The wastes of pecan nut (Carya illinoinensis (Wangenh.) K. Koch) production are increasing worldwide and have high concentrations of tannins and phenols.
AimsTo study the biodegradation of lignocellulosic wastes of pecan used as solid substrate for the cultivation of the white-rot fungus Ganoderma lucidum (Curtis) P. Karst.
MethodsSix formulations of pecan wastes were used as solid substrate: pecan shells (PS100), pecan pericarp (PP100), pecan wood-chips (PB100), and the combinations PS50+PP50, PB50+PS50 and PB50+PP50. The substrates were inoculated with a wild strain of G. lucidum collected in the Iberian Peninsula. The biodegradation capability of G. lucidum was estimated by using the mycelial growth rate, the biological efficiency, the production and the dry biological efficiency.
ResultsNotably, all solid substrates were suitable for G. lucidum growth and mushroom yield. The best performance in mushroom yield was obtained with PB100 (55.4% BE), followed by PB50+PP50 (31.7% BE) and PB50+PS50 (25.4% BE). The mushroom yield in the substrates containing pecan wood-chips (PB) was significantly higher.
ConclusionsOur study is leading the way in attempting the cultivation of G. lucidum on lignocellulosic pecan waste. These results show an environmentally friendly alternative that increases the benefits for the global pecan industry, especially in rural areas, and transforms biomass into mushrooms with nutraceutical properties and biotechnological applications.
Los residuos de la producción de pacana (Carya illinoinensis [Wangenh.] K. Koch) se distribuyen por todo el mundo y poseen elevadas concentraciones de taninos y fenoles.
ObjetivosEstudiar la biodegradación de los residuos lignocelulósicos de la pacana usados como sustrato sólido para el cultivo de Ganoderma lucidum (Curtis) P. Karst.
MétodosSe utilizaron seis formulaciones de sustratos sólidos a partir de los residuos: cáscara de la nuez (PS100), pericarpio de la nuez (PP100), astillas de ramas de poda (PB100) y las combinaciones PS50+PP50, PB50+PS50 y PB50+PP50. Los sustratos se inocularon con las hifas de una cepa silvestre de G. lucidum procedente de la península ibérica. La capacidad de biodegradación de G. lucidum se estimó mediante el ratio de crecimiento micelial, la eficiencia biológica, la producción de carpóforos y la eficiencia biológica en seco.
ResultadosNotablemente, todos los sustratos sólidos utilizados resultaron adecuados para ser colonizados por G. lucidum y producir carpóforos. Los mejores rendimientos en cultivo se obtuvieron con la formulación PB100 (55,4% BE), seguida por PB50+PP50 (31,7% BE) y PB50+PS50 (25,4% BE). La producción de carpóforos en sustratos con astillas de ramas del árbol (PB) fue considerablemente más elevada que en aquellos que no contenían este residuo.
ConclusionesEste estudio muestra la posibilidad de cultivar G. lucidum sobre residuos lignocelulósicos de pacana. Los resultados obtenidos sugieren una alternativa respetuosa con el medio ambiente para el incremento de los beneficios en la industria de la pacana a nivel internacional, especialmente en zonas rurales, al convertir biomasa en la producción de un hongo de interés nutracéutico y con aplicaciones biotecnológicas.
The cultivation of Carya illinoinensis (Wangenh.) K. Koch is increasing worldwide (i.e. United States, Mexico, Australia, South Africa, Brazil, Argentina, Chile, Egypt, Israel, Spain, etc.).30,41 Pecan nut production is valued at over USD 433 million in the United States (USDA, 2008). In 2012, the worldwide production of nuts rose to 7 million tons, of which 187,000 tons were produced in Europe and 13% were produced in the Iberian Peninsula.10 The direct disposal of waste from pecan nut yields is often neglected,38 which represents an important loss of biomass and it is a major cause of environmental pollution. In fact, the pecan nutshell processing generates an important amount of waste in the form of shells (40–50%).30,35 The waste products of pecan are rich carbon sources1 and have high concentrations of tannins and phenols.7,23 The composition of lignocellulosic wastes of pecan, in terms of percentage of cellulose, hemicellulose, lignin and ash in the shells, are 5.6, 3.8, 70 and 5.85%, respectively,1 in the pericarp are 30, 26, 41 and 1.7%,13 and in the pecan branches are 38.7, 30.2, 23.3 and 0.4%, respectively.28
The ability of Ganoderma lucidum to decompose lignocellulosic wastes has been extensively studied.18,45 So far, there have been no studies to find out whether these white-rot fungi (WRF) can utilize lignocellulosic pecan waste. The mushroom G. lucidum, known as reishi (in Japan) and lingzhi (in China), has been used for centuries in oriental traditional medicine and it is one of the most important and widely distributed WRF in the world. G. lucidum has been linked to nutraceutical mushrooms. In clinical trials with G. lucidum, hepatoprotective efficacy,5 control in type 2 diabetes42 and protection against lung cancer37 were observed. Additionally, biotechnological applications of the fungus, for example as a producer of enzymes that decolorize the synthetic dyes from effluents,16,24 or in oleo-chemical and biotechnological industries for producing lipase,3 have been proposed. Moreover, it has been studied its potential for environmental decontamination of heavy metals,39 and for the biological pre-treatment of lignocellulose for bioenergy production.20,45
In Spain, pecan cultivation has been successfully expanded over the last two decades, particularly in Valle del Guadalhorce (Malaga). In this work, we tested the capability of G. lucidum to use lignocellulosic wastes of pecan.
Materials and methodsG. lucidum strain isolationThe fruiting body of a G. lucidum (Curtis) P. Karst specimen was collected from an evergreen oak (Quercus ilex subsp. ballota) forest in Robledo de Chavela (Madrid, Spain). The fruiting body was cultured in potato dextrose agar (PDA), at 25°C in a culture chamber according to Postemsky et al.31 The wild strain was first morphologically and anatomically characterized, as described in Nithya et al.25 Secondly, the molecular analysis of the fruiting body by rDNA-ITS was obtained according to Zheng et al.46 The fungal DNA was extracted and amplified by polymerase chain reaction (PCR) of the ITS region. The obtained nucleotide sequence was compared with those of GenBank, using the NCBI BLAST program. DNA sequences were aligned by MAFFT 7 using the default settings and manually optimized with BioEdit version 7.2.3. A phylogenetic analysis was carried out in PAUP version 4.0b 10. The specimen was identified through the sequence as G. lucidum (accession number: KT805317) (Appendix A). The cultures of G. lucidum were kept at 4°C in the dark.20
The fungal radial growth (mm) in pure culture (n=10) was registered according to Imtiaj et al.15 to compare with commercial strains. Pure samples of G. lucidum cultures were kept on PDA in Petri dishes.
Spawn productionThe spawn was produced with a mixture of grains of Triticum durum (59.1%, w/w), distilled water (40%, w/w), CaCO3 (0.1%, w/w) to balance the pH, and CaSO4 (0.8%, w/w) for the texture, according to Curvetto et al.6 Two mycelial plugs of G. lucidum were inoculated. The spawn was produced after 10 days (0.25 l Erlenmeyer flasks at 25°C in the dark).
Pecan waste preparationThe wastes of C. illinoinensis were obtained from an organic certified pecan plantation in Valle del Guadalhorce (Malaga, Spain). The pecan wastes were separated into three categories: (i) shells (PS), (ii) pericarp (PP) and (iii) branches (PB). First, the lignocellulosic wastes were dried at 35°C for 3 days. Once dried, PP and PS were pressed, and PB was chopped into wood-chips. Then PS, PP and PB were sieved to obtain 2.5 and 5mm particles. Pecan wastes were prepared according to Royse and Sanchez-Vazquez32 and Lakshmi.36 Finally, the pecan wastes PS, PP and PB were washed and hydrated with distilled water for 24h. The relative humidity was adjusted between 60 and 70%.29 In this study, the wastes were PS 100% (PS100), PP 100% (PP100), PB 100% (PB100), and the paired formulations PS50+PP50, PB50+PS50 and PB50+PP50. All substrate formulations were supplemented with CaCO3 1% (w/w, dry matter) according to Yang et al.,43 Erkel8,9 and Manavalan et al.20
Experimental designThe experiment was carried out in glass tubes 20cm long and 1.6cm in diameter.31 Tubes were filled with the different substrates to a height of 13cm from the bottom (Fig. 1a). Nine replicates were prepared for each of the six substrates (eighteen tubes×3) and sterilized in an autoclave for 45min at 121°C.18,43 The glass tubes were inoculated at the top of the substrate with spawn at 5% (w/w) and were sealed with Parafilm M®. Colonization of the glass tubes with the different substrates took place in a chamber at 25±1°C. Mycelial growth rate (mm day−1) was measured by marking the advancing fronts at intervals on the tubes, using the same method as for recording fungal radial growth on Petri dishes. To induce the formation of sporophore primordia, the tubes containing the mycelium were subjected to a cold shock for 2 days at 4±1°C.29,44 Then they were placed in the growth chamber at 25°C, with 85% relative humidity, and 10-h/day of light (fluorescent lamps; 500–1000lux). A gap of 50mm was left between the substrate and the Parafilm M® until the period of fruit body formation to promote gas exchange and the proper antler development. When the primordia appeared, the upper film was perforated and, once developed, the film was completely withdrawn.
Lignocellulosic wastes of pecan studied for cultivating Ganoderma lucidum. (a) The six formulations include shells (PS100), pericarp (PP100), wood-chips of branches (PB100), and paired formulations of shells and pericarp (PS50+PP50), shells and wood-chips of branches (PP50+PS50) and pericarp and wood-chips of branches (PB50+PP50). (b) Fructification on the six formulations.
The growth of G. lucidum was measured by estimating the biological efficiency, the production, and the dry biological efficiency. Biological efficiency32 (BE) is defined as the weight of fresh mushrooms×100/weight of dry substrate. Production6 (P) is defined as the weight of fresh mushrooms×100/weight of wet substrate. Dry biological efficiency36 (DBE) is defined as the weight of dry mushrooms×100/weight of dry substrate.
First, the assumptions of independence, normality and homoscedasticity were tested for the studied variables (mycelial growth rate, BE, P and DBE). Secondly, given that the data structure comply with normality criteria, ANOVA was used to evaluate the mycelial growth rate, BE, P and DBE, which were represented in terms of mean±standard error. Analysis of variance (MIXED and REML) to test the differences in colonization and production between the substrates was carried out with Statistical Analysis System (SAS) software, version 9.2 for Windows (SAS Institute Inc., Cary, NC, USA). Significant differences were determined by paired Student-t test (LSD) with α=0.05.
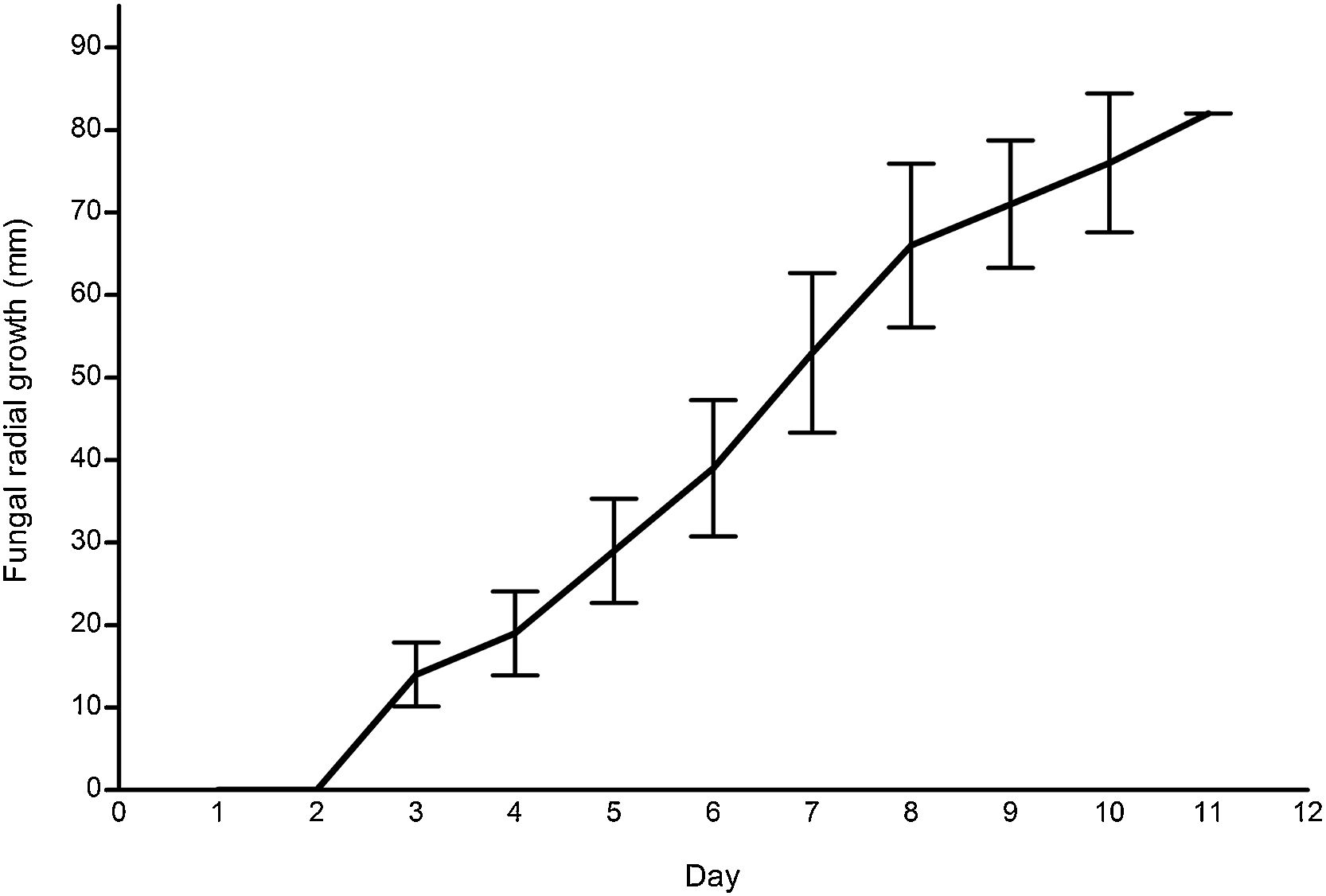
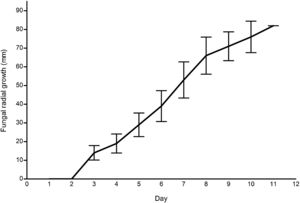
ResultsG. lucidum strain growthFig. 2 illustrates the mycelial radial growth of G. lucidum (n=10). The growth rate, 8.83mmday−1, was comparable to other wild strains, e.g., 8.70mmday−1 from South Korea,17 and 7.5–6mmday−1 from India,25 and to commercial strains.11 Similarly, the mycelium grew consistently on wheat grains.
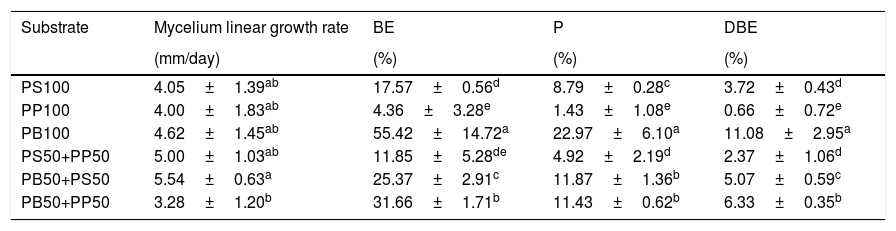
Mycelial linear growth on pecan substrateAll formulations were found to be suitable as solid substrate for G. lucidum when using 5% inoculum (Table 2). The mycelial growth was significantly higher in the PB50+PS50 substrate, 5.54±0.63mmday−1 in a total of 23.67±2.89 days to fill the tube. The lowest growth was recorded for the PB50+PP50 substrate, 3.28±1.20mmday−1 over a period of 43±13.75 days. The formulations PS100, PP100, PB100 and PS50+PP50 had similar mycelial linear growth (Table 2).
G. lucidum yieldThe six formulations were able to produce at least two flushes over the period of the experiment (Fig. 1a and b). Table 2 shows the performance in mushroom yield of G. lucidum. The BE ranged from 4.36% for substrate PP100 to 55.42% for substrate PB100; P ranged from 1.43% in the substrate PP100 to 22.97% in the substrate PB100, and DBE ranged from 0.66% in the substrate PP100 to 11.08% in the substrate PB100.
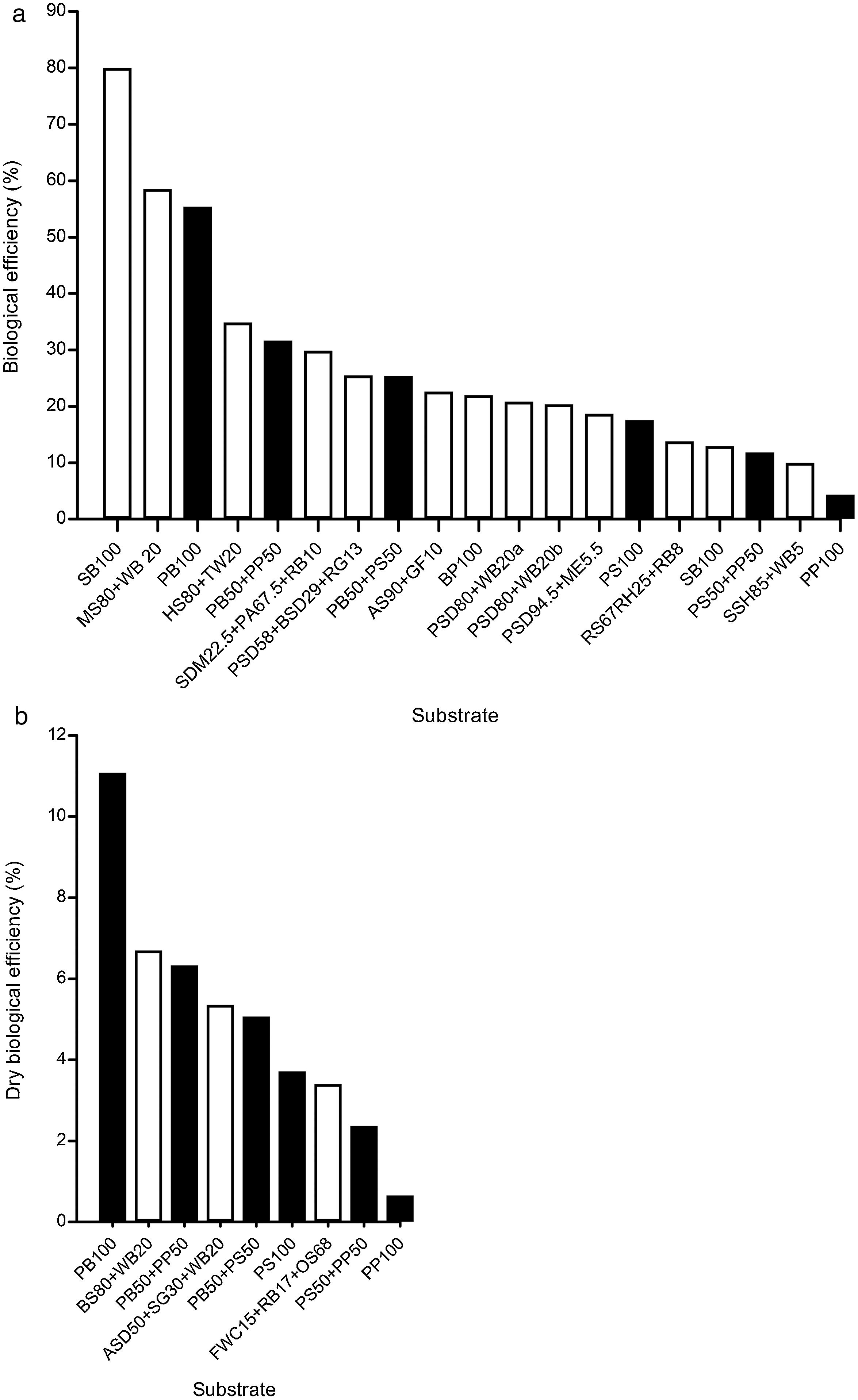
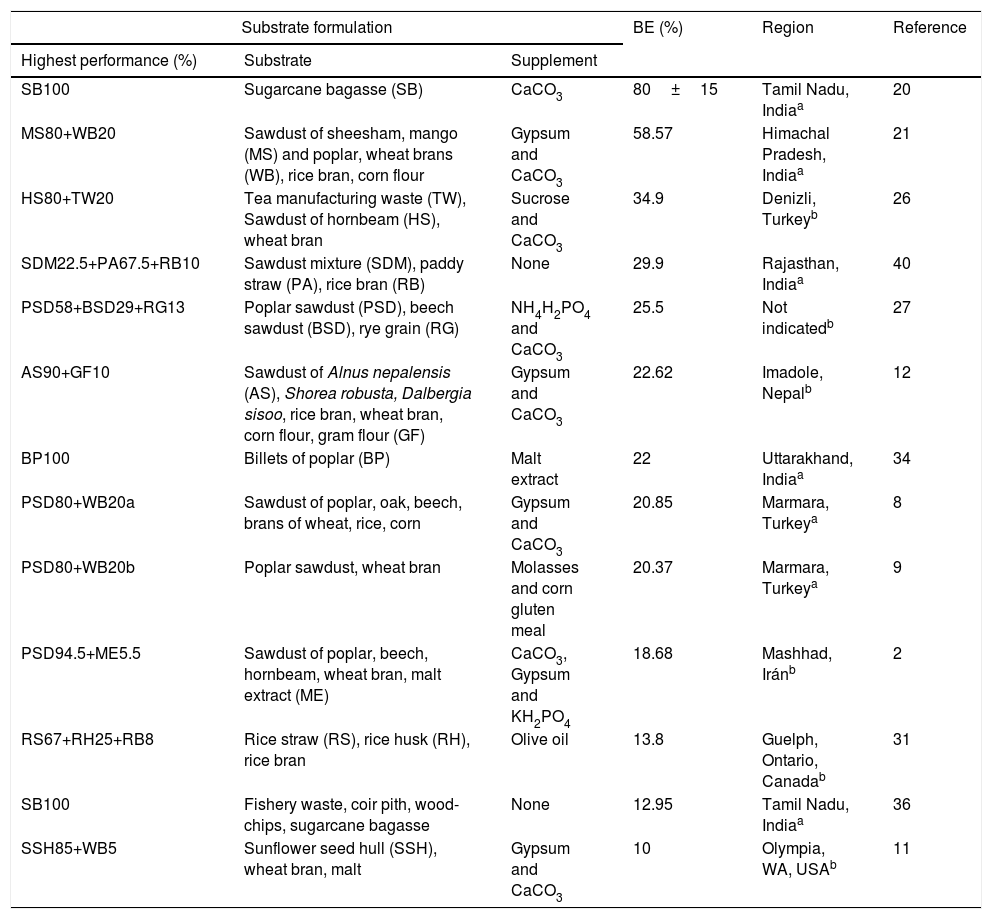
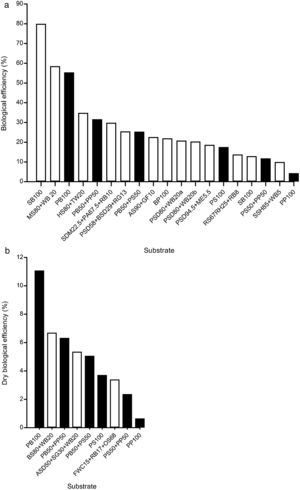
DiscussionG. lucidum has been successfully produced on a wide variety of waste materials, including corn stover residues,33 sawdust with rice straw,40 sunflower seed shells,11 tea residues,26 bagasse from sugar cane,20 soy residues,14 fish waste36 and different types of sawdust2,8,18 (poplar, beech, hornbeam, oak) (Table 1). Similarly, studies on lignocellulosic degradation ability have been conducted. Zhang et al.45 studied the biological pre-treatment of bamboo culms (Phyllostachys pubescence) with G. lucidum and 33 other WRF. After a 4-week period of cultivation, G. lucidum caused component loss of 12.10% (w/w) in weight: 10.56% (w/w) in lignin, 12.83% (w/w) in cellulose, and 15.16% (w/w) in hemicellulose. The selective delignification of G. lucidum was also observed in poplar wood.19Table 1 reviews the available studies on solid substrates for cultivating G. lucidum, using both commercial and wild strains. Comparison with the pecan wastes associated with BE and DBE is illustrated in Fig. 3a and b.
Solid substrates with the highest performance in the cultivation of the medicinal Ganoderma lucidum mushroom.
| Substrate formulation | BE (%) | Region | Reference | ||
|---|---|---|---|---|---|
| Highest performance (%) | Substrate | Supplement | |||
| SB100 | Sugarcane bagasse (SB) | CaCO3 | 80±15 | Tamil Nadu, Indiaa | 20 |
| MS80+WB20 | Sawdust of sheesham, mango (MS) and poplar, wheat brans (WB), rice bran, corn flour | Gypsum and CaCO3 | 58.57 | Himachal Pradesh, Indiaa | 21 |
| HS80+TW20 | Tea manufacturing waste (TW), Sawdust of hornbeam (HS), wheat bran | Sucrose and CaCO3 | 34.9 | Denizli, Turkeyb | 26 |
| SDM22.5+PA67.5+RB10 | Sawdust mixture (SDM), paddy straw (PA), rice bran (RB) | None | 29.9 | Rajasthan, Indiaa | 40 |
| PSD58+BSD29+RG13 | Poplar sawdust (PSD), beech sawdust (BSD), rye grain (RG) | NH4H2PO4 and CaCO3 | 25.5 | Not indicatedb | 27 |
| AS90+GF10 | Sawdust of Alnus nepalensis (AS), Shorea robusta, Dalbergia sisoo, rice bran, wheat bran, corn flour, gram flour (GF) | Gypsum and CaCO3 | 22.62 | Imadole, Nepalb | 12 |
| BP100 | Billets of poplar (BP) | Malt extract | 22 | Uttarakhand, Indiaa | 34 |
| PSD80+WB20a | Sawdust of poplar, oak, beech, brans of wheat, rice, corn | Gypsum and CaCO3 | 20.85 | Marmara, Turkeya | 8 |
| PSD80+WB20b | Poplar sawdust, wheat bran | Molasses and corn gluten meal | 20.37 | Marmara, Turkeya | 9 |
| PSD94.5+ME5.5 | Sawdust of poplar, beech, hornbeam, wheat bran, malt extract (ME) | CaCO3, Gypsum and KH2PO4 | 18.68 | Mashhad, Iránb | 2 |
| RS67+RH25+RB8 | Rice straw (RS), rice husk (RH), rice bran | Olive oil | 13.8 | Guelph, Ontario, Canadab | 31 |
| SB100 | Fishery waste, coir pith, wood-chips, sugarcane bagasse | None | 12.95 | Tamil Nadu, Indiaa | 36 |
| SSH85+WB5 | Sunflower seed hull (SSH), wheat bran, malt | Gypsum and CaCO3 | 10 | Olympia, WA, USAb | 11 |
| DBE% | |||||
|---|---|---|---|---|---|
| BS80+WB20 | Oat straw, bean straw (BS), Brachiaria grass straw, Tifton grass straw, Eucalyptus sawdust and wheat bran | CaCO3 | 6.7 | Botucatu, Brazilb | 22 |
| ASD50+SG30+WB20 | Sawdust of stalk of Acacia confusa (ASD), Stillage grain (SG), wheat bran | NH4H2PO4 and CaCO3 | 5.36 | Hsinchu, Taiwanb | 43 |
| FWC15+RB17+OS68 | Food waste compost (FWC), rice bran and oak sawdust (OS) | NaCl and Ca | 3.4±0.2 | Chuncheon, South Koreab | 18 |
Ganoderma lucidum growth on the six formulations of pecan wastes. Mycelium linear growth rate, biological efficiency (BE), production, and dry biological efficiency (DBE).
| Substrate | Mycelium linear growth rate | BE | P | DBE |
|---|---|---|---|---|
| (mm/day) | (%) | (%) | (%) | |
| PS100 | 4.05±1.39ab | 17.57±0.56d | 8.79±0.28c | 3.72±0.43d |
| PP100 | 4.00±1.83ab | 4.36±3.28e | 1.43±1.08e | 0.66±0.72e |
| PB100 | 4.62±1.45ab | 55.42±14.72a | 22.97±6.10a | 11.08±2.95a |
| PS50+PP50 | 5.00±1.03ab | 11.85±5.28de | 4.92±2.19d | 2.37±1.06d |
| PB50+PS50 | 5.54±0.63a | 25.37±2.91c | 11.87±1.36b | 5.07±0.59c |
| PB50+PP50 | 3.28±1.20b | 31.66±1.71b | 11.43±0.62b | 6.33±0.35b |
The same letters are not significantly different by the paired student-t test (α=0.05).
Ganoderma lucidum mushroom cultivation in solid substrates. (a) Biological efficiency, and (b) Dry biological efficiency. Formulations of pecan wastes (black bars) are compared with other solid substrates (white bars); abbreviations of the solid substrates are given in Table 1.
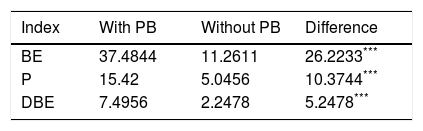
The G. lucidum strain KT805317 used for the first time in this study, is adapted to Mediterranean climate conditions, contrary to the strains reported so far (Table 1). The results were particularly significant regarding the proportion of the native inoculum used in the degradation of residues. The mycelial growth rate of G. lucidum in pecan waste was analogous to that on other solid substrates, such as agro-industrial rice residues on substrate formulations using 10% of inoculum20,43 and 8% of inoculum.31 The wood-chips formulations with shells or pericarp improved the G. lucidum yield. The biological efficiency increased from 4.36% in PP100 formulation to 31.67% in PB50+PP50, and from 8.79% in PS100 formulation to 11.87% in PB50+PS50 (Table 2). The orthogonal contrast (Table 3) corroborates the importance of formulations in the mushroom yield, with significantly higher values of BE, P and DBE in the substrates with pecan wood-chips (PB100). Ultimately, substrate PB100 was certainly the most efficient for G. lucidum yield. The higher similarity of the substrate formulations with the natural environment in which the G. lucidum grows could be possibly the key for success to obtain high BE values.
Orthogonal contrast of yields in relation to the substrates with and without wood-chips of pecan branches (PB).
| Index | With PB | Without PB | Difference |
|---|---|---|---|
| BE | 37.4844 | 11.2611 | 26.2233*** |
| P | 15.42 | 5.0456 | 10.3744*** |
| DBE | 7.4956 | 2.2478 | 5.2478*** |
BE: biological efficiency, P: production, DBE: dry biological efficiency.
No significant correlation was observed between the rate of mycelial growth and mushroom yield within the same test formulation, ρ (mycelial growth rates, P)=0.1430, p-value=0.5713 (ns) and ρ (mycelial growth rates, BE)=0.0927, p-value=0.7144 (ns). It is important to know how long G. lucidum takes to colonize each type of substrate. This is an industrial setting of great interest, but should not be taken as a measure of performance. This result indicates that a substrate should not be discarded for a long colonization time.
This study shows the novelty of using pecan wood-chips instead of sawdust to produce G. lucidum. Royse and Sanchez-Vazquez32 reported the influence of wood-chip particle size on the cultivation of the shiitake (Lentinula edodes); yields from substrates prepared with wood-chip particles of <0.85mm were compared with yields from substrates prepared with wood-chip particles of 2.8±4.0mm. Further research is required to confirm the influence of particle size of pecan wastes on G. lucidum cultivation and yield.
The pecan wood chip formulation (PB100), with 55.42% BE, emerges as one of the best solid substrates for cultivating G. lucidum (Fig. 3a), only surpassed by sugarcane bagasse (SB100), with 80±15% BE,20 and sawdust of mango and wheat brans (MS80+WB20), with 58.57% BE.21 Both the formulations PB50+PP50, with 31.7% BE, and PB50+PS50, with 25.4% BE, are listed in the top ten agro-industrial residues to obtain G. lucidum mushrooms. The only additive used in our study was the pH regulator (1% of CaCO3). Comparable results in the same conditions were those of pecan shells (PS100), with 17.6% BE, and the mixture of poplar sawdust and wheat bran without additives (PSD80+WB20), with 17.2% BE.9
The DBE index is particularly relevant for G. lucidum production because this mushroom is being sold as dry matter in all its forms (powder, capsules, whole and chopped). The best value was obtained for PB100 (11%) relative to other reported values (Fig. 3b).
ConclusionsThis study describes an attempt to cultivate the medicinal mushroom G. lucidum by using agro-industrial residues of C. illinoinensis. The results show that all six formulations of the substrates tested were suitable for the fungus growth and mushroom yield. The mushroom yield was increased with formulations including shells or pericarp with wood-chips. The best result (55.4% BE) was obtained with PB100. The cultivation of pecan is being expanded in both the northern and southern hemispheres41 due to market demand and the ability of the tree to adapt to a range of climate environments.4 The economic aspect of pecan production on a global scale could benefit considerably from the usage of the lignocellulosic wastes to produce high value products in the form of mushrooms with medicinal and biotechnological applications.
Conflict of interestsAll authors declare that they have no conflict of interest.
We are grateful for the support and materials provided by the producers of pecan nuts from the Asociación Valle del Guadalhorce, in the province of Málaga, Spain. We also thank Álvaro Rodrigo for providing the G. lucidum specimen. This work was possible due to the funding provided by the Erasmus Mundus Program under a EuroTango Project for a PhD program.