Studies have reported that coronavirus disease 2019 (COVID-19) may cause erectile dysfunction (ED), however, its role in the pathophysiology of ED has not yet been fully elucidated. We aimed to elucidate COVID-19's effects on cavernosal smooth muscle, which has a pretty important role in erection physiology, by corpus cavernosum electromyography (cc-EMG).
Materials and methodsTwenty-nine male patients aged 20–50 years who applied to the urology outpatient clinic due to ED were included in the study. Nine patients that had COVID-19 and were treated as outpatients were classified as group 1, 10 patients who were hospitalized due to COVID-19 were classified as group 2, and 10 patients who did not have COVID-19 were classified as the control group (group 3). Patients underwent diagnostic evaluation including International Index of Erectile Function (IIEF)-5 form, penile color Doppler ultrasonography (CDUS), cc-EMG, and fasting serum levels of reproductive hormones (07–11am).
ResultsAccording to penile CDUS and hormonal values results, there was no significant difference between the groups. According to cc-EMG results, amplitudes and relaxation capacities of the cavernosal smooth muscle of patients in group 3 were significantly higher than those in the other groups.
ConclusionsCOVID-19 can cause ED not only by psychogenic and hormonal factors but also with cavernosal smooth muscle damage.
Clinical Trial Registration Number: NCT04980508.
Los estudios han informado que la COVID-19 puede causar disfunción eréctil, sin embargo, su papel en la fisiopatología de la disfunción eréctil aún no se ha aclarado por completo. Nuestro objetivo era dilucidar los efectos de la COVID-19 en el músculo liso cavernoso, que tiene un papel bastante importante en la fisiología de la erección, mediante electromiografía del cuerpo cavernoso (cc-EMG).
Materiales y métodosSe incluyeron en el estudio 29 pacientes varones de 20 a 50 años de edad que solicitaron la consulta externa de urología debido a disfunción eréctil. Nueve pacientes que tenían COVID-19 y fueron tratados como pacientes ambulatorios se clasificaron como grupo 1, 10 pacientes que fueron hospitalizados debido a COVID-19 se clasificaron como grupo 2 y 10 pacientes que no tenían COVID-19 se clasificaron como grupo control (grupo 3). Los pacientes se sometieron a una evaluación diagnóstica que incluyó el índice internacional de función eréctil (IIEF)-5, ecografía Doppler color del pene (CDUS), cc-EMG y niveles séricos en ayunas de hormonas reproductivas (07-11 am).
ResultadosDe acuerdo con los resultados de los valores de CDUS y hormonales del pene, no hubo diferencias significativas entre los grupos. De acuerdo con los resultados de cc-EMG, las amplitudes y las capacidades de relajación de las actividades EMG del músculo liso cavernoso de los pacientes del grupo 3 fueron significativamente mayores que las de los otros grupos.
ConclusionesLa COVID-19 puede causar disfunción eréctil no solo por factores psicógenos y hormonales, sino también por daño del músculo liso cavernoso.
Coronavirus disease 2019 (COVID-19), which is caused by a novel coronavirus Sars-Cov-2, has been affecting people all over the world for more than one year and has resulted in many deaths.1 Although it affects patients by causing viral pneumonia and acute respiratory distress syndrome in particular,2 numerous studies have reported that it can affect almost every part of the body and cause different pathologies including anosmia,3 hypogonadism,4 myopathy,5,6 neuropathy,7 and vasculitis.8 Multiple studies have been conducted to evaluate the effects of COVID-19 on male sexual and reproductive health. Studies have reported that it may cause erectile dysfunction (ED),9 hypogonadism,4 and infertility.10 However, its role in the pathophysiology of ED has not yet been fully elucidated.
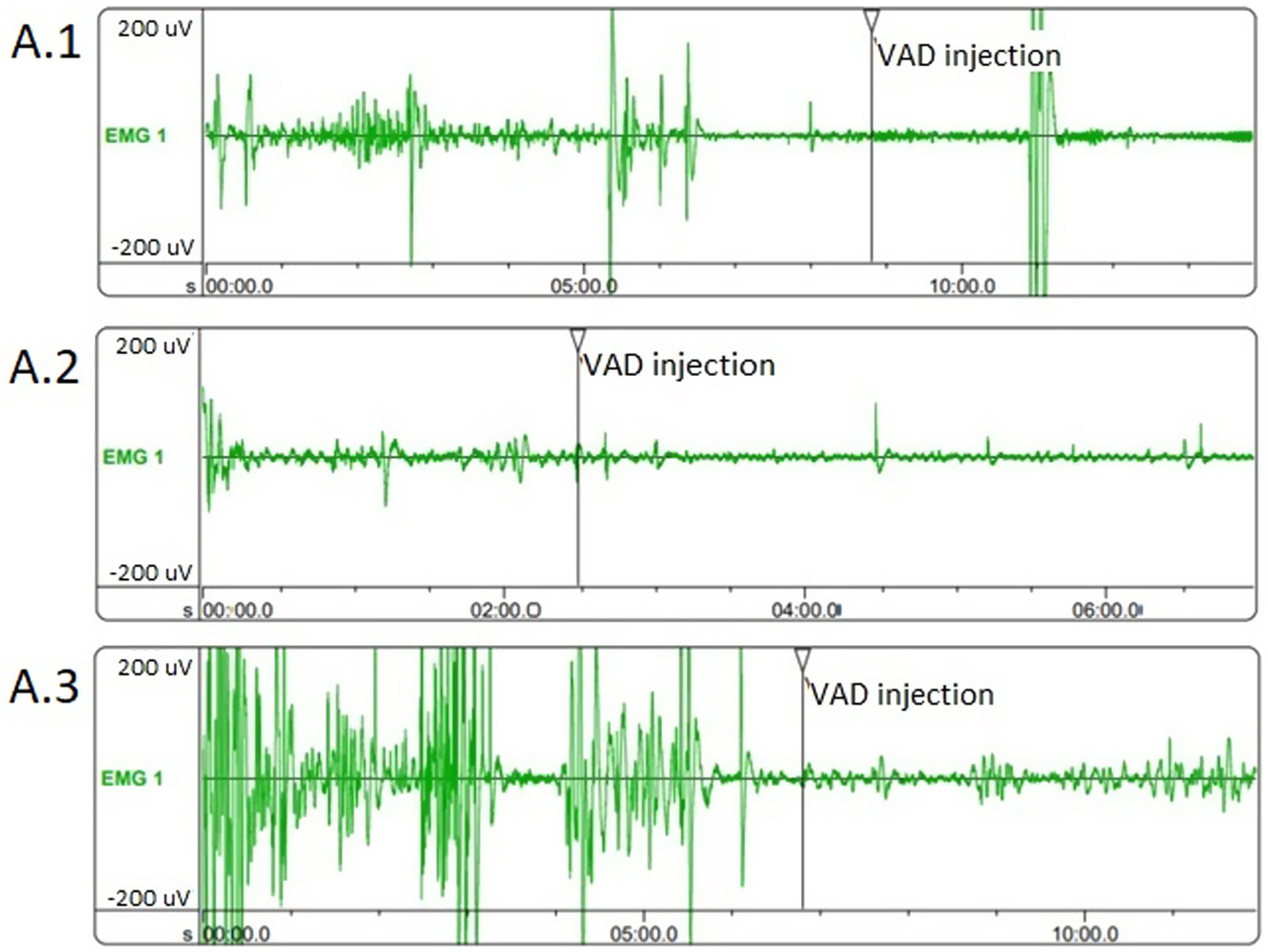
Corpus cavernosum electromyography (cc-EMG) allows us to measure cavernosal smooth muscle tone and relaxation capacity, which have an important role in erection physiology.11,12 Relaxation degree (RD) is defined as the percentage decrease in the amplitude of corpus cavernosum electrical activity (CCEA) after intracavernosal injection of vasoactive drug (VAD) (papaverine 60mg) during cc-EMG, and reflects the relaxation capacity of cavernosal smooth muscles.13
Since COVID-19 may causes endothelial, it has a potential to impair cavernosal smooth muscle physiology. We aimed to evaluate how cavernosal smooth muscles are affected by COVID-19, using cc-EMG. To the best of our knowledge, our study is the first in literature that evaluates the effect of COVID-19 on cavernosal smooth muscles.
Materials and methodsThis pilot study was reviewed and approved by the Institutional Review Board of Ankara Yildirim Beyazit University School of Medicine in February 2021, after the approval of the Ministry of Health of Turkey (26379996/10). Also, this study was retrospectively registered on Clinicaltrials.gov and approved by the US National Library of Medicine, National Institutes of Health (NCT04980508).
Patients aged 20–50 years who applied to the urology outpatient clinic due to ED between March 2021 and May 2021 and had an International Index of Erectile Function (IIEF)-514 score of ≤21 and also had COVID-19 were included in the study. Nine patients who had COVID-19 and were treated as outpatients were classified as group 1, and 10 patients who were hospitalized due to COVID-19 were classified as group 2. In addition, 10 patients aged 20–50 years, who applied to the urology outpatient clinic with the complaint of ED and had an IEF-5 score≤21 and also did not have COVID-19 were randomly selected to form the control group (group 3). Patients with concomitant neurological disease, hormonal drug use, history of pelvic trauma and history of penile, testicular, pelvic, or perineal surgery, diagnosis of malignancy, and patients that used opioids within the last 3 months were excluded from the study. Psychiatric assessment was made with Beck Depression Inventory15 and patients with>9 points in this assessment were excluded from the study. Patients underwent diagnostic evaluation including IIEF-5 form, penile color Doppler ultrasonography (CDUS), cc-EMG, and fasting serum levels of testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin measured in the morning (07–11am).
Penile color Doppler ultrasonographyPenile CDUS (GE Healthcare Systems, Milwaukee, USA) was performed with a 6–15MHz linear probe to determine arterial or venoocclusive pathology. Before the penile CDUS, papaverine HCI 60mg was injected into one of the cavernosal bodies. According to penile CDUS examination, peak systolic flow<25cm/s is determined as arterial insufficiency, and end-diastolic flow>5cm/s is determined as venous leakage.
Corpus cavernosum electromyographyThe CCEA was measured by a high-speed electromyography module (Medical Measurement Systems, Enschede, the Netherlands) connected to a computer. A band-pass filter with a sampling frequency of 200Hz and a cutoff frequency of 0.1–20Hz was used. The CCEA recordings were obtained using coaxial needle electrodes during the flaccid state of the penis and a ground electrode was also placed on the patient's foot to intercept electrical noise. The CCEA recordings were carried out for 10min and peak to peak cavernous electrical potentials were recorded. All tests were performed in a well-isolated room free of any interference. Room temperature was maintained at 20–24°C. The RD of cavernosal smooth muscles were calculated using the formula: RD=(Preinjectional CCEA−Postinjectional CCEA)/Preinjectional CCEA.13
Statistical analysesThe patients’ age, comorbidities, smoking and alcohol usage, IIEF-5 scores, hormonal profiles, as well as cc-EMG and penile CDUS results were recorded. All statistical analyses were performed with SPSS 25.0.0.1 software (IBM Corp., Armonk, NY, USA) with the one-way analysis of variance (ANOVA) and Kruskal–Wallis test. Normal distribution assumption for continuous variables was analyzed with the Shapiro–Wilk test. A value of p<0.05 was considered statistically significant. Power analysis could not be performed because there was no previous study evaluating the effect of COVID-19 on cavernosal smooth muscles.
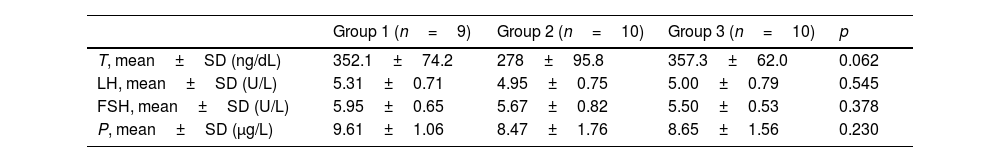
ResultsThe mean age of the patients was 36.6±7.5 years in group 1, 37.2±7.8 years in group 2, and 35.7±7.1 years in group 3. The mean time between COVID-19 and cc-EMG was 101.4±21.4 days in group 1, and 110.9±20.8 days in group 2. Although the scores of the patients in group 2 were lower than those in groups 1 and 3, this difference was not significant (p=0.34). The distribution of vasculogenic pathologies according to penile CDUS in groups was as follows: 33% in group 1 (arterial insufficiency in 2 and venous leakage in 1 patient), 40% in group 2 (arterial insufficiency in 2, mixed insufficiency in 2 patients), and 40% in group 3 (arterial insufficiency in 3, venous leakage in 1 patient). IIEF-5 scores and penile CDUS results are summarized in Table 1. There was no significant difference between groups in terms of hormonal profile. Although the testosterone level was lower in group 2 compared to groups 1 and 3, this difference was not significant (p: 0.062) (Table 2).
International Index of Erectile Function (IIEF) and penile color Doppler ultrasonography findings 15min after 60mg papaverine injection.
| Group 1 (n=9) | Group 2 (n=10) | Group 3 (n=10) | p | |
|---|---|---|---|---|
| IIEF-5, mean±SD | 10.33±3.70 | 8.90±2.64 | 11.10±3.10 | 0.304 |
| Peak-systolic flow mean±SD (cm/s) | 32.77±2.64 | 30.60±1.97 | 34.60±1.97 | 0.410 |
| End-diastolic flow mean±SD (cm/s) | 0.11±2.97 | 1.00±2.94 | 0.90±6.10 | 0.889 |
SD: standard deviation.
Reproductive hormone values.
| Group 1 (n=9) | Group 2 (n=10) | Group 3 (n=10) | p | |
|---|---|---|---|---|
| T, mean±SD (ng/dL) | 352.1±74.2 | 278±95.8 | 357.3±62.0 | 0.062 |
| LH, mean±SD (U/L) | 5.31±0.71 | 4.95±0.75 | 5.00±0.79 | 0.545 |
| FSH, mean±SD (U/L) | 5.95±0.65 | 5.67±0.82 | 5.50±0.53 | 0.378 |
| P, mean±SD (μg/L) | 9.61±1.06 | 8.47±1.76 | 8.65±1.56 | 0.230 |
T: testosterone, LH: luteinizing hormone, FSH: follicle-stimulating hormone, P: prolactin, SD: standard deviation.
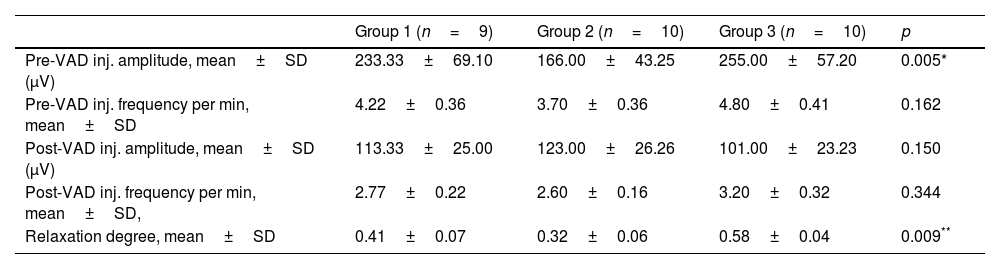
Evaluation of the cc-EMG results showed that the amplitudes before intracavernosal VAD injection were 233±69μV in group 1, 166±43.2μV in group 2, and 255±57.2μV in group 3. The pre-VAD amplitudes of the patients in group 2 were significantly lower than those in group 3 (p=0.005). Moreover, the pre-VAD amplitudes of the patients in group 1 were also significantly lower than those in group 3 (p=0.042). In addition, while the RD values of the patients in group 2 were significantly lower than those in group 3, no significant difference was observed between other groups. The cc-EMG findings are summarized in Table 3. Fig. 1 depicts cc-EMG samples of patients from 3 different groups.
Corpus cavernosum electromyography findings (VAD: vasoactive-drug (papaverine 60mg).
| Group 1 (n=9) | Group 2 (n=10) | Group 3 (n=10) | p | |
|---|---|---|---|---|
| Pre-VAD inj. amplitude, mean±SD (μV) | 233.33±69.10 | 166.00±43.25 | 255.00±57.20 | 0.005* |
| Pre-VAD inj. frequency per min, mean±SD | 4.22±0.36 | 3.70±0.36 | 4.80±0.41 | 0.162 |
| Post-VAD inj. amplitude, mean±SD (μV) | 113.33±25.00 | 123.00±26.26 | 101.00±23.23 | 0.150 |
| Post-VAD inj. frequency per min, mean±SD, | 2.77±0.22 | 2.60±0.16 | 3.20±0.32 | 0.344 |
| Relaxation degree, mean±SD | 0.41±0.07 | 0.32±0.06 | 0.58±0.04 | 0.009** |
SD: standard deviation.
According to the results of our study, pre-VAD injection cavernosal smooth muscle amplitudes of the group hospitalized due to COVID-19 were significantly lower than those of the group that did not have COVID-19. In a study performed on patients who presented with ED, the amount of cavernosal smooth muscle and CCEA were compared using corpus cavernosum biopsies and cc-EMG, and lower amplitude values were reported in patients with damaged corpus cavernosum smooth muscles.16 Therefore, the low amplitudes of cavernosal smooth muscles in cc-EMG in patients with COVID-19 support the thesis that COVID-19 causes cavernosal smooth muscle damage. Also, the results of our study suggest that the cavernous smooth muscle relaxation capacity of patients with symptomatic COVID-19 was significantly lower than those that were asymptomatic and had no disease. In a study, Kayigil et al. reported that the RD reflects the relaxation capacity of cavernosal smooth muscles, and lower values are obtained in the presence of cavernosal smooth muscle damage.13 The low RD in cc-EMG in patients with COVID-19 supports the thesis that COVID-19 causes cavernosal smooth muscle damage.
The SARS-CoV-2 virus infects cells via the membrane-bound angiotensin-converting enzyme (ACE)-2.17 The vascular endothelium is abundant in ACE-2 receptors, and thus it is one of the target tissues for SARS-CoV-2.18 Healthy vascular endothelial cells play a critical role in the coagulation system and vascular homeostasis by naturally expressing factors that induce vascular relaxation, increase blood flow, inhibit platelet aggregation and coagulation, and promote fibrinolysis. However, dysfunctional endothelial cells shift the balance toward vascular contraction and thrombus formation.19 Due to the resulting endothelial dysfunction, the nutrition and blood supply of the tissues including the cavernous tissues may be impaired. This may lead to damage of cavernous smooth muscles, which have a very important role in the erection mechanism. In erection physiology, parasympathetic stimulation leads to dilatation in penile vascular smooth muscles and an increase in blood flow, relaxation in cavernosal smooth muscles, inhibition of venous return by compression of subtunical venous plexus, and further reduction of venous return by stretching the tunica albuginea and compression of emissary veins.20 It has been shown that diseases that cause endothelial dysfunction, such as diabetes and hypercholesterolemia may impair cavernosal smooth muscle relaxation and thus lead to erectile dysfunction.21,22 In addition, in an experimental study comparing diabetic and healthy rats, it was observed that the smooth muscle rate of cavernosal tissues was significantly lower in the diabetic group.23 In another study by Mersdorf et al. in which they compared cavernosal tissue samples taken during penile prosthesis implantation, it was shown that cavernosal smooth muscle damage may occur in addition to vascular damage in conditions that cause endothelial dysfunction such as diabetes.24 It is possible that COVID-19, through its endothelial dysfunction effect, causes damage to cavernosal smooth muscles similar to diabetes and leads to erectile dysfunction via this mechanism.
Kadihasanoglu et al. compared 262 participants, including groups consist of patients who hospitalized due to COVID-19, who hospitalized due to non-COVID-19 lung infections, and an age-matched control group.4 According to the results of this study, significantly lower testosterone levels were found in the group that hospitalized for COVID-19 compared to other 2 groups. In addition, LH/FSH levels were significantly lower in patients who had severe COVID-19 compared to those with milder COVID-19. They also reported lower total testosterone levels, in patients who had severe COVID-19 compared to those with milder COVID-19.4 In a study comparing 81 men with COVID-19 and 100 men without COVID-19, authors reported lower testosterone levels in the group of patients who had COVID-19 than those who did not have COVID-19.25 In our study, the testosterone levels were lower in the group that hospitalized due to COVID-19 compared to other 2 groups, but this difference was not significant. Hypogonadism is one of the well-known causes of ED, and it is likely to play a role in the pathophysiology of COVID-19-induced ED.
During the pandemic stay-at-home orders, economic problems, staying away from their partner, and avoiding sexual intercourse to prevent the spread of the virus caused stress, anxiety, and depressive symptoms.26 A study conducted with healthcare professionals revealed the psychological effects of the pandemic on healthcare professionals and showed that they were prone to post-traumatic stress disorder.27 These psychosocial changes may cause or worsen ED.28 In a study conducted by Culha et al. on health professionals working in a pandemic hospital, they reported a significant decrease in the level of sexual desire and the number of sexual intercourse during the pandemic compared to the pre-pandemic period. In addition, the rate of sexual dysfunction has been reported to be significantly higher in individuals with higher anxiety scores.29 This psychosocial condition is likely to play a role in the pathophysiology of ED.
Our study has some limitations. Low sample size is one of them. Conceivably, this is due to lower rates of admission to the hospital for non-life-threatening conditions, which included sexual health problems during the pandemic. Another limitation is that we did not have any data regarding the erectile capacity of patients before COVID-19. We tried to ensure that the pre-COVID-19 erectile function capacities of the patients in groups were equal by creating groups with patients who have similar clinical characteristics.
COVID-19 is a systemic disease that can affect not only the respiratory tract but almost every part of the body. Numerous studies have been conducted to evaluate the effects of COVID-19 on male sexual functions. We evaluated how cavernosal smooth muscles are affected by COVID-19 using cc-EMG. It has been emphasized that psychological effects of the pandemic and quarantine as well as organic pathologies may be involved in the pathophysiology of sexual dysfunction.
ConclusionsIn conclusion, our results suggest that COVID-19 can cause ED not only by psychogenic and hormonal factors but also with cavernosal smooth muscle damage. We think that patients with ED and history of COVID-19 should be evaluated for cavernosal tissue damage. Indeed our results should certainly be supported by larger-scale prospective randomized controlled studies with longer follow-up.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestNone.