Exposure of the nematophagous fungus Pochonia chlamydosporia to solar radiation and elevated temperatures before being incorporated into the soil can reduce its survival and efficiency as biocontrol agent.
AimsA field experiment was carried out to assess the effect of the exposure period on the viability of P. chlamydosporia applied on the soil surface.
MethodsA commercial bionematicide based on P. chlamydosporia was sprayed on soil, and soil samples were collected before and at 0, 30, 60, 90, 120, and 150min after fungal application. Relative humidity (RH), the irradiance (IR), air temperature (AT), and soil temperature (ST) were recorded. The number of P. chlamydosporia colony forming units (CFUs) was evaluated after 20 days of incubation.
ResultsP. chlamydosporia survival decreased over the time of exposure on the soil surface. Overall, the number of CFUs decreased by more than 90% at 150min after application. Exposure to RH ≥61%, ST and AT between 25–35°C and 19–29°C, and IR between 1172 and 2126μmol of photons m−2s−1 induced a negative exponential effect on the survival of the fungus over the time.
ConclusionsExposure to climatic conditions on the soil surface reduces P. chlamydosporia viability.
La exposición del hongo nematófago Pochonia chlamydosporia a la radiación solar y la temperatura elevada antes de ser incorporado al suelo puede reducir su supervivencia y eficiencia como agente de biocontrol.
ObjetivosSe realizó un experimento de campo para evaluar el efecto del período de exposición a condiciones ambientales sobre la viabilidad de P. chlamydosporia en la superficie del suelo.
MétodosSe pulverizó sobre el suelo un bionematicida comercial hecho a base de P. chlamydosporia y se recogieron muestras de suelo antes y después de 0, 30, 60, 90, 120 y 150min tras la aplicación del hongo. Se registraron la humedad relativa (HR), la irradiación (IR), la temperatura del aire (TA) y la temperatura del suelo (TS). Se evaluó el número de unidades formadoras de colonias (UFC) de P. chlamydosporia después de 20 días de incubación.
ResultadosLa supervivencia de P. chlamydosporia disminuyó durante el tiempo de exposición en la superficie del suelo. En general, el número de UFC disminuyó en más de un 90% a los 150min después de la aplicación. La exposición a HR≥61%, TS y TA entre 25-35°C y 19-29°C, e IR entre 1.172 y 2.126μmol de fotones m−2 s−1 indujo un efecto exponencial negativo en la supervivencia del hongo.
ConclusionesLa exposición a las condiciones climáticas en la superficie del suelo reduce la viabilidad de P. chlamydosporia.
Pochonia chlamydosporia parasitizes phytonematodes.12,23 It produces chlamydospores that enhance its establishment and survival in the soil.12 Incorporating chlamydospores into the soil increases the probability of nematode egg parasitism and protects them from adverse surface environmental conditions.3 In some cases, however, P. chlamydosporia-based bionematicides are applied on the soil surface3 and knowledge is scarce about the effect on this fungus of high temperatures and solar radiation.
We hypothesize that temperatures higher than the optimal range of 24–28°C1 and extended exposure to ultraviolet radiation reduce P. chlamydosporia viability, as reported for other fungi,4,15,18 despite producing chlamydospores. We assessed P. chlamydosporia viability after exposure on the soil surface for 0, 30, 60, 90, 120, and 150min.
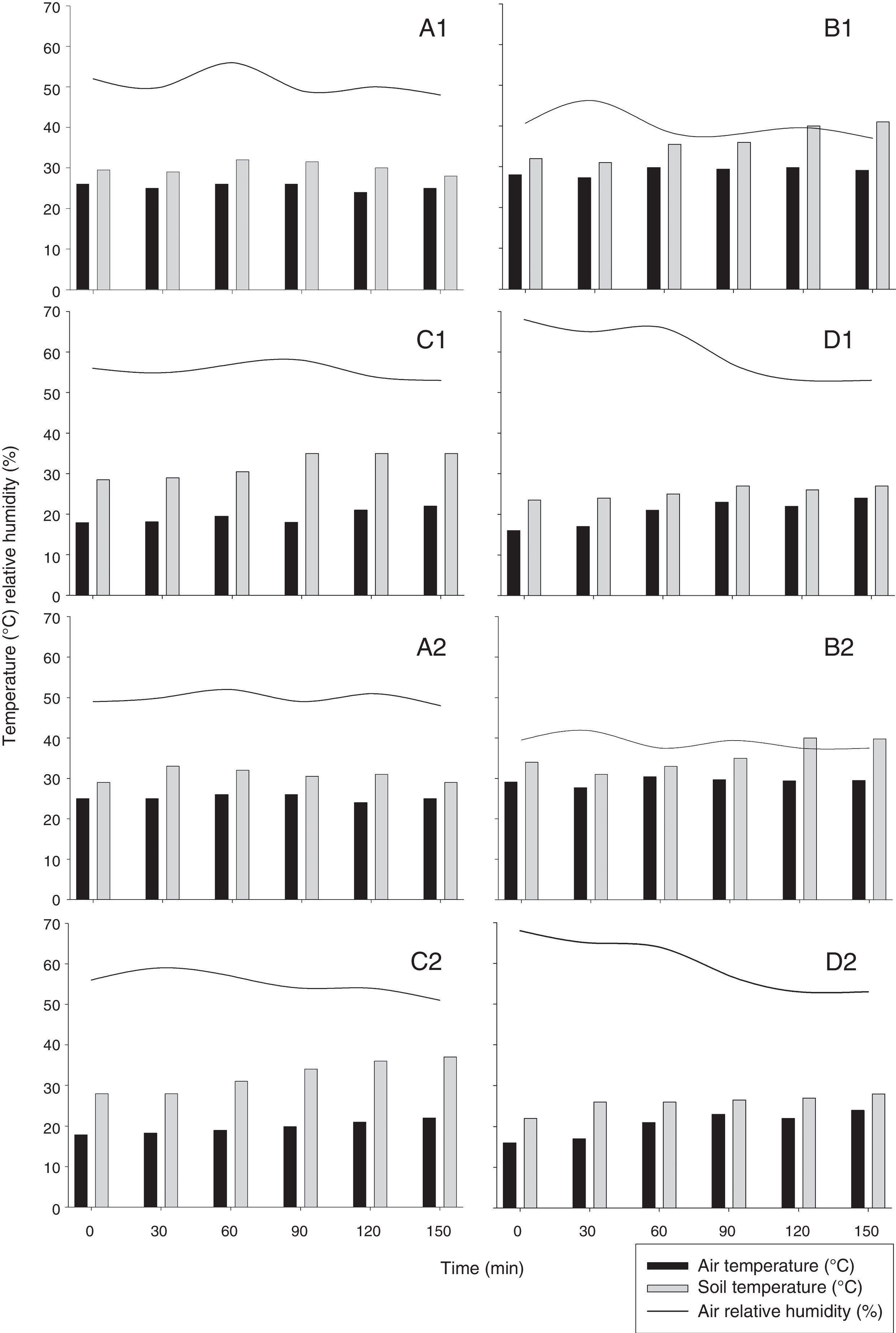
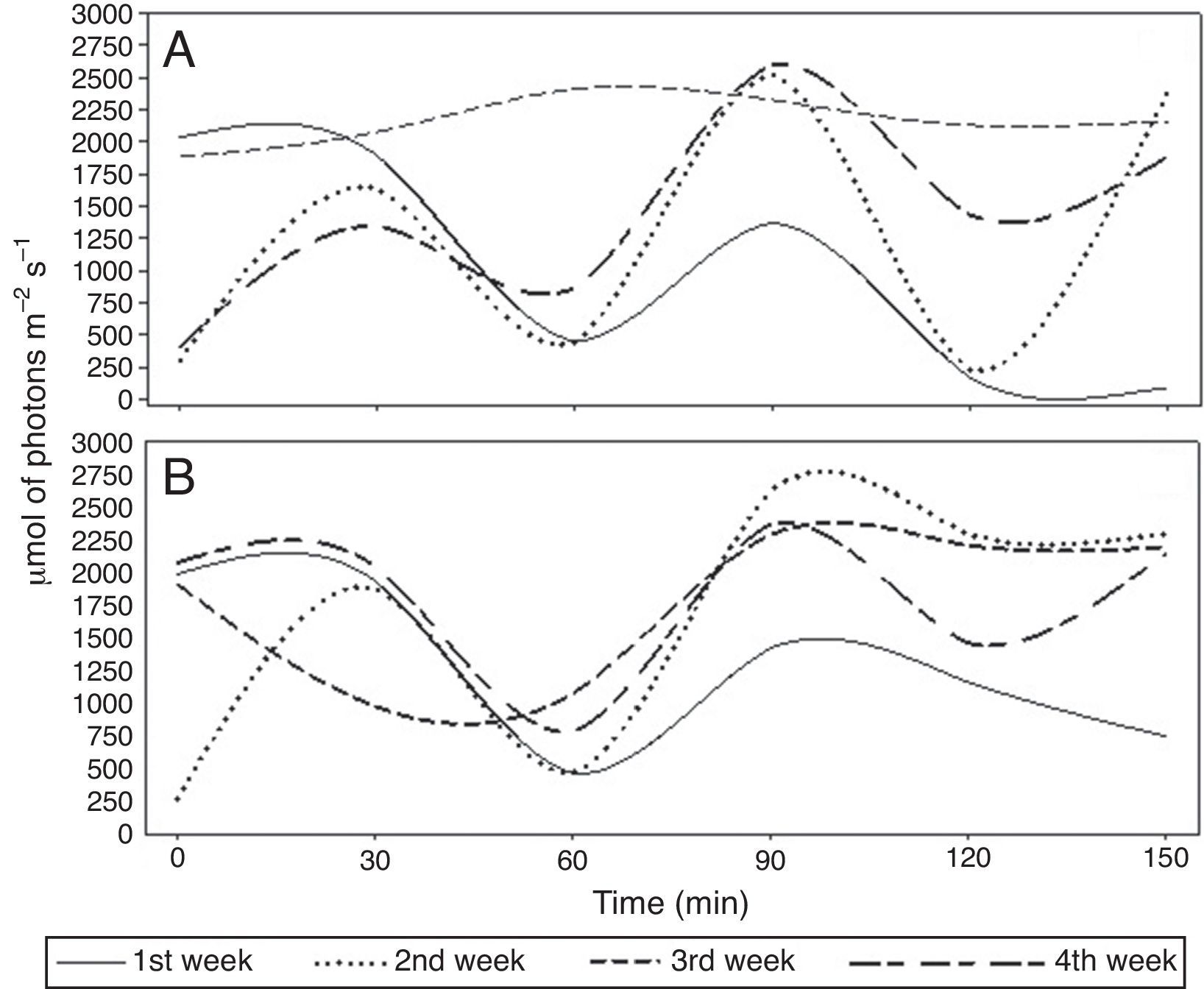
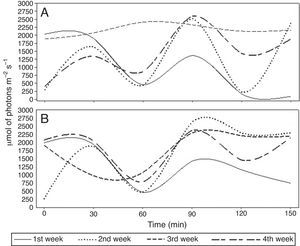
We carried out a field experiment at the UFV-CRP. P. chlamydosporia isolate Pc-10 was used as a wettable powder bionematicide (Rizotec®, Rizoflora Biotecnologia, 3×108 viable chlamydospores/g). We diluted and applied Pc-10 on raised beds (1.6m wide and 0.30 high) on the soil surface using a backpack sprayer pressurized with CO2, with spray volume of 300l/ha. We applied the bionematicide between 01:00 and 03:30 pm. We collected soil samples before and at 0, 30, 60, 90, 120, and 150min after application. We collected five soil samples per plot (2.5m length and 1.6m wide) with 50mm diameter PVC pipe inserted 5cm deep in the soil. We bulked the samples to form a composite sample and stored them at 4°C until use. We recorded relative humidity (RH, %), air temperature (AT, °C), soil temperature (ST, °C) (Fig. 1) and irradiance (IR, μmol of photons m−2s−1) (Fig. 2) at the time of soil sampling.
Air and soil temperature (°C) and relative humidity (%) at sampling time after the application of Pochonia chlamydosporia var. chlamydosporia isolate Pc-10 on the soil surface. Replica 1: 1st week (A1); 2nd week (B1); 3rd week (C1); 4th week (D1). Replica 2: 1st week (A2); 2nd week (B2); 3rd week (C2); 4th week (D2).
We repeated the applications for four weeks. The experiment was performed twice simultaneously in different locations of the field, using similar protocols. Experimental replicas will hereafter be referred to as replicas 1 and 2. We used a randomized block design with seven periods after P. chlamydosporia application and four weeks of evaluations (blocks). In both the replicas, average RH, AT, ST and IR ranged from 39.14 to 61.28%, 19.21 to 29.17°C, 25.07 to 35.36°C and 1172 to 2126μmol of photonsm−2s−1, respectively (Figs. 1 and 2). We assessed RH, IR, and AT using infrared gas analyzer (IRGA Li-Cor 6400 XT with integrated fluorescence) and recorded ST using thermometers buried 5m deep into the soil. Average soil moisture (SM), determined by the gravimetric method,2 was 16.4, 6.3, 19.2 and 20% at weeks 1, 2, 3, and 4, for both replicas. We assessed the viability of P. chlamydosporia by using colony-forming units (CFUs) assay,9 plating soil suspension onto semi-selective medium.8
We tested data for normality (Kolmogorov–Smirnov test) and homoscedasticity (Bartlett test). Regression analysis (p<0.05) was used to study the effect of P. chlamydosporia exposure periods on the soil over the number of CFUs. We used R version 3.1.1 for statistical analyses.16
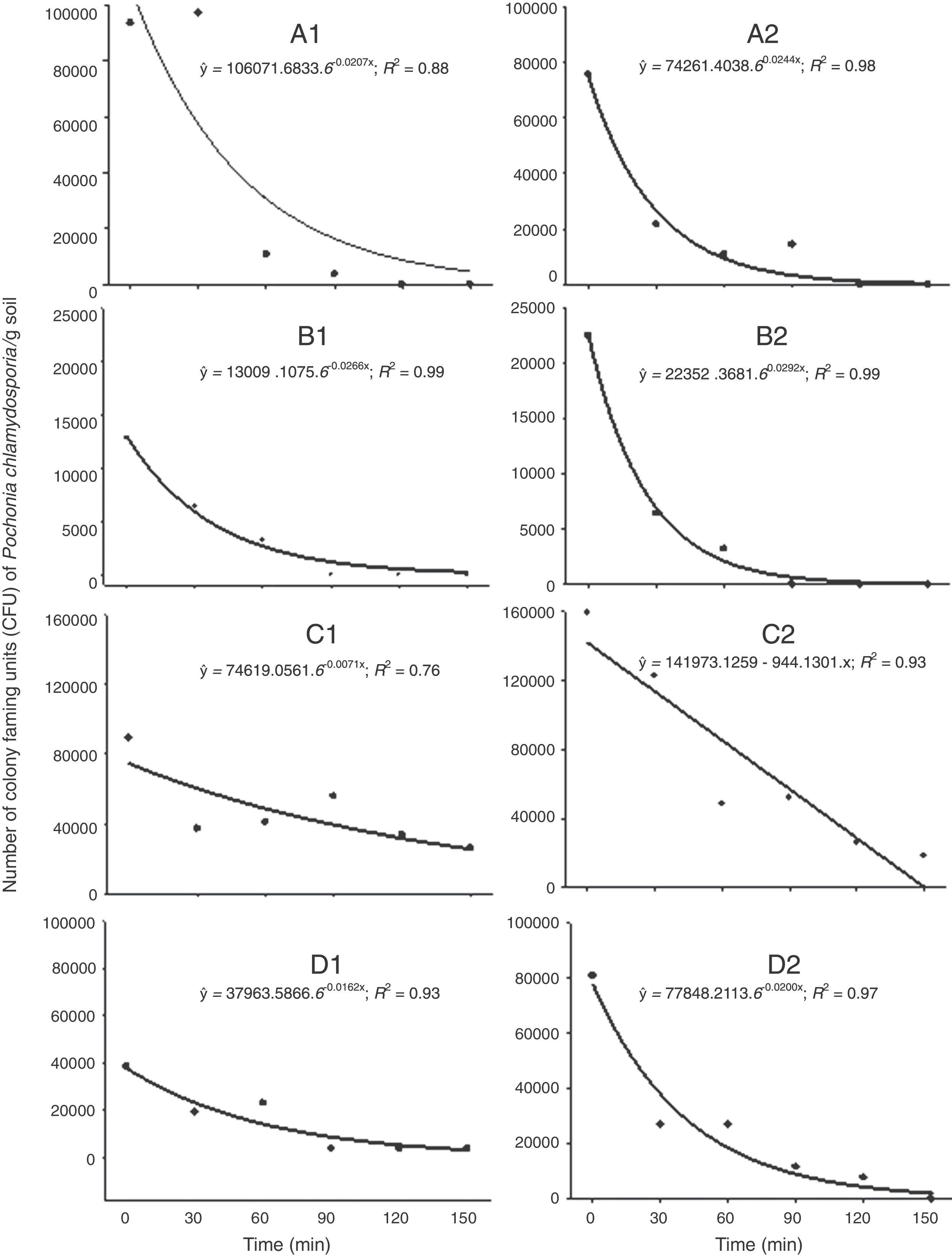
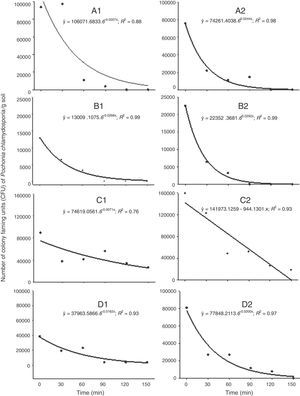
P. chlamydosporia survival was reduced exponentially or linearly over the time of exposure on the soil surface (Fig. 3). We did not recover any P. chlamydosporia colony from samples collected before P. chlamydosporia application. Therefore, CFUs recovered in the experiment were not due to the presence of native isolates. The number of P. chlamydosporia CFUs decreased by more than 90% at 150min after application, except in the third week after P. chlamydosporia surface soil application in the replica 1 (Fig. 3 C1). In the first week, the number of CFUs decrease rate (DR) was approximately 46% and 64% at every 30min of exposure in replicas 1 and 2 (Figs. 3 A1 and A2). RH, ST, AT and IR ranged 50–50.8%, 30–30.6°C, 25–25.1°C, and 1172–1378μmol of photons m−2s−1 (Figs. 1 and 2).
Number of colony forming units (CFU/g of soil) of Pochonia chlamydosporia var. chlamydosporia isolate Pc-10 recovered from soil samples collected at different periods after the application of the fungus on the surface of the seed beds. Replica 1: 1st week (A1); 2nd week (B1); 3rd week (C1); 4th week (D1). Replica 2: 1st week (A2); 2nd week (B2); 3rd week (C2); 4th week (D2).
P. chlamydosporia viability was reduced by 55 and 69% at every 30min in the second week (Figs. 3 B1 and B2), when RH, ST, AT and IR ranged 39.1–40.5%, 35.2–35.4°C, 28.8–29.2°C, and 1630–1638μmol of photons m−2s−1. Fungus survival was higher in the third week, especially in replica 1 (Fig. 3 C1), with 19% DR at every 30min. RH, ST, AT and IR ranged 54.2–55.1%, 31.4–31.9°C, 19.2–19.3°C, and 1593–2126μmol of photons m−2s−1 (Figs. 1 and 2). In the fourth week, P. chlamydosporia viability decreased by 38 and 51% at every 30min of exposure (Figs. 3 D1 and D2).
ST and AT between 25–35°C and 19–29°C, IR between 1172 and 2126μmol of photons m−2s−1 and RH ≥61% induced a negative exponential effect on P. chlamydosporia survival over time, which may reduce its ability to control nematodes.
High and low temperatures limit the survival and growth of soil fungi, especially <5°C and >30°C.22P. chlamydosporia optimal range is 24–28°C,1 although temperature tolerance may vary according to the fungus strain origin. AT in the first two weeks was around 25°C, reaching 30°C, whereas ST was around 30°C and reached 40°C. A suitable P. chlamydosporia temperature range (20–30°C) allowed higher survival rate in the third and fourth weeks, especially until 60min after application. Low water availability (LWA) in the soil also reduces P. chlamydosporia survival.19 SM and RH in the first two weeks ranged 6.3–16.3% and 40–57%, whereas they ranged 19.2–20% and 55–69% in the third and fourth weeks.
Acting synergistically with high temperatures and LWA, the cumulative effect of the exposure to solar radiation reduced P. chlamydosporia viability, as reported for other biocontrol agents.4,11,13,14 UV-A and UV-B rays cause genetic and morphological damage in fungi,17,20 reducing the longevity of the propagules.6,7,14 Melanin protects fungi against radiation.10,21P. chlamydosporia chlamydospores do not contain melanin,24 making them more vulnerable to radiation. IR levels were always above 1000μmol of photons m−2s−1 at 30min of exposure and reached 2080μmol of photonsm−2s−1 in the third week of replica 1. To enhance nematode control, P. chlamydosporia-based bionematicides must be incorporated into the soil3 or the formulation must have compounds for protecting the fungus from adverse environmental conditions,5 such as high temperatures (>25°C) and high irradiance (>1000μmol of photons m−2s−1).
Conflict of interestThe authors have no conflict of interest to declare.
E.A. Lopes thanks CNPq for grant support (Proc. 304663/2014-0) and FAPEMIG for the financial support for the research (APQ-00538-11).