This study aimed at investigating the aphrodisiac effects of aqueous extract of Carpolobia lutea root at the doses of 47, 94 and 141mg/kg body weight in paroxetine-induced sexual dysfunction in male rats.
Materials and methodsThirty sexually active male rats (148.20±3.22g) were assigned into six groups (A–F) of five animals each. Rats in group A received 0.5ml of distilled water once daily for 7 days while those in groups B, C, D, E and F which were induced with sexual dysfunction (oral administration of 10mg/kg of paroxetine suspension, once daily for 21 days) received 0.5ml corresponding to 7.14mg/kg body weight of PowmaxM, 47, 94 and 141mg/kg body weight of the extract and distilled water, respectively. Sexual behaviour parameters (frequencies of mount (ML), intromission (IF), ejaculation (EL), latencies of mount (ML), intromission (IL), ejaculation (EL) and post ejaculation interval (PEI)) were monitored 30min post administration by pairing (1:1) with receptive female rats (114.01±2.64g) on days 1, 4 and 7. The concentrations of serum testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) were determined after 7 days of administration using standard methods.
ResultsThe study revealed that the extract contained saponins (21.02mg/L), anthraquinones (5.11mg/L), alkaloids (2.93mg/L), flavonoids (1.82mg/L), tannins (0.91mg/L) and cardiac glycosides (0.09mg/L) whereas terpenes, phlobatannins and steroids were not detected. Paroxetine significantly (p<0.05) decreased mount frequency, intromission frequency, ejaculation frequency and ejaculation latency whereas it increased mount latency, intromission latency and post-ejaculatory interval for more than the baseline of 25% in each case. In contrast, all the doses of the extract significantly (p<0.05) attenuated the parameters of sexual behaviour displayed by the sexual dysfunction animals, with the 141mg/kg body weight comparing favourably (p>0.05) with the sexual dysfunction animals treated with Powmax. In addition, the extract significantly (p<0.05) elevated the levels of serum luteinizing hormone, follicle stimulating hormone and testosterone which were hitherto reduced by paroxetine.
ConclusionThe study concludes that the aqueous extract of C. lutea root especially the doses of 94 and 141mg/kg body weight restored various components of sexual arousal and performance as well as the reproductive hormones in the sexually sluggish male rats with the highest dose being the most effective. Present findings provide experimental evidence to support the folkloric claim of the plant in the management of sexual inadequacies in males.
el objetivo del presente estudio fue investigar el efecto afrodisíaco del extracto acuoso de la raíz de Carpolobia lutea en dosis de 47, 94 y 141mg/kg de masa corporal para casos de disfunción sexual inducida por paroxetina en ratas macho.
Materiales y métodosse distribuyeron treinta machos sexualmente activos (148,20±3,22g) en seis grupos (A-F) de 5 animales cada uno. Las ratas del grupo A recibieron 0,5ml de agua destilada al día durante 7 días, mientras que las de los grupos B, C, D, E y F—a quienes se había inducido una disfunción sexual con paroxetina (se administró una suspensión oral de paroxetina, 10mg/kg una vez al día durante 21 días)—recibieron respectivamente 0,5ml de PowmaxM en dosis de 7,14mg/kg de masa corporal, el extracto en dosis de 47, 94 y 141mg/kg de masa corporal, y agua destilada. Se observaron los parámetros de comportamiento sexual—frecuencia de cópula (ML), penetración (IF), eyaculación (EL), latencia de las cópulas (ML), penetración (IF), eyaculación (EL) e intervalo posteyaculación (PEI)—durante los 30 minutos subsiguientes a la administración comparando (1:1) con las ratas hembra (114,01±2,64g) en los días 1, 4 y 7. Las concentraciones de testosterona sérica, folitropina (FSH) y lutropina (LH) se fijaron con métodos normalizados después de 7 días de administración.
Resultadosel estudio reveló que el extracto contenía saponinas (21,02mg/l), antraquinonas (5,11mg/l), alcaloides (2,93mg/l), flavonoides (1,82mg/l), taninos (0,91mg/l) y glucósidos cardiacos (0,09mg/l), si bien no se detectaron terpenos, florotaninos ni esteroides. La paroxetina redujo de manera significativa (p<0,05) la frecuencia de cópula, frecuencia de penetración, frecuencia de eyaculación y latencia de las cópulas, aunque aumentó la latencia de las cópulas, latencia de penetración y el intervalo posteyaculación en más de un 25% respecto a los valores de referencia en casa caso. Por el contrario, todas las dosis del extracto atenuaron de manera significativa (p>0,05) el comportamiento sexual de los animales con la disfunción sexual; aquellos con 141mg/kg de masa corporal arrojaron resultados favorables (p>0,05) en comparación con los que fueron tratados con Powmax. Asimismo, el extracto aumentó enormemente (p<0,05) los niveles séricos de lutropina, folitropina y testosterona que hasta el momento se veían reducidos por la paroxetina.
Conclusiónel estudio llega a la conclusión de que el extracto acuoso de la raíz de C. lutea, con las dosis de 94 y 141mg/kg de masa corporal en particular, recuperaron varios componentes de la excitación y el rendimiento sexual, así como las hormonas reproductivas en las ratas macho sexualmente lentas. La dosis más alta resultó ser la más eficaz. Los presentes hallazgos suponen una demostración experimental para apoyar las reivindicaciones populares del uso de la planta en la gestión de incapacidades sexuales en varones.
Sexual relationships/interactions are among the most important social and biological activities in animals. Male sexual behaviour consists of both appetitive (motivational) and consummatory components that are regulated by gonadal steroid hormones secreted from the testes.1 Male sexuality is mainly governed by a well organized neural circuit that connects a variety of brain areas including the medial preoptic area, nuclear accumbens and the bed nucleus of stria terminals which appear to control the different components of mating behaviours.2,3 However, at one time or the other, this sexual relationship or sexuality may be bedevilled with some challenges such as sexual dysfunction. Sexual dysfunction in males (MSD) is the repeated inability to perform satisfactory sexual intercourse in 25% of all attempted sexual encounter. It also refers to hindrance/problems during any phase of the sexual response cycle that prevents individuals or couples from performing or enjoying normal sexual activity. MSD presents as erectile dysfunction or impotence (inability to have or maintain an erection sufficient for sexual functioning); ejaculation disorders including premature, inhibited, retarded or retrograde ejaculation; orgasmic disorder (inability to reach orgasm/climax with a partner, absence of orgasm during sexual intercourse and/or absence of orgasm without lengthy sexual contact), inhibited or hypoactive sexual desire (a disinterest in sexual contact or complete lack of sexual desire) and priapism (prolonged erection unaccompanied by sexual desire). Sexual dysfunctions are highly prevalent in men worldwide and Rosen4 puts it at 31% while Lewis et al.5 estimated it to be 20–30% and it increases with age. Several management options for MSD which included medications, mechanical aids such as penile implants, psychological and hormonal interventions are not without their shortcomings such as aching in the copulatory organ, urethral burning, infections, pains and bleeding.6 Therefore, there is the need to search for cheaper, effective options with reduced side effects in medicinal plants.
Carpolobia lutea G. Don (family: polygalaceae) is also known as cattle stick (English), ikpafum (Ibibio, Southern Nigeria), Agba or Angalagala (Igbo-Eastern Nigeria) and Oshunshun (Yoruba-Western Nigeria).7 It is widely found in tropical Africa where it grows in the rainforest and Guinea savannah of Sierra Leone and Cameroon as a dense overgrowth or an evergreen shrub or small tree of 5m high.8 Ethnobotanically, various parts of the plant have been claimed to be used in the management of several ailments. For instance, the leaves are used to cure fever, ulcer, malaria, dermal infection, venereal diseases, sterility, vermifuge, taenifuge, stomach problem, diarrhoea, headache, leprosy, snakebite and wounds. The leaves have also been used to promote child birth while the root bark have been implicated to be used for treating rheumatism, fever, general pain and insanity.9–13 The stem bark is dried and taken as snuff to cure migraine9. Furthermore, the decoction of the root is reputed in Western and Southern Nigeria (Yoruba ethnicity and Ibibios of Akwa Ibom State of Nigeria) as “ogun aleko” meaning sex invigorating tonic or aphrodisiac.12,14
Pharmacological studies reported in the open scientific literature on C. lutea are limited to anti-diarrhoeal and anti-ulcerogenic activities of the ethanolic extract and ethyl acetate fractions of the leaf,14,15 gastroprotective effect of the leaf extract,16 neuropharmacological activity of the cinnamoyl and coumaroyl glucosides from leaf fractions,17in vitro antiplasmodial,18 antimicrobial activity of the leaves,19in vivo antimalarial activity of the ethanolic extract and fractions of leaf and root,20 analgesic,21,22 contraceptive,23in vitro anti-trypanosomal and anti-leishmanial activities,24 antidiabetic and hypoglycaemic activities of ethanolic leaf extract and fractions.8
Although, myriads of scientific studies on the efficacy of the plant have been reported, there is no scientific report that has evaluated the sexual function enhancing effect of the plant, notwithstanding the acclaimed use of the plant as an aphrodisiac in some regions of Nigeria and Cote D’ Ivore. Therefore, the present study aimed at evaluating the aphrodisiac effect of the aqueous extract of C. lutea root in paroxetine-induced sexually impaired male rats.
Materials and methodsPlant materialsFresh roots of C. lutea were purchased from herb sellers at a market (Oja tuntun) in Ilorin, Nigeria. The plant was authenticated at the Department of Plant Biology, University of Ilorin, Ilorin, Nigeria and a voucher specimen (UIH No. 030) was deposited at the Herbarium Unit of the Department.
AnimalsThirty healthy, in-bred, sexually active, male Wistar rats weighing (148.20±3.22)g and equal number of female rats (114.01±2.64g) were obtained from the Animal Holding Unit of the Department of Biochemistry, University of Ilorin, Ilorin, Nigeria. The animals were placed in their respective cages and housed in a well-ventilated Animal House under the following conditions: temperature: 22±3°C; 12:12h light and dark cycle; humidity: 45–50%).25
Assay kitsAssay kits for testosterone, luteinizing and follicle stimulating hormones were products of Monobind Inc., Lake Forest, USA. Progesterone and estradiol benzoate were from Ningbo Tisun Medic Biochemic Co. Ltd., Ningbo, Peoples Republic of China and Sigma Chemical, St. Louis, USA, respectively. All other reagents used were of analytical grade and were prepared in volumetric flask using all glass-distilled water.
Preparation of extractThe fresh roots of C. lutea were sliced very thinly, oven-dried at 40°C for 48h and pulverized. A known weight (50g) of the powder was extracted in 200ml of distilled water for 48h at room temperature with constant shaking. The lyophilized (Vir Tis Benchtop K, Vir Tis Co., Gardiner, NY) filtrate yielded 5.12g (percentage yield of 10.24%). Calculated amounts of the yield were constituted in distilled water to give the required doses of 47, 94, and 141mg/kg body weight. The dose of 94mg/kg body weight corresponded to what is normally used in the folk medicine by an adult male of 70kg for the management of sexual dysfunction while other doses of 47 and 141mg/kg body weight were half of 94mg/kg body weight and three fold of 47 mg/kg body weight.
Phytochemical screeningThe extract of C. lutea roots was screened for the presence of some secondary metabolites as described for alkaloids, saponins, tannins, anthraquinones, cardiac glycosides, flavonoids, terpenes, phlobatannins and steroids.26 The amount of the detected metabolites was determined as described for saponins,27 anthraquinones and cardiac glycoside,28 alkaloids,29 flavonoids30 and tannins.31
Induction of sexual dysfunction and assessment of mating behaviour indices in male ratsTwenty-five male rats were orally administered 10mg/kg of paroxetine hydrochloride suspension (prepared daily in Tween-80 [BDH Chemicals Ltd., Poole, England], suspended in 0.9% saline solution) using a metal oropharyngeal cannula, once daily for 21 days to induce sexual dysfunction.32,33 Similarly, intact, healthy female rats were artificially brought into oestrus (since female rats allows indiscriminate mating only during this period and for the data to be reliable) by a single injection of 50μg of estradiol benzoate 36h before testing with male rats.32 Rats in the oestrus phase were confirmed by vaginal smears examinations as described by OECD.34 The primed female rats that exhibited profound receptivity were introduced into the cages containing the male rats after which the following sexual behaviour parameters were observed directly from the cage side and also using camera (Cannon PowerShot A560 Digital, Canon Inc., USA) in dim light, for 30min: MF (number of times the male assumes copulatory position but failed to achieve intromission-characterized by lifting of the males fore body over the hind quarter of the female and clasping her flanks with its fore hand); IF (number of intromissions from the time of introduction of the female until ejaculation); EF (number of ejaculations made during the observatory period); ML (time interval between the introduction of the female and the first mount by the male); IL (time interval from the introduction of the female to the first intromission by the male, usually characterized by pelvic thrusting and springing dismounts); EL (time interval between the first intromission and ejaculation, usually characterized by longer, deeper pelvic thrusting and slow dismount followed by a period of inactivity or reduced activity) and PEI (time interval between ejaculation and erection of the male copulatory organ for the next phase). The frequencies and latencies of these behaviour parameters were determined electronically from cassette transcription and were reconciled with camera recording. Male rats which showed minimum reduction of 25% in MF, IF, EF and EL as well as minimum increase of 25% in ML, IL and PEI were declared as having sexual dysfunction and used for the subsequent study.35
Animal grouping and extract administrationThirty male rats, after two weeks of acclimatization were assigned into six groups (A–F) of five animals each. Rats in group A (control group) were orally administered 0.5ml of distilled water, once daily with the aid of a metal oropharnygeal cannula while those in groups B, C, D, E and F, apart from being induced with sexual dysfunction (10mg/kg of paroxetine suspension (prepared daily in Tween-80 [BDH Chemicals Ltd., Poole, England], suspended in 0.9% saline solution)32,33 also received 0.5ml each of distilled water, 7.14mg/kg body weight of PowmaxM (a reference male sexual stimulant and energy enhancer polyherbal drug consisting of Panax ginseng, Camelia sinensis, Cnidium monnier, Epimedium brevicornum, Songaria cynomorium, Gingko biloba, Dahurian angelica, Salvia miltiorrhiza root, l-arginine hydrochloride and Gamma aminobutyric acid), 47, 94 and 141mg/kg body weight of the extract respectively. Exactly 30min after treatment, the male rats were monitored for sexual behaviour on days 1, 4 and 7 as previously described. The study was conducted following the guidelines on the care and use of laboratory animals of the Ethical Committee of the Department of Biochemistry, University of Ilorin. The guidelines on the use of experimental animals by the European Convention and other Scientific purposes-ETS-123 were also strictly adhered to.36
Preparation of serumThe procedure described by Yakubu et al.37 was adopted for the preparation of serum. Twenty-four hours after seven daily doses of the distilled water, extract and PowmaxM to the animals, the rats were anaesthetized in diethyl ether fumes. When they became unconscious, the jugular veins were cut and 5ml of the blood was collected in centrifuge tubes, allowed to clot for 10min at room temperature and centrifuged at 685×g for 10min. The serum was collected with the aid of Pasteur pipette and used within 12h of preparation for the hormone analyses.
Determination of serum male reproductive hormonesThe concentrations of testosterone, FSH and LH in the serum of the animals were determined according to the test procedures outlined in the Instruction Manual contained in the assay kits. Although the hormone assay kits were designed for use on human serum by a microplate immunoenzymometric assay (EMA/ELISA), the analyzer was calibrated and validated for use with animal sera. In the case of the testosterone, the within- and between-assay precision coefficients of variation were 4.1% and 7.3%, respectively, and the correlation coefficient, sensitivity of the assay, and cross-reactivity were 0.805, 0.445ng/mL, and 0.8, respectively. Furthermore, the within- and between-precision assay coefficients of variation for FSH were 3.5% and 7.0%, respectively whereas those for LH were 3.6% and 8.4% respectively. The correlation coefficient, sensitivity, and cross-reactivity for LH were 0.878, 0.003mIU/well, and <0.0001 while 0.908, 0.006mIU/well and <0.0001 were for FSH.
Statistical analysisData were expressed as the mean±SEM of five determinations except otherwise stated. The data were subjected to statistical analysis using Duncan's multiple range test and complemented with Students t-test. The analyses were done with Students Package for Social Science, version 17.0 (SPSS Inc.; Chicago, USA). Differences were considered statistically significant at p<0.05.
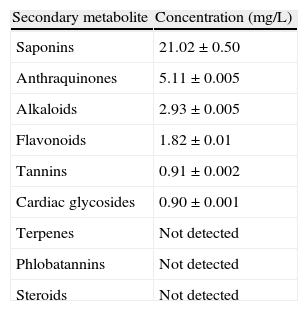
ResultsPhytochemical screening of the aqueous extract of C. lutea root revealed the presence of saponins, anthraquinones, alkaloids, flavonoids, tannins and cardiac glycosides whereas terpenes, phlobatannins and steroids were not detected (Table 1). Saponins occurred as the most abundant in the root extract while glycosides and tannins were present in trace amount. In addition, alkaloids, anthraquinones and flavonoids were present in moderate amount (Table 1).
Secondary metabolite constituents of aqueous extract of Corpolobia lutea roots.
| Secondary metabolite | Concentration (mg/L) |
| Saponins | 21.02±0.50 |
| Anthraquinones | 5.11±0.005 |
| Alkaloids | 2.93±0.005 |
| Flavonoids | 1.82±0.01 |
| Tannins | 0.91±0.002 |
| Cardiac glycosides | 0.90±0.001 |
| Terpenes | Not detected |
| Phlobatannins | Not detected |
| Steroids | Not detected |
Values are means±three determination.
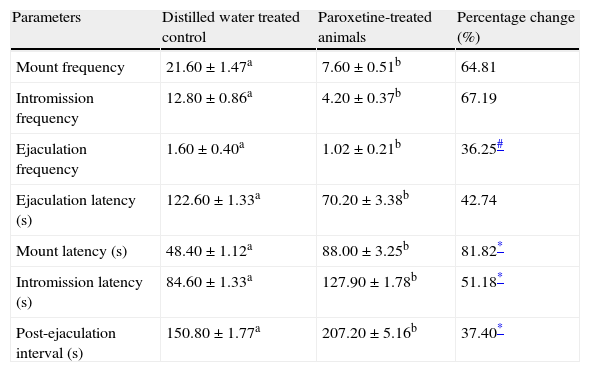
Treatment of the sexually active male rats with paraoxetine hydrochloride significantly reduced (p<0.05) the MF, IF, EF and EL and also increased the ML, IL and PEI; the change in each of the parameter was more than the baseline of 25% (Table 2).
Effects of paroxetine hydrochloride on the sexual behaviour parameters of male rats.
| Parameters | Distilled water treated control | Paroxetine-treated animals | Percentage change (%) |
| Mount frequency | 21.60±1.47a | 7.60±0.51b | 64.81 |
| Intromission frequency | 12.80±0.86a | 4.20±0.37b | 67.19 |
| Ejaculation frequency | 1.60±0.40a | 1.02±0.21b | 36.25# |
| Ejaculation latency (s) | 122.60±1.33a | 70.20±3.38b | 42.74 |
| Mount latency (s) | 48.40±1.12a | 88.00±3.25b | 81.82* |
| Intromission latency (s) | 84.60±1.33a | 127.90±1.78b | 51.18* |
| Post-ejaculation interval (s) | 150.80±1.77a | 207.20±5.16b | 37.40* |
Data are mean of five determinations±SEM.
Values carrying superscripts different from the control for each parameter are significantly different (p<0.05).
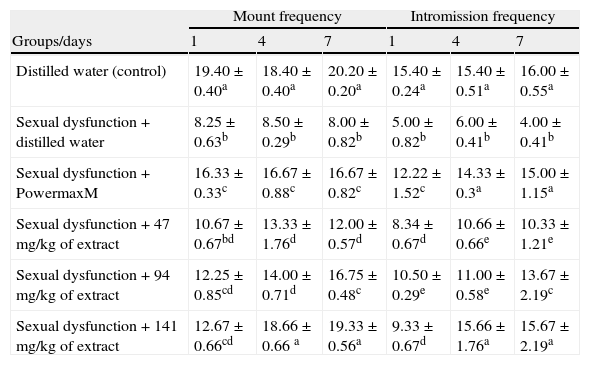
The extract significantly and progressively increased the MF and IF in the paroxetine-induced sexually sluggish animals with the values of MF and IF that were not significantly different (p>0.05) from those of sexually impaired animals treated with the reference drug, PowmaxM (Table 3).
Effects of aqueous extract of Carpolobia lutea root on mount and intromission frequencies of paroxetine-induced sexual dysfunction in male rats.
| Mount frequency | Intromission frequency | |||||
| Groups/days | 1 | 4 | 7 | 1 | 4 | 7 |
| Distilled water (control) | 19.40±0.40a | 18.40±0.40a | 20.20±0.20a | 15.40±0.24a | 15.40±0.51a | 16.00±0.55a |
| Sexual dysfunction+distilled water | 8.25±0.63b | 8.50±0.29b | 8.00±0.82b | 5.00±0.82b | 6.00±0.41b | 4.00±0.41b |
| Sexual dysfunction+PowermaxM | 16.33±0.33c | 16.67±0.88c | 16.67±0.82c | 12.22±1.52c | 14.33±0.3a | 15.00±1.15a |
| Sexual dysfunction+47mg/kg of extract | 10.67±0.67bd | 13.33±1.76d | 12.00±0.57d | 8.34±0.67d | 10.66±0.66e | 10.33±1.21e |
| Sexual dysfunction+94mg/kg of extract | 12.25±0.85cd | 14.00±0.71d | 16.75±0.48c | 10.50±0.29e | 11.00±0.58e | 13.67±2.19c |
| Sexual dysfunction+141mg/kg of extract | 12.67±0.66cd | 18.66±0.66 a | 19.33±0.56a | 9.33±0.67d | 15.66±1.76a | 15.67±2.19a |
Data are mean of five determinations±SEM.
Values carrying superscripts different from control from the control down the group for each day and parameter are significantly different (p<0.05).
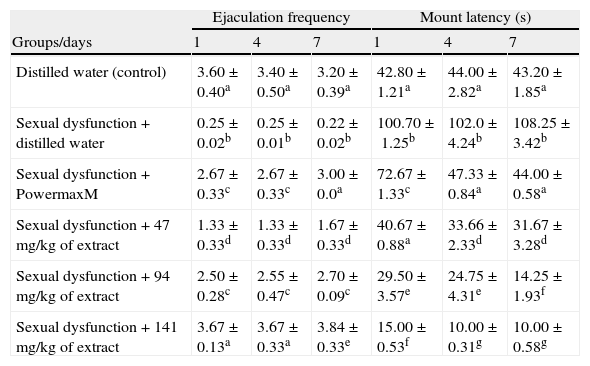
Extract administration significantly (p<0.05) attenuated and increased the EF of the paroxetine-induced sexual dysfunction in the animals (Table 4). In contrast, the ML of the sexually impaired animals was reduced in a manner related to the doses and exposure periods (Table 4).
Effects of aqueous extract of Carpolobia lutea root on ejaculation frequency and mount latency of paroxetine-induced sexual dysfunction in male rats.
| Ejaculation frequency | Mount latency (s) | |||||
| Groups/days | 1 | 4 | 7 | 1 | 4 | 7 |
| Distilled water (control) | 3.60±0.40a | 3.40±0.50a | 3.20±0.39a | 42.80±1.21a | 44.00±2.82a | 43.20±1.85a |
| Sexual dysfunction+distilled water | 0.25±0.02b | 0.25±0.01b | 0.22±0.02b | 100.70±1.25b | 102.0±4.24b | 108.25±3.42b |
| Sexual dysfunction+PowermaxM | 2.67±0.33c | 2.67±0.33c | 3.00±0.0a | 72.67±1.33c | 47.33±0.84a | 44.00±0.58a |
| Sexual dysfunction+47mg/kg of extract | 1.33±0.33d | 1.33±0.33d | 1.67±0.33d | 40.67±0.88a | 33.66±2.33d | 31.67±3.28d |
| Sexual dysfunction+94mg/kg of extract | 2.50±0.28c | 2.55±0.47c | 2.70±0.09c | 29.50±3.57e | 24.75±4.31e | 14.25±1.93f |
| Sexual dysfunction+141mg/kg of extract | 3.67±0.13a | 3.67±0.33a | 3.84±0.33e | 15.00±0.53f | 10.00±0.31g | 10.00±0.58g |
Data are mean of five determinations±SEM.
Values carrying superscripts different from control from the control down the group for each day and parameter are significantly different (p<0.05).
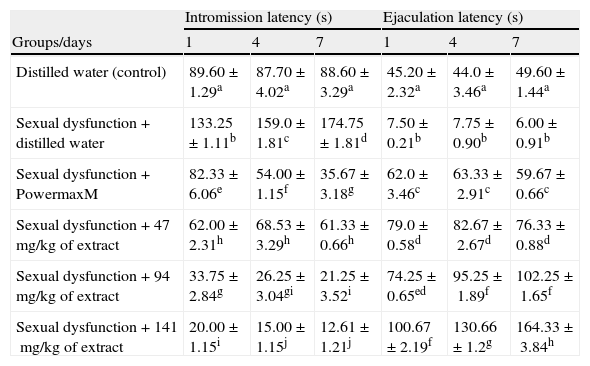
In addition, all the doses of the extract significantly decreased (p<0.05) the IL of the sexually sluggish animals whereas the EL was significantly prolonged (p<0.05) (Table 5). The alterations in the IL and EL by the extract did not compare favourably (p>0.05) with the non-sexually impaired animals treated with distilled water (Table 5).
Effects of aqueous extract of Carpolobia lutea root on latencies of intromission and ejaculation of paroxetine-induced sexual dysfunction in male rats.
| Intromission latency (s) | Ejaculation latency (s) | |||||
| Groups/days | 1 | 4 | 7 | 1 | 4 | 7 |
| Distilled water (control) | 89.60±1.29a | 87.70±4.02a | 88.60±3.29a | 45.20±2.32a | 44.0±3.46a | 49.60±1.44a |
| Sexual dysfunction+distilled water | 133.25±1.11b | 159.0±1.81c | 174.75±1.81d | 7.50±0.21b | 7.75±0.90b | 6.00±0.91b |
| Sexual dysfunction+PowermaxM | 82.33±6.06e | 54.00±1.15f | 35.67±3.18g | 62.0±3.46c | 63.33±2.91c | 59.67±0.66c |
| Sexual dysfunction+47mg/kg of extract | 62.00±2.31h | 68.53±3.29h | 61.33±0.66h | 79.0±0.58d | 82.67±2.67d | 76.33±0.88d |
| Sexual dysfunction+94mg/kg of extract | 33.75±2.84g | 26.25±3.04gi | 21.25±3.52i | 74.25±0.65ed | 95.25±1.89f | 102.25±1.65f |
| Sexual dysfunction+141mg/kg of extract | 20.00±1.15i | 15.00±1.15j | 12.61±1.21j | 100.67±2.19f | 130.66±1.2g | 164.33±3.84h |
Data are mean of five determinations±SEM.
Values carrying superscripts different from control from the control down the group for each day and parameter are significantly different (p<0.05).
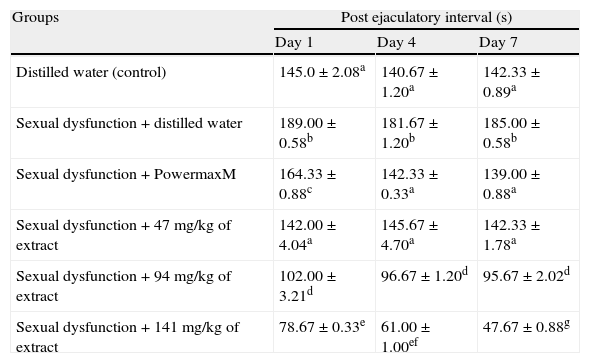
The computed post-ejaculatory interval of the sexual dysfunction male rats was decreased (p<0.05) following the administration of the extract in a manner related to the doses and the exposure periods (Table 6).
Effect of aqueous extract of Carpolobia lutea roots on post ejaculation interval of paroxetine-induced sexual dysfunction in male rats.
| Groups | Post ejaculatory interval (s) | ||
| Day 1 | Day 4 | Day 7 | |
| Distilled water (control) | 145.0±2.08a | 140.67±1.20a | 142.33±0.89a |
| Sexual dysfunction+distilled water | 189.00±0.58b | 181.67±1.20b | 185.00±0.58b |
| Sexual dysfunction+PowermaxM | 164.33±0.88c | 142.33±0.33a | 139.00±0.88a |
| Sexual dysfunction+47mg/kg of extract | 142.00±4.04a | 145.67±4.70a | 142.33±1.78a |
| Sexual dysfunction+94mg/kg of extract | 102.00±3.21d | 96.67±1.20d | 95.67±2.02d |
| Sexual dysfunction+141mg/kg of extract | 78.67±0.33e | 61.00±1.00ef | 47.67±0.88g |
Data are mean of five determinations±SEM. Values carrying superscripts different from control from the control down the group for each day and parameter are significantly different (p<0.05).
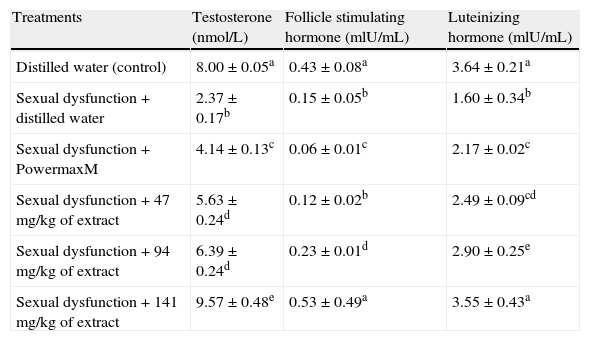
All the doses of the extract significantly elevated the levels of testosterone, FSH and LH in the serum of the sexual dysfunction animals when compared with the paroxetine-induced sexually dysfunction animals (Table 7). The most profound increase in the serum testosterone which was higher than the non-dysfunction distilled water treated animals was obtained with the 141mg/kg body weight of the extract (Table 7). Unlike the testosterone, the elevated levels of FSH and LH in the serum of the animals were not significantly different from those of the non-sexually impaired distilled water treated animals (Table 7).
Effect of aqueous extract of Carpolobia lutea roots on serum hormone concentration of paroxetine-induced sexual dysfunction in male rats.
| Treatments | Testosterone (nmol/L) | Follicle stimulating hormone (mlU/mL) | Luteinizing hormone (mlU/mL) |
| Distilled water (control) | 8.00±0.05a | 0.43±0.08a | 3.64±0.21a |
| Sexual dysfunction+distilled water | 2.37±0.17b | 0.15±0.05b | 1.60±0.34b |
| Sexual dysfunction+PowermaxM | 4.14±0.13c | 0.06±0.01c | 2.17±0.02c |
| Sexual dysfunction+47mg/kg of extract | 5.63±0.24d | 0.12±0.02b | 2.49±0.09cd |
| Sexual dysfunction+94mg/kg of extract | 6.39±0.24d | 0.23±0.01d | 2.90±0.25e |
| Sexual dysfunction+141mg/kg of extract | 9.57±0.48e | 0.53±0.49a | 3.55±0.43a |
Data are mean of five determinations±SEM. Values carrying superscripts different from control from the control down the group for each day and parameter are significantly different (p<0.05).
Several substances of plant and animal origin which have been used in many cultures of the developed and developing countries as aphrodisiacs are yet to be substantiated or refuted with scientific data/evidence. To understand the scientific basis/rationale behind the acclaimed use of C. lutea root as an enhancer of libido, potency and sexual pleasure, this study was evaluated in sexually deficient rat model.
Induction of sexual dysfunction in rats by paroxetine hydrochloride has been described as a useful experimental model to study sexual dysfunction in male animals33,35 and is gaining wide acceptance. Paroxetine, used for the management of depression and anxiety is a selective-serotonin reuptake inhibitor that delays or abolishes orgasm or libido by inhibiting the activity of nitric oxide synthase. Systemic inhibition of nitric oxide synthase impairs copulation and decrease penile erection in rats.38 The production of nitric oxide catalyzed by nitric oxide synthase facilitates sexual behaviour possibly by mediating the release of neurotransmitter and galvanizing genomic changes and protein synthesis or some combination thereof in the medial preoptic area of male rats.39 Paroxetine manifest its effects on sexual function by delaying orgasm/sex drive, copulating and ejaculatory behaviours (difficulties in maintaining an erection and inhibiting ejaculations).33 It also substantially decreases the concentration of testosterone in male animals. Therefore, the reduction in MF, IF, EF and EL as well as prolonged ML, IL, and PEI following the administration of paroxetine in the present study confirmed that sexual activity in the male rats has been abolished/impaired, suggesting a model of sexual dysfunction in rats. Paroxetine might have induced sexual dysfunction in the animals through any or all of the aforementioned mechanisms. The findings with respect to induction of sexual dysfunction in male rats by paroxetine in the present study are similar to those previously reported by Malviya et al.33 and Yakubu and Nurudeen.35
MF and IF are useful indices of vigour, libido and potency. An increase in MF reflects enhanced sexual motivation while a similar increase in the IF indicates efficiency of erection, penile orientation and the ease of activation of ejaculatory reflexes.40 Furthermore, increase in EF gives an idea of the number of successful expulsion of semen into the vagina of the female rats. Therefore, the significantly and progressive increases in the MF and IF as well as the increased EF in the paroxetine induced sexually impaired animals by the extract in this study suggest that the extract has restored sexual competence in the sexually impaired male rats. The restoration of sexual function in the paroxetine-induced sexual dysfunction was similar to that of the reference drug, a polyherbal Powmax. The restoration of sexual function was evidenced in the enhanced anogenital sniffing and licking of the vaginal of the female by the male rats. The display of ear-wiggling, darting, hopping and lordosis by the oestradiol benzoate treated female rats in this study implied that oestrus was induced in the animals which culminated into behaviours of proceptivity and receptivity.
Mount and intromission latencies which are inversely proportional to sexual motivation can also be used to assess enhancement of sexual motivation and sexual appetitive behaviour in animals.40 The progressive reduction in the ML and IL suggests enhanced sexual appetitive behaviour. The stimulation of intromission and ejaculation were evident from the profound pelvic thrusting in the extract treated male animals during the observation period. The reduction in mount latency may also connote increased orientation activities towards the receptive females and restricted movement to a particular area of the cage where the female was. Decrease in ML also implies increase in sexual urge that is controlled by CNS mediated action. Furthermore, the prolonged EL suggests that the extract promoted coitus for sufficiently desired time. Such a delay in EL accompanied by sustained penile erection suggests involvement of nitric oxide in the whole process of sexual behaviour and performance.41 Again, the prolonged EL suggests that the extract could assist sexual dysfunction rats in controlling the timing of ejaculation through the modulation of serotonin and its receptors.
PEI is a widely acceptable index of potency, libido and rate of recovery from exhaustion after first series of mating.42 The significant reduction in the PEI by the extract indicates significant and sustained increase in sexual activity. The reduction in PEI by the extract suggests enhanced libido and potency, as well as increased copulatory efficiency of the male rats after treatment with the extract. All these are indications of sexual function/performance improving effects of the extract. The restoration of sexual activity by the extract in the paroxetine-induced sexual dysfunction in male rats by the extract in the present study is similar to the findings earlier reported by Malviya et al.33 and Yakubu and Nurudeen35 following the administration of alcoholic extract of Allium sativa bulbs and Cnestis ferruginea roots respectively. The extract has therefore restored sexual arousal, the state of sexual excitement or desire during the period of sexual interaction.
Experimental studies using plant extracts such as Tribulus terrestris and Fadogia agrestis have revealed that an increase in sexual function in rats could be attributed to a corresponding increase in testosterone, dihydrotestosterone and dehydroepiandrosterone contents of the animals.43–45 Clinical study has also demonstrated that T. terrestris extract increased body's natural testosterone levels, improved male sexual performance and build muscle.46 Therefore, the restored/enhanced sexual arousal and performance by the aqueous extract of C. lutea roots in the present study may not be unconnected with the increase in testosterone content of the sexual dysfunction animals. Aphrodisiacs, at the level of the central nervous system, act by altering specific neurotransmitter or increasing the level of sex hormone concentration, testosterone, in this instance. Thus, the improved serum testosterone level after administration of C. lutea extracts could be considered as one of the contributing factors responsible for an overall increase of sexual performance in the extract treated animals. The enhanced levels of testosterone after treatment with the extract could be foreseen as a possibility for using the herb in the restoration of sexual function in the sexually deficient rats. The modulation of testosterone, attributed to the presence of saponins is suggested to be mediated through its metabolic conversion into dehydroepiandrosterone.47 Besides central effects, testosterone has been shown to act peripherally by facilitating the nitrergic neurotransmission, accentuating nitric oxide synthase activity and releasing nitric oxide in the cavernosa, all of which contribute to penile erection48,49 that preceded intromission.
A concern that arises with sustained elevation in testosterone levels is the feedback inhibition of gonadotropin release.50 Although at this moment, it is not clear why the increase in testosterone content of the extract treated sexually impaired male rats was accompanied by similar elevated levels of the gonadotropins; the absence of this feedback inhibition in the present study could be due to LH and FSH secretory and/or releasing property. This proposition will have to await further studies.
The main components of C. lutea roots included saponins, alkaloids and flavonoids which have been implicated as agents that can facilitate sexual behaviours in animals. For instance, saponins act centrally to stimulate endogenous testosterone levels by acting as intermediate in steroidal pathway of androgen production and raising the levels of LH.44,51–53 In addition, the elevation in the androgen content by the saponins may in turn increase nitric oxide levels in penile tissues by modulating nitric oxide synthase isoenzymes. The nitric oxide then relaxes the corpus cavernosum smooth muscle by increasing guanosine monophosphate levels.54,55 Alkaloids on the other hand may act both peripherally and centrally to relax corpus cavernosum smooth muscle in the male rat copulatory organ or increase the release of nitric oxide from endothelia and nerve endings that induce vasodilation of blood vessels in sexual organ of male rats which eventually results in erection,56 a prerequisite for successful intromission. Flavonoids have been reported to increase mRNA and protein expression of neuronal nitric oxide synthase and inducible nitric oxide synthase as well as inhibit phosphodiesterase (PDE) types 5 and 6 and cyclic adenosine monophosphate specific PDE.57,58 Since saponins, alkaloids and flavonoids were found in the aqueous extract of C. lutea roots, the pro-sexual effects of the extract may be attributed to these secondary metabolites which might have acted either centrally and/or peripherally as well as singly and or in combination. Therefore, the use of the extract might be helpful in restoring sexual dysfunction. Further studies are, however, needed to clarify the specific role(s) of these metabolites in facilitating and/or restoring sexual dysfunction in the animals.
The present study concludes that the aqueous extract of C. lutea roots has restored androgen dependent sexual performance in sexually deficient male rats. The effects of the plant as an aphrodisiac were exerted on both the mechanism of sexual arousal and performance. The results, therefore, corroborate the hype of the plant as herbal cure for sexual dysfunction. The seemingly involvement of alkaloids, flavonoids and saponins from C. lutea roots and their mechanism of action as aphrodisiac will have to await the outcome of further studies.
Conflict of interestThe authors declare no conflict of interest.