Varicocele is one of the reasons for testicular dysfunction and is frequently known to accompany infertility. The basic pathology of varicocele is the development of endothelial dysfunction. The most important factors in development of endothelial dysfunction are impaired endothelial-linked vasodilatation, increase in free oxygen radicals, reduced synthesis and release of nitric oxide (NO), abnormal vasoconstriction and increased levels of dimethyl arginine. Our aim was to identify and illustrate the relationship between asymmetric dimethyl arginine (ADMA) and NO levels in testicular tissue and plasma of rats with induced experimental varicocele.
Materials and methodsTwenty-one adolescent (average 6 weeks) male rats were included in the study and randomly divided into 3 groups. Group 1 (control, n=6) did not undergo any procedure. Group 2 (sham, n=6) had the left renal vein circled proximally but ligation was not performed. Group 3 (varicocele-induced, n=9) had partial ligation of the proximal left renal vein to induce left varicocele. Superoxide dismutase (SOD) enzyme activity and levels of end-products of NO, nitrite and nitrate salts were investigated in testis tissue. Nitrite/nitrate and ADMA levels were investigated in plasma. Histopathological examination was completed with routine hematoxylin–eosine and TUNEL dyes.
ResultsTissue SOD and plasma ADMA values were clearly increased in the varicocele group compared to the other groups; tissue and plasma nitrite/nitrate levels were clearly reduced in the varicocele group and this was observed to be statistically significant between the groups.
ConclusionWe believe our study has opened an important window on the relationship between infertility observed in varicocele patients and ADMA. We believe that broad-series prospective studies to support this are required.
El varicocele es uno de los causantes de la disfunción testicular y comúnmente cursa con infertilidad. La patología de base del varicocele es el desarrollo de la disfunción endotelial. Los factores más importantes para el desarrollo de la disfunción endotelial son la vasodilatación dependiente del endotelio, el aumento de los radicales libres del oxígeno, la reducción en la síntesis y liberación de óxido nítrico (NO), la vasoconstricción anormal y los niveles altos de dimetilarginina. Nuestro objetivo fue identificar e ilustrar la relación existente entre la dimetilarginina asimétrica (ADMA) y los niveles de NO en el tejido testicular y el plasma de ratas con varicocele inducido en laboratorio.
Material y métodosEn el estudio se incluyeron veintiuna ratas macho adolescentes (6 semanas de media), que se dividieron en 3 grupos al azar. El Grupo 1 (control, n=6) no fue sometido a ninguna operación. Al Grupo 2 (grupo quirúrgico de referencia, n=6) se le rodeó de manera proximal la vena renal izquierda, pero no se realizó la ligadura. En el Grupo 3 (varicocele inducido, n=9) se realizó una ligadura parcial de la vena renal izquierda para inducir varicocele izquierdo. Se investigaron en el tejido testicular los niveles de actividad de la enzima superóxido dimutasa (SOD) y de productos finales del NO, sales de nitritos y nitratos. Se investigaron los niveles de nitrito/nitrato y de ADMA en el plasma. El análisis histopatológico se completó con tinciones hematoxilina-eosina y TUNEL rutinarias.
ResultadosLos valores hísticos de SOD y plasmáticos de ADMA habían sufrido un aumento claro en el grupo de varicocele comparado con los otros grupos; los niveles hísticos y plasmáticos de nitrito/nitrato se habían visto claramente reducidos en el grupo de varicocele, lo cual se entendió como un valor estadístico significativo entre los grupos.
ConclusiónOpinamos que nuestra investigación ha abierto una puerta importante a la relación entre la infertilidad en casos de pacientes con varicocele y la ADMA. Para respaldarla, deberían llevarse a cabo amplios estudios prospectivos.
The relationship between varicocele and testicular dysfunction has been known for many years. Varicocele is diagnosed in about 10% of young males and is caused by enlargement of the pampiniform plexus, most frequently on the left side.1,2 About 65–70% of patients have significant reduction in sperm density and movement.3 Infertility is frequently observed and fertility returns after varicocele treatment for a significant portion of patients. Though varicocele is accepted as the most frequent cause of rectifiable infertility, its pathophysiological mechanism is not fully known.4,5
Nitric oxide (NO) is a heterodynamic molecular, colorless, odorless toxic gas with low molecular weight (30Da) and a range of effects in human biology recognized in recent years.6 NO is recognized as a physiologic mediator of cell relaxation and has been shown to play a role in vasoregulation, thrombocyte aggregation, immunity and neurotransmission.7,8 In studies on NO and fertility it has been shown that some situations that cause NO synthesis dysfunction affect fertility.9,10 The main problem in varicocele is enlarged veins which lead to disruption of testicular blood flow and development of endothelial dysfunction of the plexus pampiniform.11 The most important factors in development of endothelial dysfunction are impaired endothelial-linked vasodilatation, increase in free oxygen radicals, reduced synthesis and release of nitric oxide (NO), abnormal vasoconstriction and increase in dimethyl arginine levels.
Recent studies have emphasized the importance of endogenously released asymmetric dimethyl arginine (ADMA) which exhibits effects by inhibiting nitric oxygen synthesis in vascular pathologies. Asymmetric dimethyl arginine is an inhibitor of endogenous nitric oxide synthesis (NOS).12 The function of NOS in the body is to provide nitric oxide synthesis from l-arginine.13 In this reaction in the vascular endothelium ADMA inhibits NOS activity preventing l-arginine uptake into cells. In other words ADMA regulates the speed of formation of nitric oxide.14 Disruption of endothelial-linked vasodilatation, increased aggregation ability of platelets and increased monocyte adhesion are clinical evidence of endothelial dysfunction, which stimulate ADMA increase.
Our aim was to measure ADMA and NO levels in testicular tissue and plasma of rats with induced experimental varicocele to statistically research the relationship between them.
Materials and methodsExperimental animals and study groupsThis study was completed after permission was granted by Canakkale Onsekiz Mart University (COMU) Experimental Animal Ethics Committee. A total of 21 adolescent (average 6 weeks of age) Sprague–Dawley male rats weighing between 220 and 240g were included in the study. The animals were housed in a standard laboratory environment at constant temperature (18–21°C) and humidity with 12h daylight, 12h darkness. There were 3–4 rats in every cage fed with standard rat food and tap water.
The rats were randomly divided into 3 groups. Group 1 (control, n=6) did not undergo any procedure. Group 2 (sham, n=6) had the left renal vein circled proximally but ligation was not performed. Group 3 (varicocele, n=9) had partial ligation of the proximal left renal vein to induce left varicocele. All groups were sacrificed 8 weeks after the procedure.
Surgical proceduresAll rats due for induction of experimental left varicocele and for the sham operation were starved and only allowed water for 6h before the procedure. The rats were taken for the operation in accordance with antiseptic rules, maintaining body temperature under laboratory conditions. After anesthesia was induced by ether inhalation all rats were laid on their backs and on the front face of the abdomen a 2cm midline incision was made. Passing through the skin, subcutaneous, abdominal front wall and peritonea above the linea alba, the interior of the abdomen was reached. The intestines were pushed medially and the vena cava, fat and connective tissue were dissected to provide appropriate visuals of the left renal vein and the left renal vein was freed proximally. In rats of the induced varicocele group a thin clamp was used to circle around the renal vein proximal followed by 4/0 silk suture. Then a 0.85mm thickness L-shaped metal rod was placed above and parallel to the left renal vein and using 4/0 silk suture the renal vein and metal rod were wrapped to narrow the renal vein. Later the metal rod was retracted leaving the renal vein narrowed by 50%. In the sham group the renal vein was circled with a thin clamp and then 4/0 silk was passed around it; however, it was not tied (the narrowing procedure was not performed) and the procedure was completed. Later 3/0 catgut and 3/0 silk suture were used to close the 2 layers of the abdomen. Eight weeks after this procedure the rats in all the groups were sacrificed.
Biochemical testsThe testis of all rats were dissected and after excision, stored at −80°. To identify biochemical changes the superdioxide dismutase (SOD) enzyme activity and NO endproducts of nitrite and nitrate salt levels in the testis were measured. To investigate the nitrite–nitrate levels in plasma 5cc intracardiac blood samples were taken, centrifuged at 3000rpm for 10min and stored at −40°C until sample studies.
Tissue homogenizationAfter tissue samples were weighed, for total nitrite/nitrate and SOD assays they were homogenized with 0.9% NaCl and 10% homogenate was prepared. For total nitrite/nitrate and SOD assays the prepared homogenates were centrifuged at 15,000rpm for 15min. Samples were taken of the supernatant liquid for both assays.
Nitrite/nitrateFor measurement the “nitric oxide colorimetric assay” (Boehringer Mannheim) kit was used spectrophotometrically at 540nm.
Tissue superoxide dismutase activity assayFor tissue SOD activity the method of Sun et al.15 was modified and measurement was at 560nm using spectrophotometric methods.
Asymmetric dimethyl arginineTo measure the amount of ADMA levels in serum the BioVendor Research and Diagnostic Products (Cat. No: REA 201/96) manufactured by DLD Diagnostica GMBH (Germany) kit was used. The results were determined using the ELISA method and are given as nanogram per milliliter (ng/mL).
Histological examinationThe preparations were examined using both routine histological (H+E) and using TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick end labeling) to determine apoptosis. Examination used Oncogene Research Products, San Diego, CA, USA, firms QIA 33 (colorimetric) catalog number DNA fragmentation identification kits.
Statistical evaluationThe results of this study were evaluated using the Kruskal Wallis and Mann–Whitney U tests.
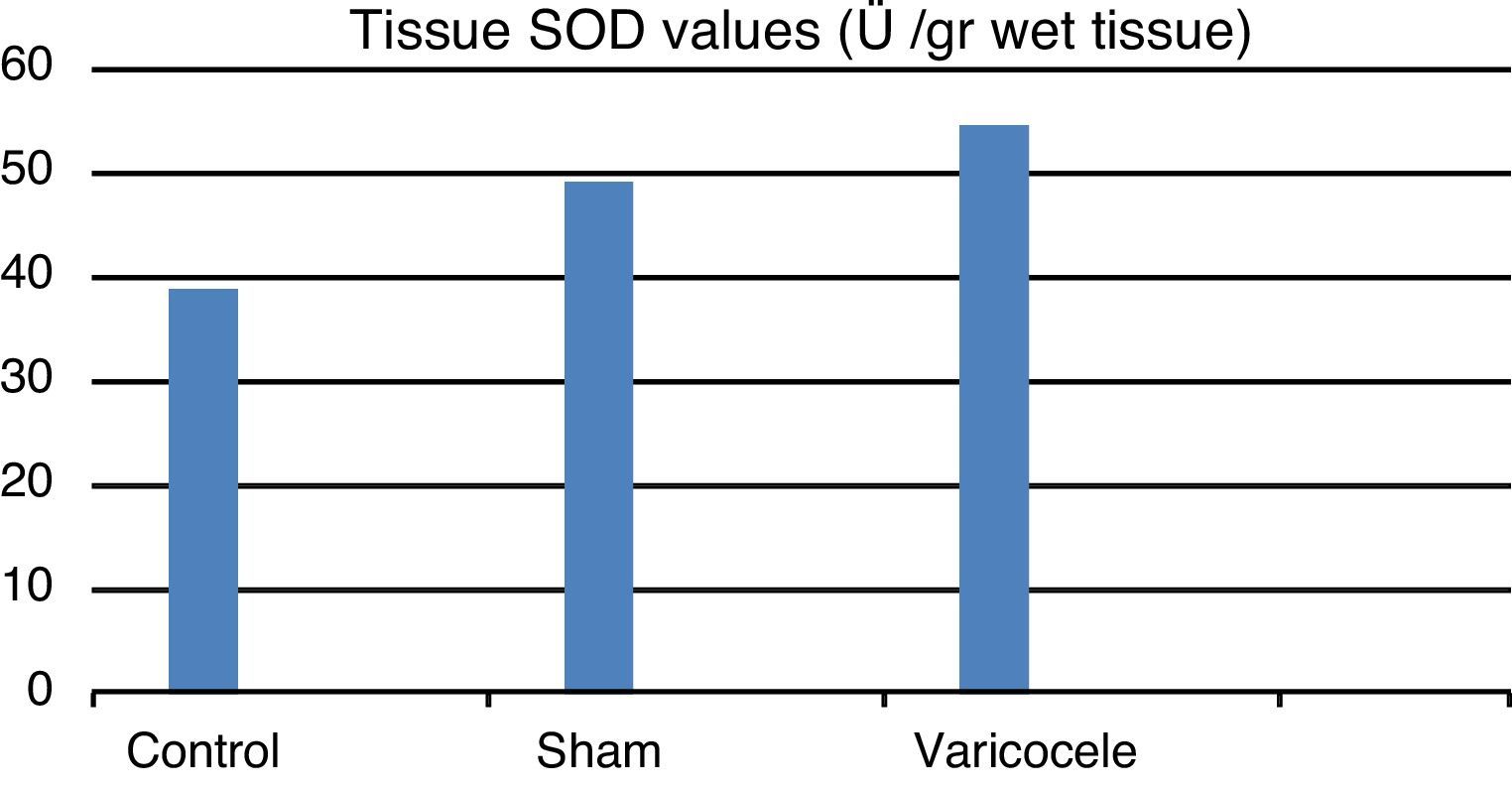
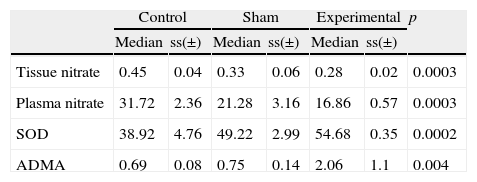
ResultsLinked to increased oxidant stress SOD, the enzyme with primary antioxidant properties, values for the 3 groups were found to be: control group 38.92±37.53 mean and standard deviation; sham group 49.22±2.99; and varicocele group 54.68±54.68 (Fig. 1). Pairwise comparison of all groups found a statistically significant difference (p=0.0002). We believe that this increase is a response to oxidative stress.
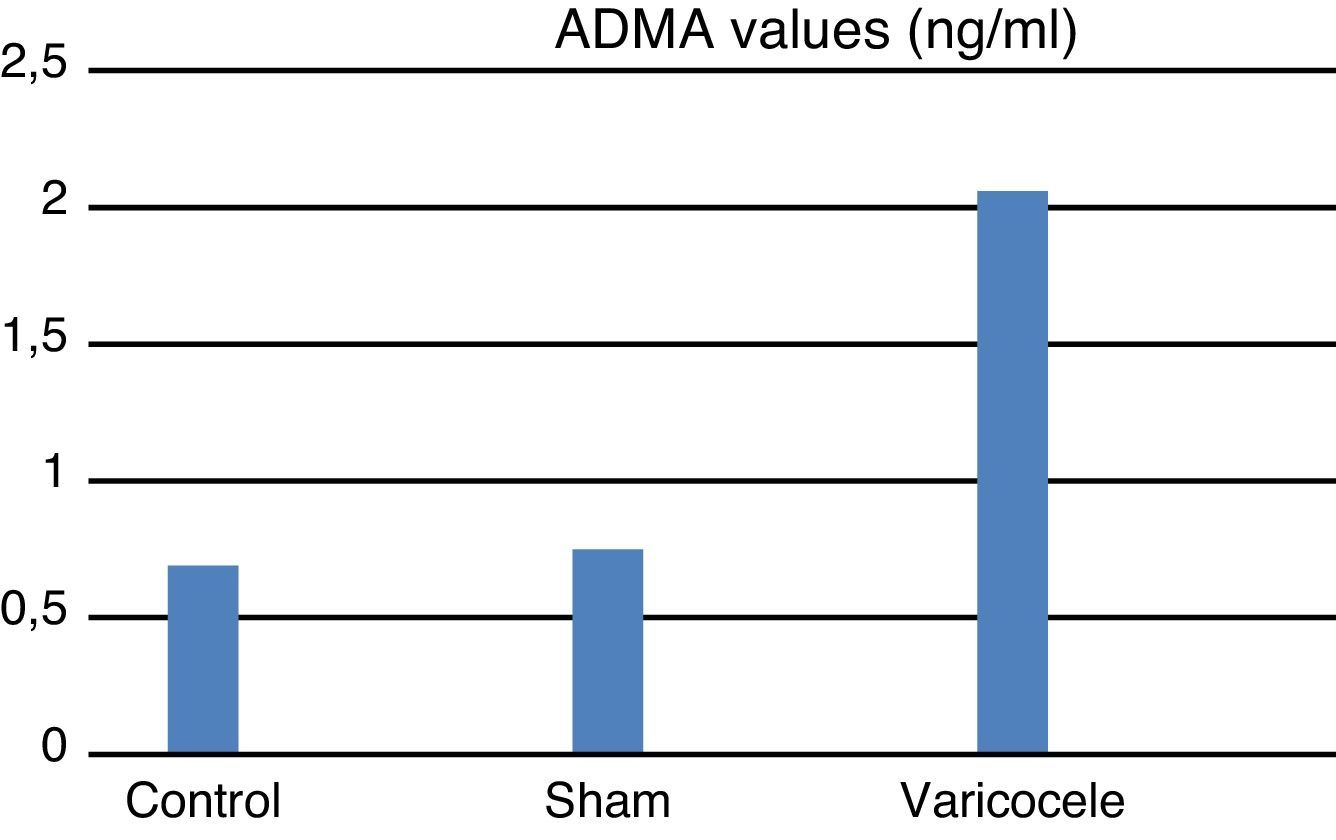
The ADMA values measured in blood samples for the groups were found to be: varicocele group 2.06±1.1 mean and standard deviation; sham group 0.75±0.14; and control group 0.69±0.08 (Fig. 2). ADMA values in the varicocele experimental group were significantly higher than the sham and control groups (p=0.004). The sham group had higher ADMA values than the control group. This shows that surgical interventions that create oxidative stress can raise ADMA levels.
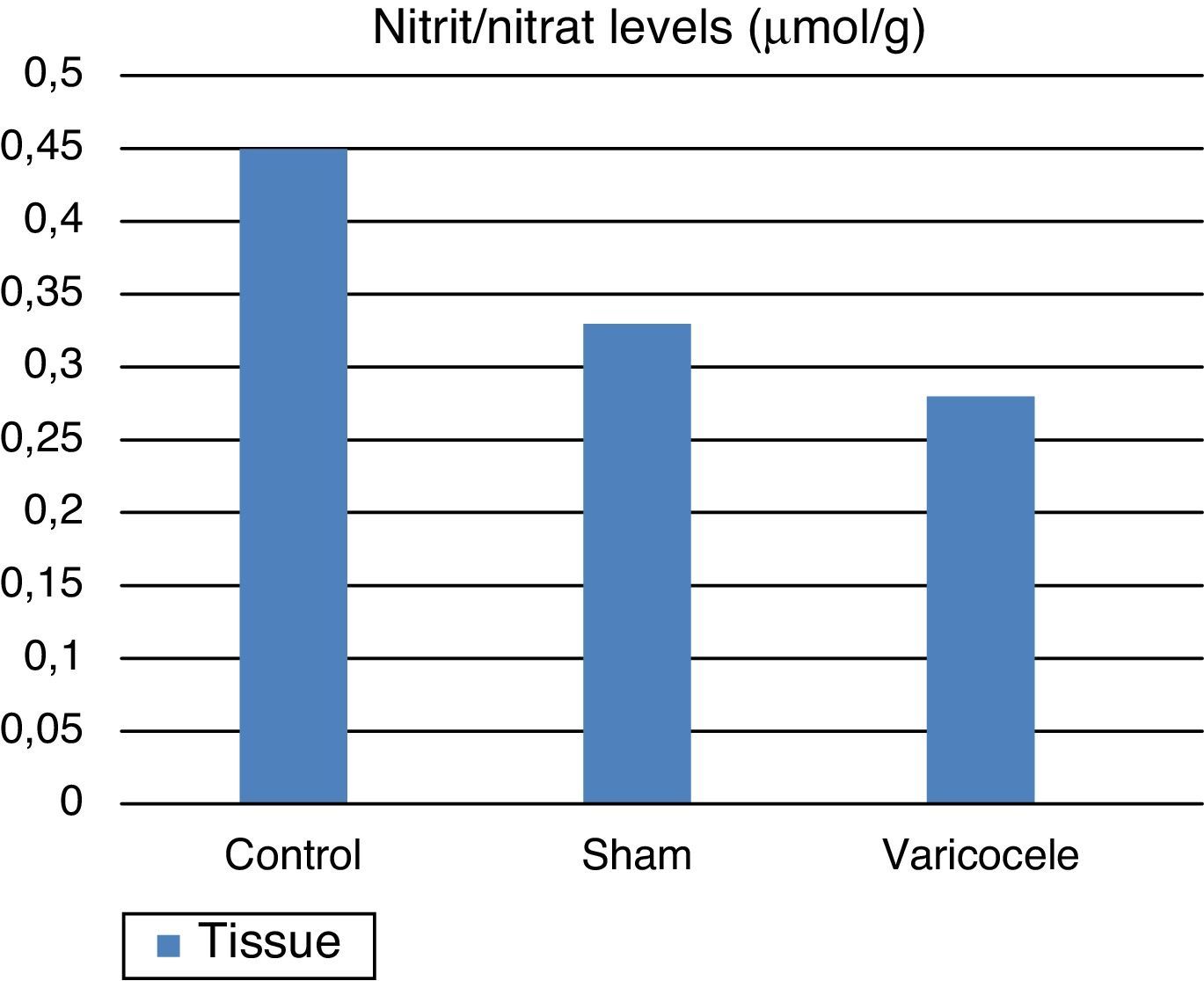
Nitrite/nitrate levels, NO end-products, in testis tissue were found to be: control group 0.45±0.04 mean and standard deviation; sham group 0.33±0.06; and varicocele group 0.28±0.02 (Fig. 3). According to these results the NO end-product salts in the varicocele group and sham group were statistically lower than the control group (p=0.0003). It is thought that the environmental NOS inhibition is responsible for this reduction.
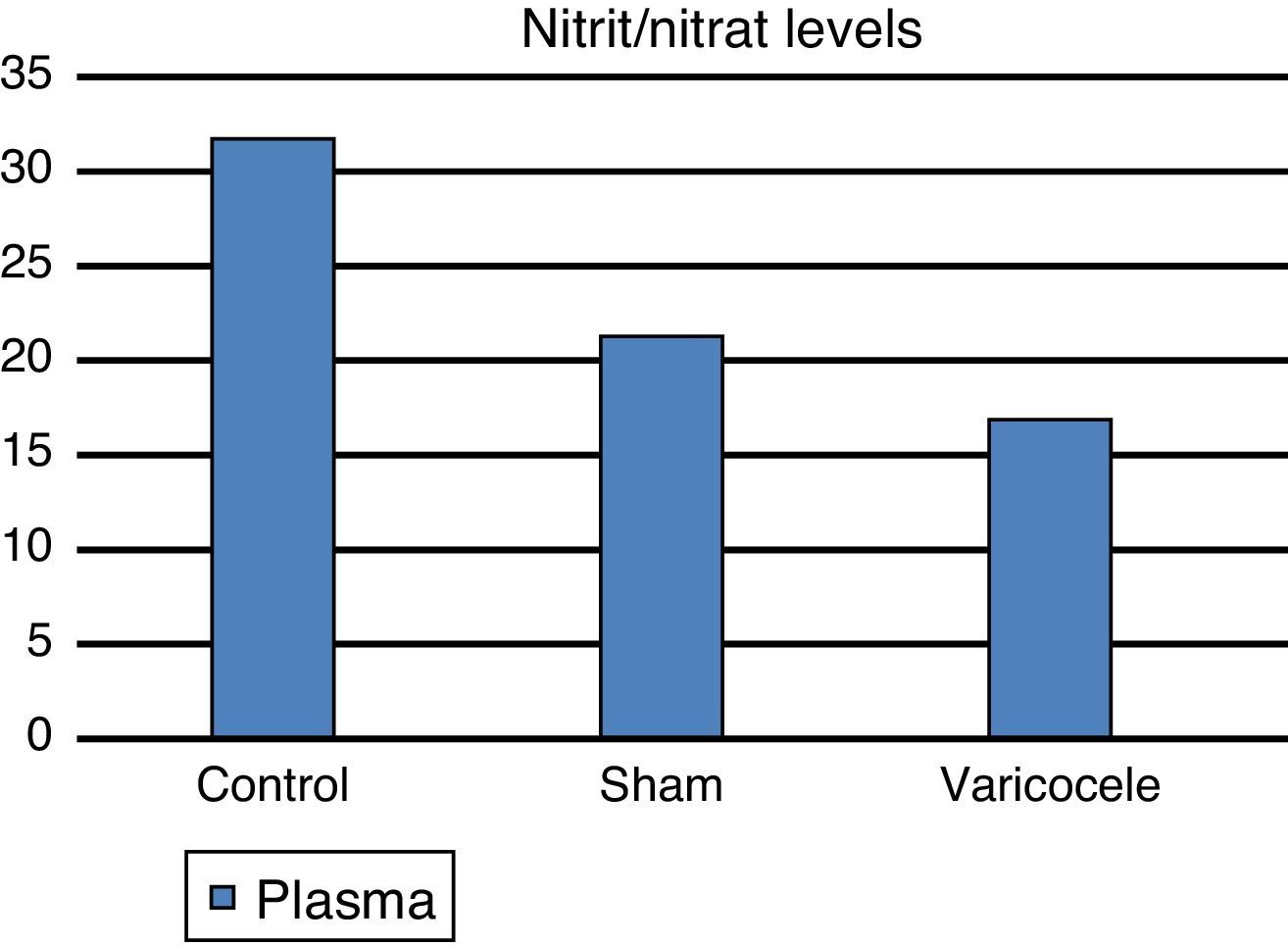
Plasma nitrite/nitrate level assay results for the control group were 31.72±2.36 mean and standard deviation, for the sham group 21.28±3.16 and for the varicocele group 16.86±0.57 (Fig. 4). These results had lowest plasma levels in the varicocele group, followed by the sham group and the highest values were in the control group, with differences found between all groups (p=0.0003) (Table 1).
Biochemical results are summarized in the table the results for the three groups.
| Control | Sham | Experimental | p | ||||
| Median | ss(±) | Median | ss(±) | Median | ss(±) | ||
| Tissue nitrate | 0.45 | 0.04 | 0.33 | 0.06 | 0.28 | 0.02 | 0.0003 |
| Plasma nitrate | 31.72 | 2.36 | 21.28 | 3.16 | 16.86 | 0.57 | 0.0003 |
| SOD | 38.92 | 4.76 | 49.22 | 2.99 | 54.68 | 0.35 | 0.0002 |
| ADMA | 0.69 | 0.08 | 0.75 | 0.14 | 2.06 | 1.1 | 0.004 |
At the histopathological examination, counting of apoptotic germ cell was performed in light microscopy with colorimetric staining (TUNEL dyes). Indexes of apoptotic change in groups were calculated as mean and standard deviation. Mean±SD was found to be 0.124±0.033 in control groups; 0.117±0.023 in sham group; and 0.312±0.043 in varicocele group. Between control and sham groups were not significant (p>0.05). In comparison of the varicocele group with the control and sham groups, apoptotic cells were increased significantly (p<0.05).
DiscussionThe basic problem that causes infertility in varicocele is disrupted testicular blood flow due to dilated veins, increased testicular temperature and release of free oxygen radicals. Studies in recent years have emphasized the importance of endogenously released asymmetric dimethyl arginine, which has the effect of inhibiting nitric oxide synthesis in vascular pathologies. Endothelium is a single-layer of surficial cells that surrounds the vascular lamina in the whole body. Diagnosis of endothelial dysfunction is defined by loss of the characteristic properties of the endothelial layer necessary to protect organ function.16,17 Endothelial dysfunction is characterized by a reduction in NO production and/or bioavailability. Apart from this damage to the endothelial cells themselves reduces biological NO activity, forming a negative cycle of atherosclerosis development and has been shown to cause progression.18,19 One of the factors in insufficient NO is the increase in reactive oxygen species (ROS).19 In situations of increased oxidative stress in the body the increase in ADMA levels may be linked to reduction in dimethylarginine dimethylaminohydrolase (DDAH) enzyme activity. The reduction in activity develops due to the oxidation of the cysteine in the active region of DDAH. This also reduces NO levels.20,21
ADMA plays the role of endogenic inhibitor to NO synthesis. Vallance et al.22 in 1992 found ADMA and l-NMMA (a nitric oxide synthase inhibitor) in human plasma and urine and reported that they acted as competitive endogenous inhibitors of NOS. l-NMMA is found in human plasma at very low levels and its inhibitory effect on NOS activity is minimum, so ADMA is thought to be the real inhibitor of NOS activity through produced methylated arginine.23,24 ADMA inhibits all three isoforms of NOS. In vitro studies have shown that at physiologically appropriate levels ADMA inhibits NOS by an important amount, and later reduces NO production by endothelial cells (EC) in culture and in isolated human veins.25–27 Inflammation produces a large amount of NO. The produced NO combines with superoxide (O2−) radicals to form peroxynitrite (ONOO−) and reduces the half-life of NO. Peroxynitrite formation develops quicker than SOD can catch superoxide radicals. Peroxynitrite links to the active region of DDAH reducing activity, thus causing increase in ADMA amounts and reducing NO levels.28 As NO is a gas it is difficult to correctly identify in biochemical studies. Indirect methods may detect its presence in tissues. One of these methods is to show the nitrite nitrate levels in tissue, destruction products of NO. In our study while a statistically significant increase was found in the ADMA and SOD levels in the varicocele model compared to the control and sham groups, there was a statistically significant reduction in nitrate levels in tissue and plasma compared to the sham and control groups. The reduction in tissue and plasma nitrate levels is an important marker of endothelial dysfunction due to the reduction in NO levels.
Patients with varicocele have been shown to have increased MDA and SOD levels, markers of oxidative stress.29 In varicocele it has been shown that there is oxidative stress in the internal spermatic vein and increased antioxidant enzyme activity. Increased antioxidant enzyme activity may be a response to balance the negative effects of free oxygen radicals.30 Ozbek et al.31 evaluated the glutathione peroxidase (GSH-Px) and SOD activity using enzymatic methods after separating sperm from seminal plasma in 15 patients with varicocele (25–36 years) who applied to a urology clinic due to infertility and 12 normal fertile patients (24–36 years). While the patient group had lower preoperative levels compared to the control group (p<0.05), postoperative values showed no definite difference to the control group (p>0.05). In conclusion Ozbek et al. declared that in varicocele patients reduced antioxidant enzyme activity in seminal fluids may be responsible for sperm dysfunction in these patients and that after operation these values may return to normal. Aitken et al.32 researched the effects of free oxygen radicals on infertility. They reported that SOR increase had negative effects on sperm function and thus on fertilization rates. In our study SOD levels in tissue were examined and found to be significantly higher in the experimental varicocele group compared to the sham and control groups (p=0.0002). At the same time in the sham group SOD levels were significantly high compared to the control group. These results show the activity in the antioxidant capacity of testicular tissue in rats with induced experimental varicocele.
In conclusion, NO synthesis degradation plays an important role in the development of infertility in individuals with varicocele. ADMA, synthesis of which increases with endothelial dysfunction, is an endogenous NOS inhibitor. In our study we showed the ADMA levels were increased in varicocele induced rats compared to sham and control groups. Increased ADMA levels, by showing the inhibitory effect on NOS, causes a fall in NO levels in the testis. We believe that our study is important in revealing the relationship between infertility observed in varicocele patients and AMDA. To support this broad-series prospective studies are required.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare no conflict of interest.