The aim of this study is to evaluate the anatomical factors influencing elasticity values of normal testicular parenchyma using shear wave elastography (SWE).
MethodsThis study examined 68 healthy male volunteers (117 testes in which standard transverse axis ultrasonography views could be obtained) via conventional scrotal ultrasonography and SWE. Both the mean (EMean) and standard deviation (ESD) elasticity values were acquired.
ResultsIn the standard transverse axis view of the rete testis at the mid-lateral edge of the testes, the EMean values in 2mm the testicular parenchyma from the rete testis and the testicular capsule at the same level as the rete testis were all significantly larger than in the central zone (P<0.001, P<0.001, respectively). The EMean value in the testicular parenchyma 2mm from the testicular capsule on the line formed approximately 45° below the horizontal line of the rete testis was significantly larger than in the rete testis approximately 45° above the horizontal line (P<0.001). In two standard transverse axis views, the ESD values in other regions were significantly larger than those in the central zones (all P<0.001). Also, the EMean values in the transmediastinal arteries were larger than those of the surrounding normal testicular parenchyma (P<0.001).
ConclusionBased on SWE, factors including the testicular capsule, the density of testicular fibrous septa, the depth of the Q-Box™, and the transmediastinal artery may influence the testes elasticity measurement.
Avaliar os factores anatómicos que influenciam os valores de elasticidade do parênquima testicular normal usando a elastografia de onda de cisalhamento (SWE).
MétodosEste estudo examinou 68 voluntários saudáveis do sexo masculino (117 testes em que foi possível obter vistas de ultrassonografia de eixo transversal padrão) através de ultrassonografia escrotal convencional e SWE. Foram adquiridos tanto os valores de elasticidade média (EMean) como de desvio-padrão (ESD).
ResultadosNa vista padrão do eixo transversal do teste rete na borda média-lateral dos testes, os valores EMean em 2mm do parênquima testicular do teste rete e a cápsula testicular ao mesmo nível que o teste rete eram todos significativamente maiores do que na zona central (p<0,001, p <0,001, respectivamente). O valor EMean no parênquima testicular a 2mm da cápsula testicular na linha formada aproximadamente 45° abaixo da linha horizontal do testículo reticular era significativamente maior do que no testículo reticular aproximadamente 45° acima da linha horizontal (p <0,001). Em duas vistas de eixo transversal padrão, os valores ESD em outras regiões eram significativamente maiores do que os das zonas centrais (todos p <0,001). Além disso, os valores EMean nas artérias transmediastinais eram maiores do que os do parênquima testicular normal circundante (p <0,001).
ConclusãoCom base no SWE, factores incluindo a cápsula testicular, a densidade do septo fibroso testicular, a profundidade da Q-Box™ e a artéria transmediastinal podem influenciar a medição da elasticidade dos testículos.
Elastography, first described by Ophir et al. (1991) for its potential application in the noninvasive assessment of the mechanical characteristics of tissues, has, in recent years, undergone rapid development.1,2 Shear wave elastography (SWE), as one of the elastographic techniques, can quantify the modulus of elasticity or Young's modulus, which represents the stiffness of tissues. SWE has significant advantages compared with strain elastography, such as high repeatability.3 SWE has been widely used in liver fibrosis, breast tumors, and thyroid nodules.4,5 However, there are few studies on testicular diseases. In patients with complete testicular torsion, a higher Young's modulus was reported in the central and border areas of torsional testes than in those of normal testes.6 Elsewhere, an experiment conducted by Zhang et al.7 on rabbits demonstrated that the SWE method might be utilized for testicular spermatogenesis evaluation after torsion.
There is a need to elucidate the elasticity values of normal testicular parenchyma and limitations in elasticity measurements when interpreting SWE on testicular pathology. Previous studies found that the peripheral zones of normal testicular parenchyma are stiffer than the central zones. For instance, a single study reported 6.73±1.27 as the strain rate between the central and peripheral zones of normal testicular parenchyma.8 Besides, the mean values of shear wave velocity were similar in the inferior and superior parts of the testicle (1.15m/s) but were significantly lower in the central part (0.90m/s).9 Measurements using elastography between normal testicular parenchyma's central and peripheral zones differ. Nevertheless, an understanding of the elasticity of testes is limited, and the mechanism of different elasticity values in different zones of normal testicular parenchyma remains unclear. Moreover, the measurement point requires standardization.5 This prompted us to analyze anatomical factors influencing the elasticity values of normal testicular parenchyma measured by SWE.
Materials and methodsThe Research Ethics Committee of our institution approved this study. Informed consent was obtained from participants before the examination.
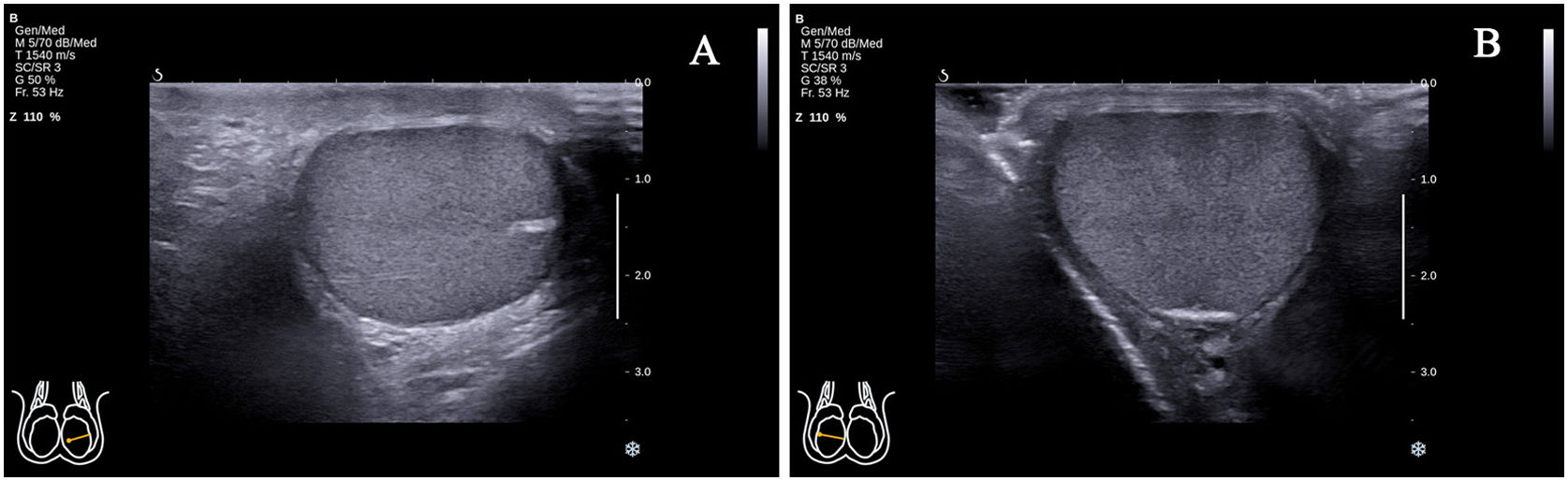
Study participantsThis study enrolled 68 healthy male volunteers (with 117 testes in which standard transverse axis ultrasonography views could be obtained). The study was conducted from May 2020 to October 2020. The standard transverse axis view of the testicle was defined as the rete testis located at the mid-lateral edge (Fig. 1A) or middle zone of the posterior lateral edge (Fig. 1B). In some parts of the testes, the above two standard transverse axis views could be obtained by changing the position of the ultrasonic probe. However, despite the change of the probe position, in the vast majority of cases, only the standard transverse axis view of the rete testis at the mid-lateral edge of the testes could be obtained. The exclusion criteria included: (1) presence of testicular, epididymal, or spermatic cord diseases; (2) history of testicular, epididymal, or spermatic cord diseases, including male infertility; (3) presence of sex hormone abnormality, including testosterone (TES), luteinizing hormone (LH) and follicle stimulating hormone (FSH); (4) Abnormal spermiogram data; (5) failure to obtain a standard transverse axis view regardless of the change in probe position.
Ultrasonographic examinationVolunteers were put in a supine position to perform scrotal ultrasonography (US). Images of conventional US and SWE were obtained using an Aixplorer ultrasound scanner (SuperSonic Imagine, Aix-en-Provence, France) with a 4–15MHz linear transducer. First, we confirmed the absence of testicular, epididymal, and spermatic cord diseases and the possibility of obtaining a standard transverse axis view using conventional US. Second, the volumes of the testes were measured in three dimensions and calculated according to the formula: length×width×height×0.523.9 Third, SWE was performed using the penetration mode with an elasticity range of 0 to 90kPa. The diameter of the measurement sampling box (Q-Box™; SuperSonic Imagine) was 2mm, which we used to measure elasticity values. The locations of the Q-Box™ were as follows.
- 1.
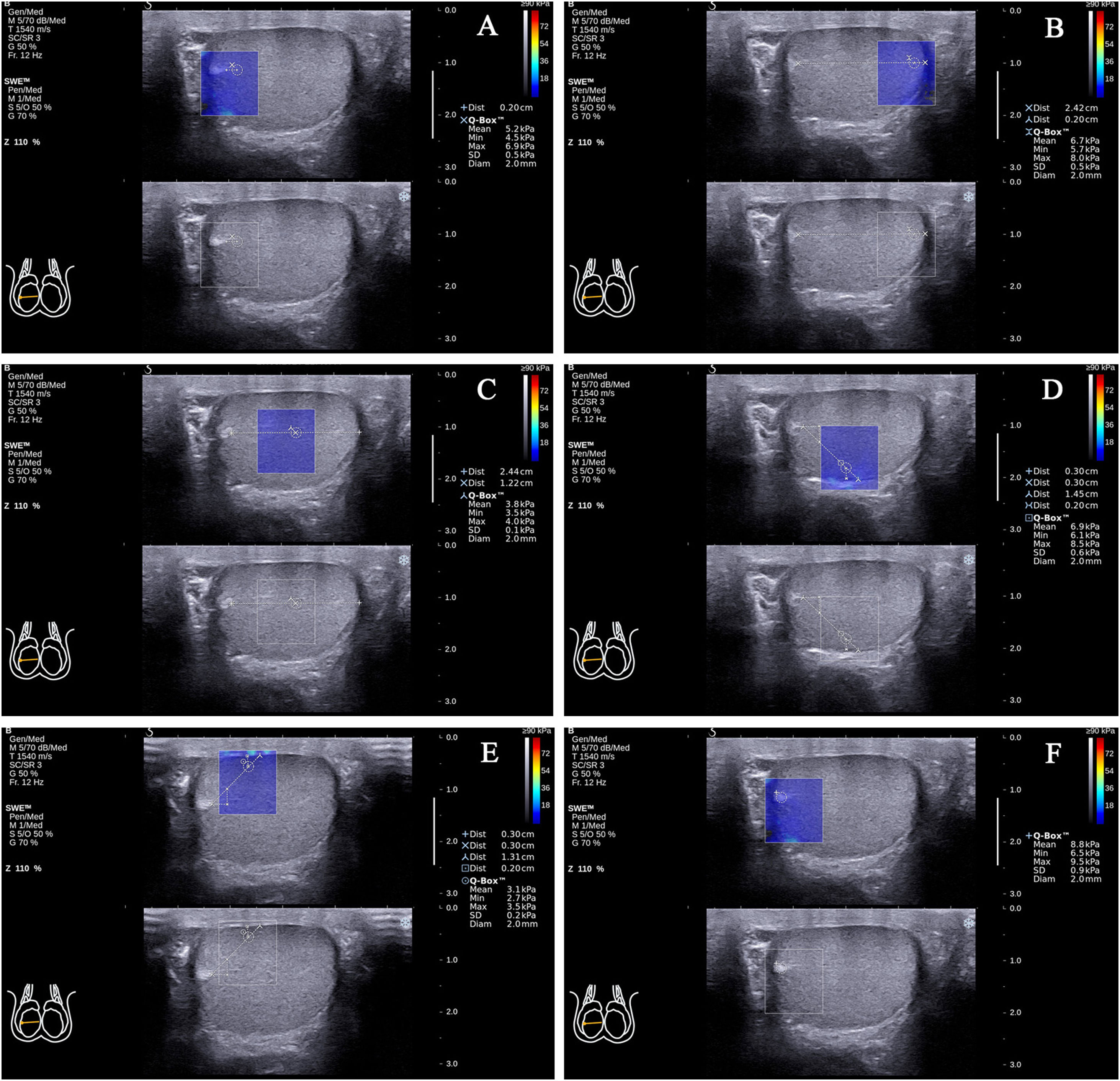
In the standard transverse axis view of the rete testis at the mid-lateral edge of the testes, there were (1) testicular parenchyma 2mm from the rete testis at the same level as the rete testis (the propagation direction of the shear wave was approximately 0° with the testicular lobular septum; the following will be abbreviated as angle values; Fig. 2A); (2) testicular parenchyma 2mm from the testicular capsule at the same level as the rete testis (0°; Fig. 2B); (3) testicular parenchyma at half of the distance from the rete testis to the testicular capsule at the same level as the rete testis (0°; Fig. 2C); (4) testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° below the horizontal line of the rete testis (45°; Fig. 2D); (5) testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° above the horizontal line of the rete testis (45°; Fig. 2E); (6) the rete testis (Fig. 2F).
Figure 2.The locations of the Q-Box™ in the standard transverse axis view of the rete testis at the mid-lateral edge of the right testicle. The location of the Q-Box™ (A) is testicular parenchyma 2mm from the rete testis at the same level as the rete testis. The location of the Q-Box™ (B) is testicular parenchyma 2mm from the testicular capsule at the same level as the rete testis. The location of the Q-Box™ (C) is testicular parenchyma at half of the distance from the rete testis to the testicular capsule at the same level as the rete testis. The location of the Q-Box™ (D) is testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° below the horizontal line of the rete testis. The location of the Q-Box™ (E) is testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° above the horizontal line of the rete testis. The location of the Q-Box™ (F) is the rete testis.
(0.86MB). - 2.
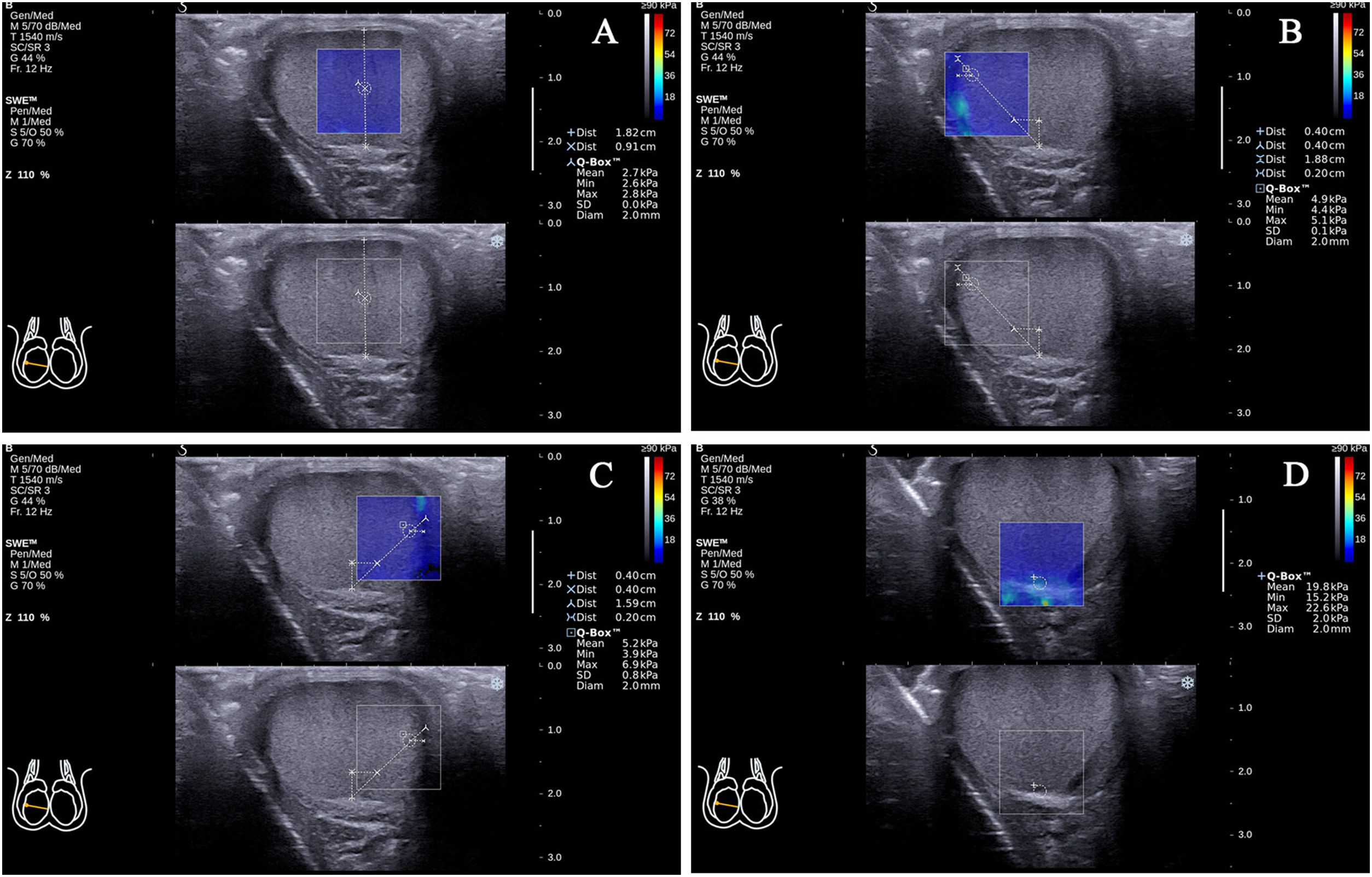
In the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the testes, there was (7) testicular parenchyma at half of the distance from the rete testis to the testicular capsule on the vertical line of the rete testis (90°; Fig. 3A); (8) testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the right side of the vertical line of the rete testis (45°; Fig. 3B); (9) testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the left side of the vertical line of the rete testis (45°; Fig. 3C); (10) the rete testis (Fig. 3D).
Figure 3.The locations of the Q-Box™ in the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the right testicle. The location of the Q-Box™ (A) is testicular parenchyma at half of the distance from the rete testis to the testicular capsule on the vertical line of the rete testis. The location of the Q-Box™ (B) is testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the right side of the vertical line of the rete testis. The location of the Q-Box™ (C) is testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the left side of the vertical line of the rete testis. The location of the Q-Box™ (D) is the rete testis.
(0.44MB).
A trained sonographer with three years of experience conducted all US examinations. Without any compression on the scrotal surface, the Q-Box™ was maintained at the above positions for at least 3s to generate stable elastic images. Both the EMean and ESD values were recorded. Two experienced sonographers analyzed the US images (with 5 and 15 years’ experience) in consensus. Notably, the values of the EMean and ESD represented the averages of three Q-Box™ measurements at each position.
Statistical analysisAll statistical data were analyzed using SPSS 18.0 software (IBM, Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation (SD) or median and interquartile range where appropriate. Continuous parameters were checked for the normality of distribution using the Shapiro–Wilk test and compared using the unpaired t-test or Mann–Whitney U test with normal and non-normal distributions, respectively. The association of the testicular volume with the EMean value in the central zones of normal testes was evaluated using regression analysis. Furthermore, Spearman's rank correlation coefficient was used to evaluate the association between the two groups. P<0.05 was considered statistically significant.
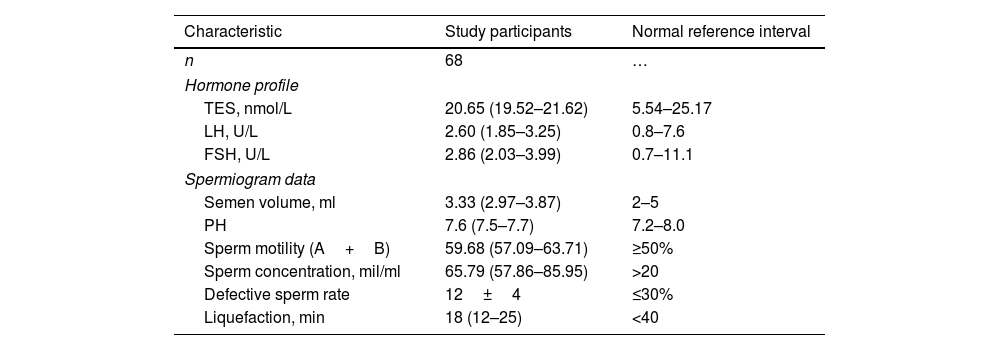
ResultsCharacteristics of study participantsA total of 68 healthy male volunteers aged 19–57 years (median age, 28.00; interquartile range, 25.25–32.50) were included in the analysis. This study excluded 19 testes that failed to show a standard transverse axis view despite changing the probe position; thus, 117 normal testes (left testicle, n=55; right testicle, n=62) were included. Hormone profile and spermiogram data of study participants are shown in Table 1. The testes were symmetric ovoid structures with medium homogeneous echogenicity and no hydrocele. Color Doppler showed the star-like or cord-like blood flow in the testes. The spectral waveform of the intratesticular arteries characteristically had a normal low-resistance pattern. Among them, in 28 testes, we obtained two standard transverse axis views by changing the position of the ultrasonic probe, whereas in 76 testes, only the standard transverse axis view of the rete testis at the mid-lateral edge of the testes was acquired. Additionally, in 13 testes, only the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge was obtained.
Hormone profile and spermiogram data of study participants.
| Characteristic | Study participants | Normal reference interval |
|---|---|---|
| n | 68 | … |
| Hormone profile | ||
| TES, nmol/L | 20.65 (19.52–21.62) | 5.54–25.17 |
| LH, U/L | 2.60 (1.85–3.25) | 0.8–7.6 |
| FSH, U/L | 2.86 (2.03–3.99) | 0.7–11.1 |
| Spermiogram data | ||
| Semen volume, ml | 3.33 (2.97–3.87) | 2–5 |
| PH | 7.6 (7.5–7.7) | 7.2–8.0 |
| Sperm motility (A+B) | 59.68 (57.09–63.71) | ≥50% |
| Sperm concentration, mil/ml | 65.79 (57.86–85.95) | >20 |
| Defective sperm rate | 12±4 | ≤30% |
| Liquefaction, min | 18 (12–25) | <40 |
Data are expressed as mean±SD or median (interquartile range). TES indicates testosterone; LH, luteinizing hormone; FSH, follicle stimulating hormone; PH, potential of hydrogen; A, grade A ratio; B, grade B ratio; mil, million.
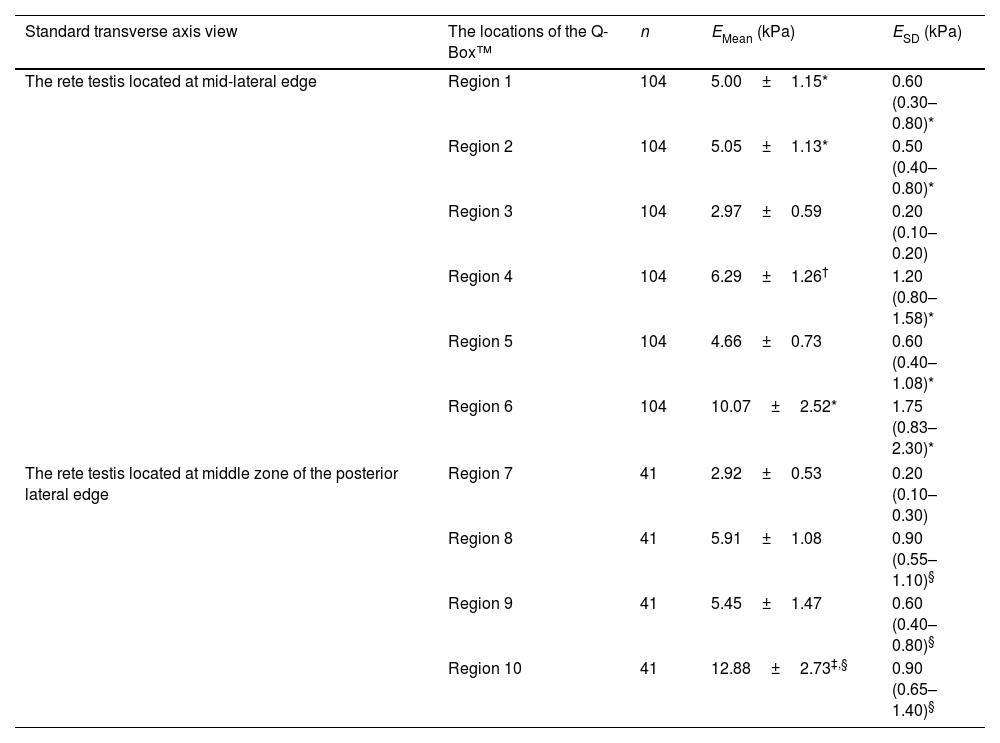
Elasticity values in different zones of normal testes measured by SWE are summarized in Table 2. In the standard transverse axis view of the rete testis at the mid-lateral edge of the testes, the EMean values in regions 1, 2, and 6 were significantly larger than those in region 3 (5.00±1.15 versus 2.97±0.59kPa, 5.05±1.13 versus 2.97±0.59kPa, 10.07±2.52 versus 2.97±0.59kPa; P<0.001, P<0.001, P<0.001, respectively). The EMean value in region 4 was significantly larger than in region 5 (6.29±1.26 versus 4.66±0.73kPa; P<0.001). Compared with region 3, the values of ESD in other regions, including regions 1, 2, 4, 5, and 6 were significantly larger (0.60 [0.30–0.80] versus 0.20 [0.10–0.20]kPa, 0.50 [0.40–0.80] versus 0.20 [0.10–0.20]kPa, 1.20 [0.80–1.58] versus 0.20 [0.10–0.20]kPa, 0.60 [0.40–1.08] versus 0.20 [0.10–0.20]kPa, 1.75 [0.83–2.30] versus 0.20 [0.10–0.20]kPa; P<0.001, P<0.001, P<0.001, P<0.001, P<0.001, respectively).
Elasticity values in different zones of normal testes.
| Standard transverse axis view | The locations of the Q-Box™ | n | EMean (kPa) | ESD (kPa) |
|---|---|---|---|---|
| The rete testis located at mid-lateral edge | Region 1 | 104 | 5.00±1.15* | 0.60 (0.30–0.80)* |
| Region 2 | 104 | 5.05±1.13* | 0.50 (0.40–0.80)* | |
| Region 3 | 104 | 2.97±0.59 | 0.20 (0.10–0.20) | |
| Region 4 | 104 | 6.29±1.26† | 1.20 (0.80–1.58)* | |
| Region 5 | 104 | 4.66±0.73 | 0.60 (0.40–1.08)* | |
| Region 6 | 104 | 10.07±2.52* | 1.75 (0.83–2.30)* | |
| The rete testis located at middle zone of the posterior lateral edge | Region 7 | 41 | 2.92±0.53 | 0.20 (0.10–0.30) |
| Region 8 | 41 | 5.91±1.08 | 0.90 (0.55–1.10)§ | |
| Region 9 | 41 | 5.45±1.47 | 0.60 (0.40–0.80)§ | |
| Region 10 | 41 | 12.88±2.73‡,§ | 0.90 (0.65–1.40)§ | |
Data are expressed as mean±SD or median (interquartile range).
Region 1: Testicular parenchyma 2mm from the rete testis at the same level as the rete testis.
Region 2: Testicular parenchyma 2mm from the testicular capsule at the same level as the rete testis.
Region 3: Testicular parenchyma at half of the distance from the rete testis to the testicular capsule at the same level as the rete testis.
Region 4: Testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° below the horizontal line of the rete testis.
Region 5: Testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° above the horizontal line of the rete testis.
Region 6: The rete testis.
Region 7: Testicular parenchyma at half of the distance from the rete testis to the testicular capsule on the vertical line of the rete testis.
Region 8: Testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the right side of the vertical line of the rete testis.
Region 9: Testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the left side of the vertical line of the rete testis.
Region 10: The rete testis.
In the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the testes, the EMean value in region 10 was significantly larger than in region 7 (12.88±2.73 versus 2.92±0.53kPa; P<0.001). No significant difference in the EMean value was reported between regions 8 and 9 (5.91±1.08 versus 5.45±1.47kPa; P=0.107). Compared with region 7, the ESD values in other regions, including regions 8, 9, and 10, were significantly larger (0.90 [0.55–1.10] versus 0.20 [0.10–0.30]kPa, 0.60 [0.40–0.80] versus 0.20 [0.10–0.30]kPa, 0.90 [0.65–1.40] versus 0.20 [0.10–0.30]kPa; P<0.001, P<0.001, P<0.001, respectively).
In addition, the EMean value in region 3 in the standard transverse axis view of the rete testis at the mid-lateral edge of the testes was comparable with region 7 in the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the testes (2.97±0.59 versus 2.92±0.53kPa; P=0.625). The EMean value in region 10 was significantly larger than in region 6 (12.88±2.73 versus 10.07±2.52kPa; P<0.001).
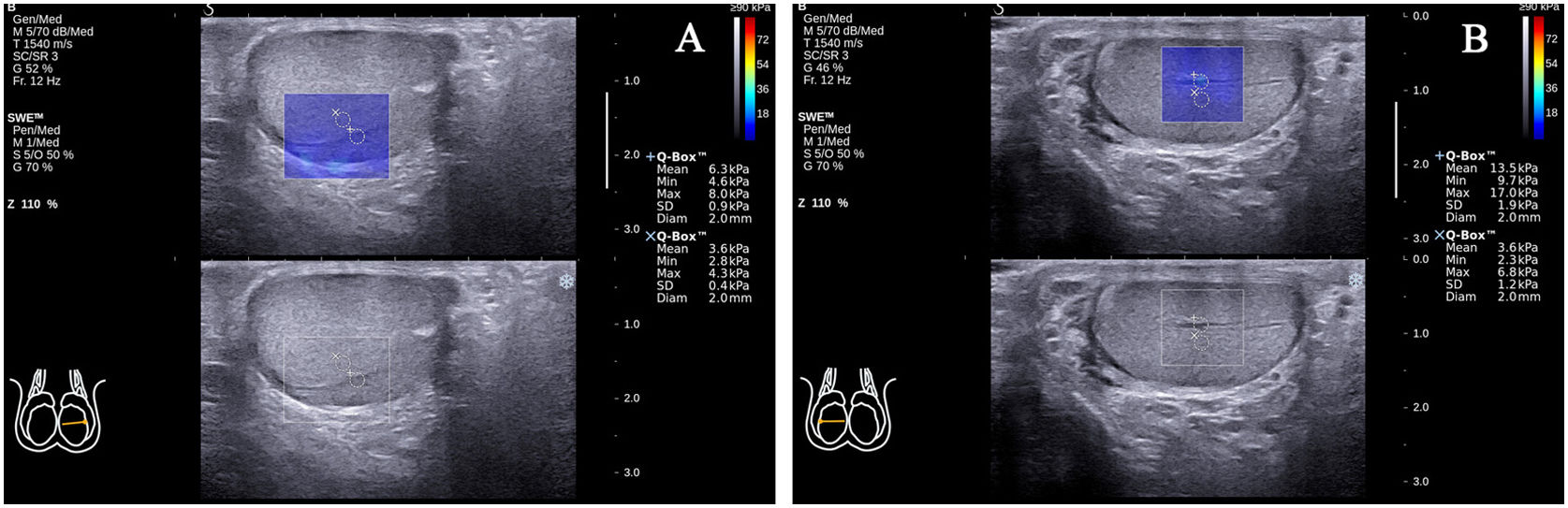
During the SWE examination, there were 25 cases of standard transverse axis views that showed a transmediastinal artery entering through the mediastinum and coursing toward the periphery of the testicular gland (25/117, 21.4%; Fig. 4A, B). The EMean value in the transmediastinal arteries was larger than in the surrounding normal testicular parenchyma (9.51±3.79 versus 4.16±1.21kPa; P<0.001).
Transmediastinal arteries in two standard transverse axis views. The EMean value in the transmediastinal artery of the left testicle (A) was larger than the surrounding normal testicular parenchyma (6.30 versus 3.60kPa). The EMean value in the transmediastinal artery of the right testicle (B) was larger than the surrounding normal testicular parenchyma (13.50 versus 3.60kPa).
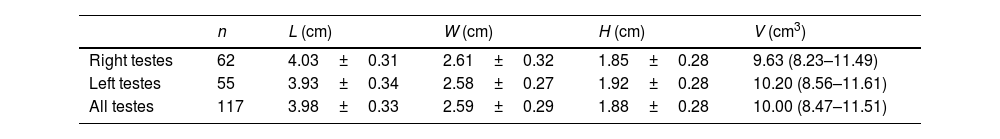
Measurements of the diameter and volume of testes are shown in Table 3. We reported no correlation between the testicular volume and the EMean value in the central zones of all normal testes (r=−0.034, P=0.719).
Measurements of diameter and the volume of testes.
| n | L (cm) | W (cm) | H (cm) | V (cm3) | |
|---|---|---|---|---|---|
| Right testes | 62 | 4.03±0.31 | 2.61±0.32 | 1.85±0.28 | 9.63 (8.23–11.49) |
| Left testes | 55 | 3.93±0.34 | 2.58±0.27 | 1.92±0.28 | 10.20 (8.56–11.61) |
| All testes | 117 | 3.98±0.33 | 2.59±0.29 | 1.88±0.28 | 10.00 (8.47–11.51) |
Data are expressed as mean±SD or median (interquartile range). L, length; W, width; H, height; and V, volume. V is calculated according to the formula: L×W×H×0.523.
Following previous reports, the peripheral zones of normal testicular parenchyma were stiffer than the central zones.8,9 However, the mechanism of different elasticity values in various zones of normal testicular parenchyma is still unclear. In this study, through assessment and comparison of the elasticity values in different zones in the standard transverse axis views of normal testes using SWE, we could reveal the anatomical factors influencing the elasticity values of normal testicular parenchyma.
In the standard transverse axis view of the rete testis at the mid-lateral edge of the testes, the EMean value in the testicular parenchyma 2mm from the testicular capsule at the same level as the rete testis was significantly larger than the testicular parenchyma at half of the distance from the rete testis to the testicular capsule at the same level as the rete testis. In these two regions, both the depth and angle between the propagation direction of the shear wave and the testicular lobular septum (approximately 0°) were approximately the same. This study, therefore, suggests that the testicular capsule critically influences the elasticity values of normal testicular parenchyma, and the normal testicular parenchyma is stiffer in the subcapsular area.
Besides, in the standard transverse axis view of the rete testis at the mid-lateral edge of the testes, the EMean value in the testicular parenchyma 2mm from and at the same level as the rete testis was significantly larger than in the testicular parenchyma at the same level as and at half of the distance from the rete testis to the testicular capsule. The rete testis is a network of epithelium-lined spaces embedded in the fibrous stroma of the mediastinum. Notably, numerous fibrous septa radially extend into the testis from the mediastinum, splitting it into around 250–400 lobules, each composed of 1–3 seminiferous tubules.10 The EMean value in the rete testis was also significantly larger than in the testicular parenchyma at the same level and at half of the distance from the rete testis to the testicular capsule. This observation concurs with the previously reported findings.11 Thus, the current study suggests that the elasticity values of normal testicular parenchyma may be affected by the density of testicular fibrous septa; the closer the rete testis, the denser the testicular fibrous septa and the stiffer the testicular parenchyma.
In the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the testes, we found no significant difference in the EMean values between testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° to the right side of the vertical line of the rete testis and that formed approximately 45° to the left side of the vertical line of the rete testis. However, in the standard transverse axis view of the rete testis at the mid-lateral edge of the testes, the EMean value in the testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° below the horizontal line of the rete testis was significantly larger than in the testicular parenchyma 2mm from the testicular capsule on the line that formed approximately 45° above the horizontal line of the rete testis. The propagation directions of the shear wave in these 4 regions were all approximately 45° with the testicular lobular septum. In addition, the EMean value in the rete testis in the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the testes was significantly larger than in the rete testis in the standard transverse axis view of the rete testis at the mid-lateral edge of the testes. Therefore, this study suggests the depth of the Q-Box™ may affect the elasticity values of normal testicular parenchyma. However, further investigation should clarify how depth influences elasticity values of normal testicular parenchyma measured by SWE.
Based on a previous study, the median stiffness value in the mid-region of the kidney (shear wave 90° to the pyramid axis) was significantly lower than at the upper pole of the kidney (shear wave 0° to the pyramid axis).12 However, in this study population, we report no significant difference in the EMean values between the central zones in two standard transverse axis views. The propagation direction of the shear wave in the central zone in the standard transverse axis view of the rete testis at the mid-lateral edge of the testes was approximately 0° with the testicular lobular septum. Whereas that of the standard transverse axis view of the rete testis at the middle zone of the posterior lateral edge of the testes was approximately 90°. This observation reveals that the angle between the propagation direction of the shear wave and the testicular lobular septum in the central zone of normal testicular parenchyma was not significantly correlated with the elasticity values.
In two standard transverse axis views, the ESD values in other regions were significantly larger than those in the central zones. Thus, this study suggests that the measurement of elasticity values in the central zones is the most stable and reproducible. Besides, the EMean values in the transmediastinal arteries were larger than those of the surrounding normal testicular parenchyma. Therefore, the elasticity measurement of diffuse testicular lesions should choose the central zones and avoid the transmediastinal artery.
In this study population, no correlation between the testicular volume and the EMean values in the central zones of normal testes was reported, an observation that is consistent with a previous study.9 This may have been attributed to the idea that our study population only includes adults but no children (children have immature and small-sized testes that may affect SWE measurements). Therefore, further investigation should clarify how immature testicular volume influences elasticity values of normal testicular parenchyma measured by SWE.
There were some limitations to this study. First, a relatively limited study population was evaluated. Second, the present study could not provide histological data to explain the results but only inferred. Third, this study was conducted using one type of commercially available equipment; thus, the results might vary according to the type of equipment and algorithm used for elastography. Fourth, a single US examiner may represent bias. Finally, we did not analyze which factor had the greatest influence on the elasticity measurements of normal testicular parenchyma.
ConclusionThis study demonstrated that the testicular capsule, density of testicular fibrous septa, depth of the Q-Box™, and the transmediastinal artery are potential factors influencing testes’ elasticity measured by SWE. These factors should be considered in the elasticity measurement of focal testicular lesions. Moreover, the elasticity measurement of diffuse testicular lesions should choose the central zone and avoid the transmediastinal artery.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Authors’ contributionsConception and design of the research: Shao-Dong Qiu, Fei Chen.
Acquisition of data: Yun-Yong Lin, Lin Mao, Jin Li, Zhi-Min Zhu, Yan-Hua Luo.
Analysis and interpretation of the data: Yun-Yong Lin, Shao-Dong Qiu, Fei Chen.
Statistical analysis: Yun-Yong Lin, Jin Li, Fei Chen.
Obtaining financing: None.
Writing of the manuscript: Yun-Yong Lin, Lin Mao, Shao-Dong Qiu, Fei Chen.
Critical revision of the manuscript for intellectual content: Xiao-Hua Zhou, Yun-Yong Lin, Fei Chen.
All authors read and approved the final draft.
Ethics approvalThis study was conducted with approval from the Ethics Committee of The Second Affiliated Hospital of Guangzhou Medical University. This study was conducted in accordance with the declaration of Helsinki.
Consent for publicationAll participants signed a document of informed consent.
Availability of data and materialsWe declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
FundingThis study was supported by Science and Technology Program of Guangzhou City, China [Grant No. 202102010049] and Science and Technology Planning Project of Guangdong Province, China [Grant No. 2012B061700045].
Conflict of interestsThe authors declare that they have no competing interests.
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study. Especially the help of Dr. Shao-Na Chen, Dr. Yi-Wen Wu and Dr. Wen-Jie Lu to this paper.