | Treatment failure | Partial response |
| 13 | 17 years/M | AML, allo PBSCT, GVHD | Lung | Rhizopusstolonifer | Prophylactic Itraconazole, AMBL, surgery | Treatment failure | Partial response |
| 36 | 12 years/F | DM, ketoacidosis | Rhino-cerebral | Rhizopus sp. | AMB, GCSF, surgery, hyperbaric oxygen | Treatment failure | Complete response |
| 15 | 10 years/M | Liver transplant, voriconazole treatment | Liver, stomach, diaphragm, pericardium, spleen | Rhizopus oryzae | AMB, surgery | Treatment failure | Complete response |
| 21 | 4 years/M | ALL, voriconazole prophylaxis | Liver | Absidiacorymbifera | AMBL, surgery | Combined treatment | Complete response |
| 8 | 12 years/F | Polytrauma | Left leg necrotizing fasciitis | Mucor sp., Trichosporon sp. | AMB, surgery, hyperbaric oxygen | Treatment failure | Complete response |
| 6 | 10 years/F | Aplastic anemia | Right thigh | Rhizopusoryzae | AMBL, GCSF, surgery | Ambulatory treatment | Complete response |
| 1 | 3 years/F | ALL | Paranasal sinus and left orbit | Rhizopus sp. | AMBL, surgery, caspofungin, hyperbaric oxygen | Combined treatment | Complete response |
| 30 | 15 months/M | Acute promyelocitic leukemia | Liver and lung | Rhizopusmicrosporus var. rhizopodiformis | AMBLC, surgery | Combined treatment | Complete response |
| 33 | 10 years/F | Severe aplastic anemia | Rhino-cerebral | Absidiacorymbifera | AMBL, caspofungin, voriconazole, GCSF, surgery | Treatment failure | Dead (adenovirus). |
| 12 | 10 years/F | AML, allogenic BMT, skin and gastrointestinal GVHD. Voriconazole treatment | Lung | Cunninghamellabertholletiae | AMBL, surgery | Adverse event | Dead (GVHD) |
| 35 | 21 months/M | Previously healthy | Rhino-sinusal | Saksenaeavasiformis | AMBL, surgery | Ambulatory treatment | Complete response |
| 10 | 2 years/M | ALL | Rhino-cerebral-orbital | Rhizopus sp. | AMBL, caspofungin, surgery, hyperbaric oxygen | Treatment failure | Partial response |
| 22 | 10 years/M | Allogenic BMT and ALL relapse, Down syndrome, micafungin and voriconazole prophylaxis. | Rhino-cerebral-orbital | Rhizopusarrhizus | AMBL, voriconazole, micafungin, surgery | Combined treatment | Complete response |
| 22 | 19 months/M | ALL, allogenic cord blood transplant,mMicafungin and voriconazole prophylaxis | Right cheek and lung | Rhizopus sp. | AMBLC, voriconazole, micafungin, topic AMB, surgery | Adverse event | Complete response |
| 24 | 12 years/F | DM | Rhino-cerebral-orbital | Rhizopus sp. | AMB, AMBL, surgery | Treatment failure | Complete response |
| 28 | 13 years/F | Osteosarcoma | Lung, internal yugular vein | Absidiacorymbifera | AMB, AMBL, caspofungin, surgery | Combined therapy | Complete response |
| 3 | 16 years/F | Multivisceral transplantation | Right tight | Cunninghamella bertholletiae | AMB, AMBL, GCSF, drainage | Change to oral treatment | Complete response |
| 31 | 8/F | DM | Rhinocerebral, internal carotid artery and cavernous sinus thrombosis | Mucor sp. | AMBL, GCSF, INF gamma, surgery, hyperbaric oxygen, GCSF | Combined therapy | Complete response |
| 42 | 2 years/M | Chronic granulomatous disease | Skin near right nipple | Rhizopus | ABLC, surgery | Adverse event | Complete response |
| 19 | 3 years/F | ALL | Rhino-Orbital-Cerebral | Lichtheimia corymbifera | Surgery, AMB, posaconazole, terbinafine, hyperbaric oxygen | Combined treatment | Complete response |
| 38 | 9 | ALL | Hepatic | No growth in culture | Liposomal amphotericin B, posaconazole, surgery | Empiric combined therapy | Complete response |
| 32 | 2 years/M | AML | Cerebral, hepatic, lung, spinal cord | Absidiacorymbifera | AMB, posaconazole | Combined therapy | Complete response |
| 23 | 2 years/M | ALL | Rhino-cerebral | Mucor sp. | Surgery, AMB, posaconazole, micafungin | Combined therapy | Complete response |
| 9 | 12 years/F | ALL | Rhino-cerebral, palatal | No growth in culture | Surgery, AMB, caspofungin | Change to oral treatment | Complete response |
| Present case | ALL/Down syndrome/DM | Rhino-cerebral | Rhizopus oryzae | ABCL, posaconazole | Treatment failure | Complete response |
DM: diabetes mellitus, AML: acute myeloid leukemia, GVHD: graft versus host disease, ALL: acute lymphoblastic leukemia AMB: amphotericine B deoxycholate, AMBL: liposomal amphotericine B, AMBLC: amphotericin B lipid complex GCSF: granulocyte colony stimulating factor, allo-PBSCT: allogenic peripheral blood stem cell transplant, BMT: bone marrow transplant, INF gamma: interferon gamma.
In our case, posaconazole was prescribed in a dose of 400mg twice a day (30mg/kg/day) because we could not monitor its concentration in the plasma. Unfortunately, although developing countries have availability to novel drugs, they lack the needed infrastructure for monitoring plasmatic concentration, therefore it will be ideal to have more information on the posology of posaconazole. In conclusion, posaconazole can be considered as an option for salvage treatment in children with non-responding mucormycosis, with a partial or complete response in 62–80% of adults and nearly 100% of children with mild side effects even during long-term treatment.