In the past decades, great interest has been shown in the development of new therapies for erectile dysfunction. Stem cell therapy has generated promising results in numerous preclinical trials in animal models, which is why has led to the development of the first clinical trials in humans. The main cause involved in the pathophysiology of erectile dysfunction is vascular damage related to endothelial and neuronal injury. The interest in stem cell therapy is justified by their capability to differentiate into specific damaged tissues, including endothelium and nervous tissue, and induction of the host own cell proliferation. Despite the great effort of the many studies carried out to date, knowledge about biological effects, therapeutic efficacy and safety of stem cells therapy for erectile dysfunction is still very limited.
En las últimas décadas, ha habido gran interés en el desarrollo de nuevos tratamientos para tratar la disfunción eréctil. El tratamiento con células madre ha arrojado prometedores resultados en numerosos estudios preclínicos en modelos animales, lo cual ha generado el desarrollo de los primeros ensayos clínicos en seres humanos. Puesto que la principal causa implicada en la fisiopatología de la disfunción eréctil es una lesión vascular asociada con lesión endotelial y neuronal, el interés por el tratamiento con células madre se justifica por la capacidad que tienen para diferenciarse en los distintos tejidos dañados, incluyendo endotelio y tejido neuronal, y en la inducción de la reparación de las propias células del huésped. A pesar del gran esfuerzo de los distintos estudios realizados hasta el momento actual, el conocimiento sobre los efectos biológicos, la eficacia terapéutica y la seguridad del tratamiento con células madre aún se encuentra muy limitado.
Erectile dysfunction (ED) is defined as the inability to attain or maintain a penile erection satisfactory for sexual intercourse.1 The incidence of ED has been increasing in the past decades, affecting 19.2% of male global population. There is a clear association with age, affecting from 2.3 to 53.4% of men aged 30 to 80 years, respectively.2
Risk factors for ED are similar to those associated with cardiovascular disease, including hypertension, hyperlipidemia, diabetes, smoking, and obesity. The main components in the physiology of erection are the endothelial cells (EC), the cavernous smooth muscle cells (CSMC), and the neuronal nitric oxide (NO) synthase-positive cavernous nerves.3 The high prevalence of ED in patients with cardiovascular diseases, along with the fact that endothelial dysfunction and reduced availability of NO often underlie vascular diseases, suggests the essential role of the EC in the pathogenesis of ED.4
The present work is a review of the available information and evidence of stem cell (SC) therapy in clinical trials published and those that are currently under development.
Pathophysiology of erectile dysfunction of vascular etiologyThe main cause of erectile dysfunction is of vascular etiology. Patients with Diabetes Mellitus (DM) type 1 and 2 exhibit impaired new blood vessel development in response to ischemia, so they are prone to present vascular complications leading to end organ damage, including ED. The hallmark of these vascular complications is endothelial dysfunction, causing cellular apoptosis.5
In hyperlipidemia, atherosclerosis of cavernous vessels is a common event, but it is also associated with the decreased levels of cavernous NO, and the subsequent impairment of cavernous nerves and EC function.6
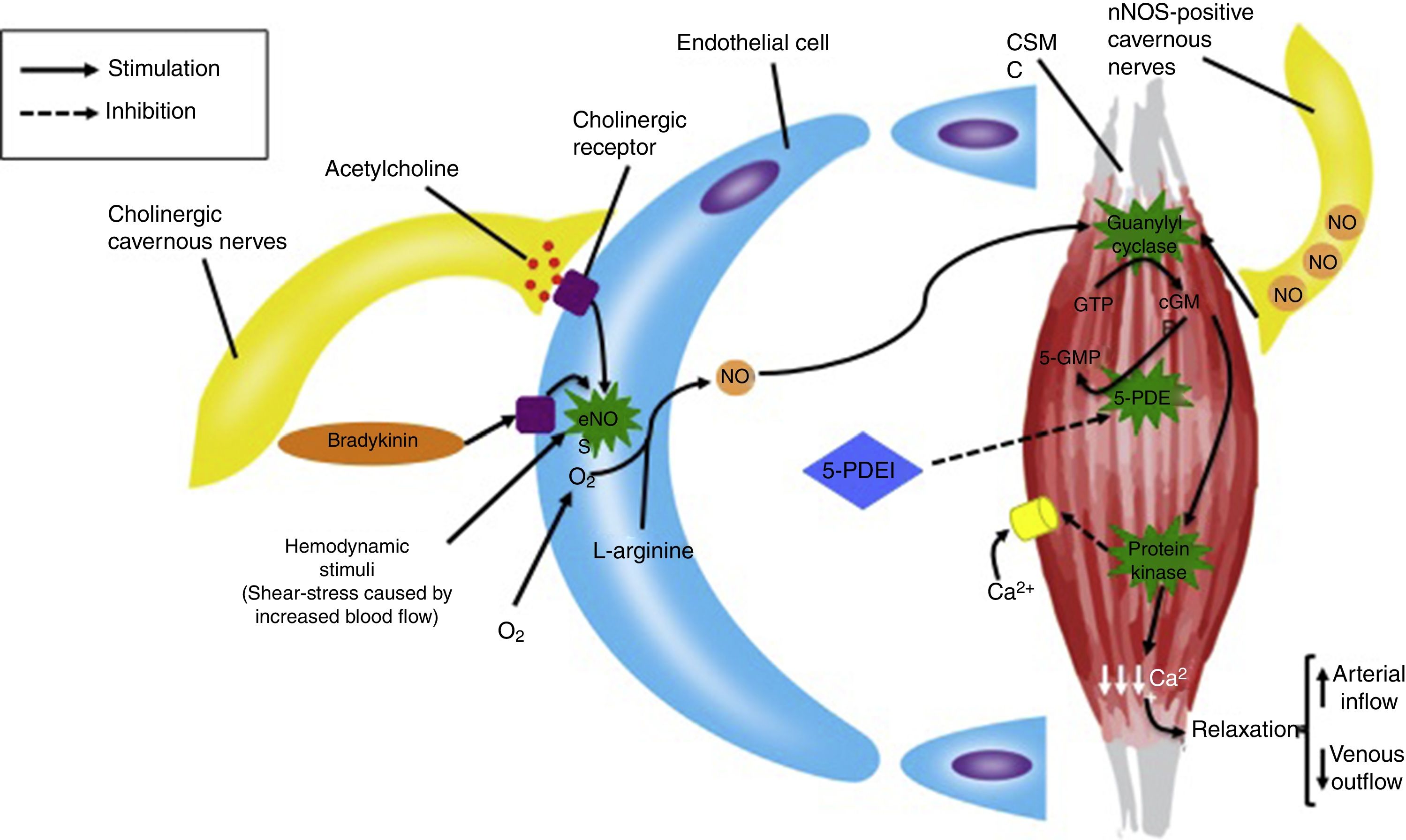
Currently, the first line treatment for ED is 5-phosphodiesterase inhibitors (5-PDEI). These drugs are competitive and reversible inhibitors of cyclic guanosine monophosphate (cGMP) hydrolysis. The clinical effect is a potentiation of NO function, caused by intracellular accumulation of cGMP, which reduces cytosolic levels of calcium inside CMSC. The final result is an increased relaxation of smooth muscle and blood accumulation into the corpus cavernosum by an increased arterial inflow and a decreased venous outflow (Fig. 1).7
Regulation of cavernous nerves, endothelial cells and cavernous smooth muscle, and the role of 5-PDEI in erectile function. CSMC=cavernous smooth muscle cell, GTP=guanosine triphoshpate, cGMP=cyclic guanosine monophosphate, 5-PDEI=5-phosphodiesterase inhibitor, NO=nitric oxide, eNOS=endothelial nitric oxide synthase, nNOS=neuronal nitric oxide synthase.
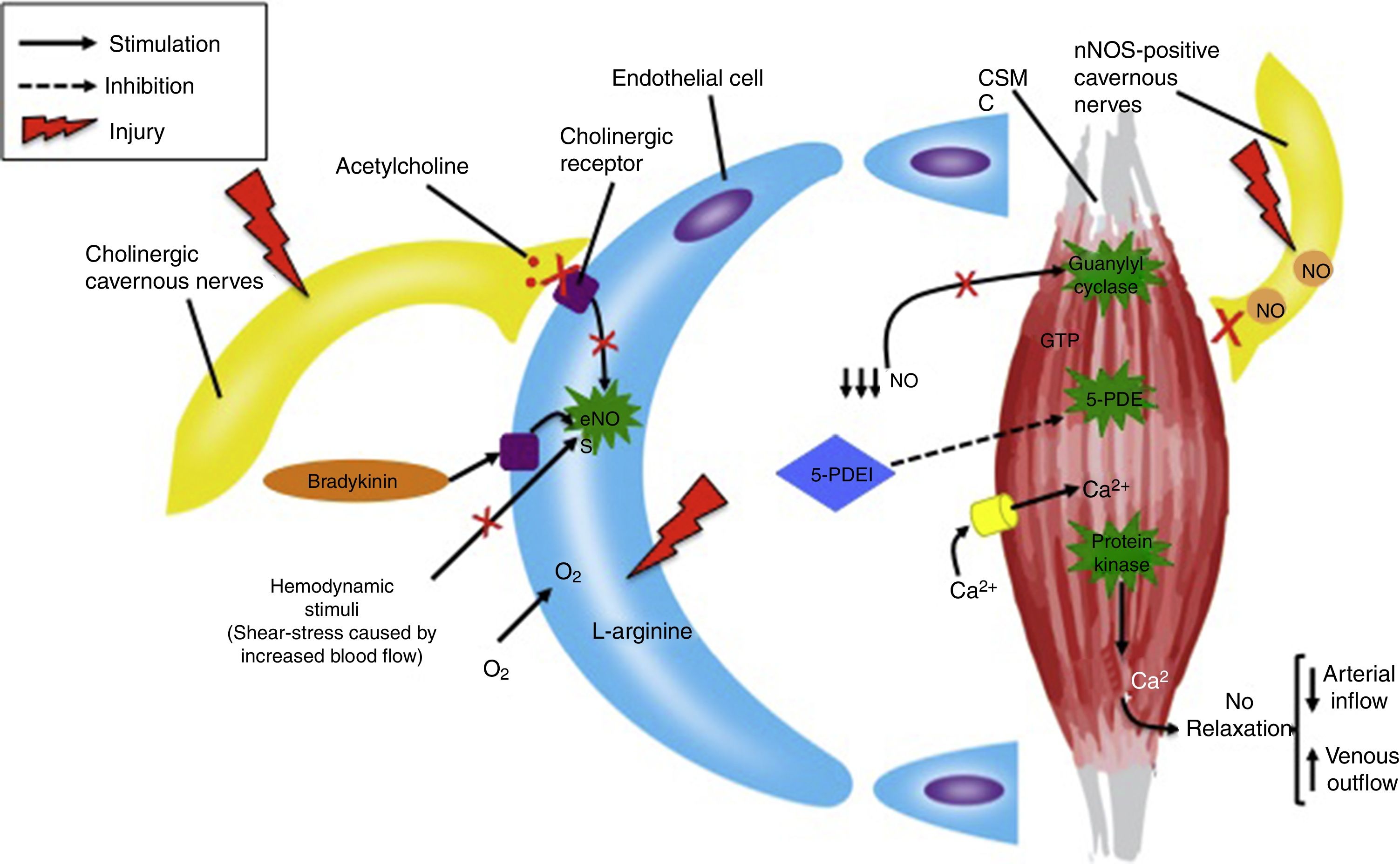
In order to obtain the therapeutic effect of 5-PDEI, neuronal nitric oxide synthase (nNOS)-positive cavernous nerves and EC function must be partially or completely preserved, in order to produce the sufficient blood concentrations of NO to activate guanylyl cyclase and produce cGMP inside CSMC.8 For this reason, diseases such as DM, hyperlipidemia and radical prostatectomy, in which cavernous nerves and EC are severely damaged, the efficacy of 5-PDEI is limited and usually unsatisfactory (Fig. 2).9
Pathophysiology of severe endothelial and nerve dysfunction in erectile function and nitric oxide synthesis. CSMC=cavernous smooth muscle cell, GTP=guanosine triphoshpate, 5-PDEI=5-phosphodiesterase inhibitor, NO=nitric oxide, eNOS=endothelial nitric oxide synthase, nNOS=neuronal nitric oxide synthase.
Except for lifestyle changes and penile revascularization, the outcome of the available treatments is not the expected due to the subjacent cause of the disease. In other words, conventional therapies do not intervene in the recovery of nerve and endothelial tissue function, being the role of the drugs described above only a palliative measure.10
Therefore, invasive therapies, such as intracavernousal injection of prostaglandin analogs, and surgical procedures, such as the penile prosthesis implantation, are considered to be of choice in ED related to these diseases. The inconvenience of these therapeutic modalities lies in the high risk of adverse events, such as local pain and bruising related to prostaglandin analogs injection, and infections and complications related to the surgical procedure in penile prosthesis.11,12 In these group of patients stem SC therapy seems to be a promising treatment.13
Penile revascularization deserves a special consideration. This therapeutic option consists of surgical anastomosis, similar to cardiac bypass, between the epigastric and cavernous artery. Nowadays, these techniques are limited to young men with localized obstruction of the internal pudendal artery or common penile artery secondary to trauma. It is a complicated procedure and is not performed routinely.14
Selection of the source and type of stem cellsFor a long time, it was believed that vasculogenesis, the process by which new vessels are formed by endothelial progenitor cells (EPCs), was thought to be restricted to blood vessel formation in utero. This dogma was invalidated by the discovery of circulating EPCs by Asahara et al.15
EPCs are thought to be a subset of cells derived from bone marrow that play a crucial role in neovascularization of ischemic tissue and maintenance of endothelial cell integrity. They can promote adult vasculogenesis and contribute to the recovery of perfusion by homing to, differentiating, proliferating and incorporating into new vessels, producing angiogenic factors, and even through a paracrine effect influencing neighboring cells, thereby enhancing collateral vessel formation.16–18 Mobilization of EPCs out of the bone marrow and, therefore circulating EPC number are lower in diabetic patients and animal models of DM when compared with normoglycemic subjects. The result is a diminished capacity of neovascularization and perfusion of ischemic tissue.19
Many types of adult SCs are available, including mesenchymal stromal cells (MSCs) and hematopoietic stem cells (HSCs), derived from several sources, including bone marrow (BM), adipose tissue and umbilical cord blood.20
In addition to HSCs, different types of progenitor cells can be obtained from human BM, including endothelial, MSCs and very small embryonic-like SCs.21,22 Due to the increasingly successful and safe experiences with HSC transplantation using granulocyte colony-stimulating factor (G-CSF) stimulated BM, alternative routes for cell therapy research are available.23 G-CSF-stimulated BM allows to obtain higher cell concentrations of different progenitor cell phenotypes in a lower volume, compared with adipose-derived and umbilical cord-derived SCs. MSCs have been reported to increase their numbers in situ after G-CSF administration.24,25 Additionally, stimulated BM HSCs have been described to be in a proliferating state, whereas peripheral blood HSCs (adipose and umbilical cord-derived) remain in the G0/G1 phase of the cell cycle, which causes a limited capacity of cellular differentiation.22 For the reasons mentioned above, G-CSF-stimulated BM is a compelling progenitor cell source that might be a potential treatment for ED, compared with other sources of SCs.
The role of stem cells as a therapy for erectile dysfunctionLimited evidence of SC therapy for ED has been published in the last decade. SCs are able to differentiate into various cell types, including EC, smooth muscle cells, Schwann cells, and neurons, among other cell types, depending on the environment in which SCs are exposed.26 Recent studies have guided their research based on this knowledge about SCs with the aim of being used as a therapy for ED, through the regeneration of dysfunctional EC, cavernous nerves, and CSMC. Additional evidence shows that transplanted SC might encourage regeneration of the host's own EC and CSMC, or by the stimulation of appropriate interactions between EC and CSMC through a paracrine effect.27
As described previously, many prevalent diseases reduce the bioavailability of NO, including severe diabetes and denervation of erectile tissue due to radical prostatectomy or neuropathy of other causes, resulting in degeneration of nitrergic nerves of the corpus cavernosum and vasculature. Men with these underlying disorders commonly do not respond satisfactorily to 5-PDEI.28
Many animal studies on SC therapy for cavernous nerve injury, aging and diabetes mellitus-related ED rat models have shown the utility of both BM and adipose-derived SCs. Soebadi et al. summarized more than 40 preclinical studies with animal models using SCs for ED. Most of the studies showed an improvement in erectile function. However, there are many discrepancies in structural outcomes showed between studies, including evidence of nerve and CSMC regeneration, increased cavernous smooth muscle and endothelial intracellular content, and decreased fibrotic tissue.10
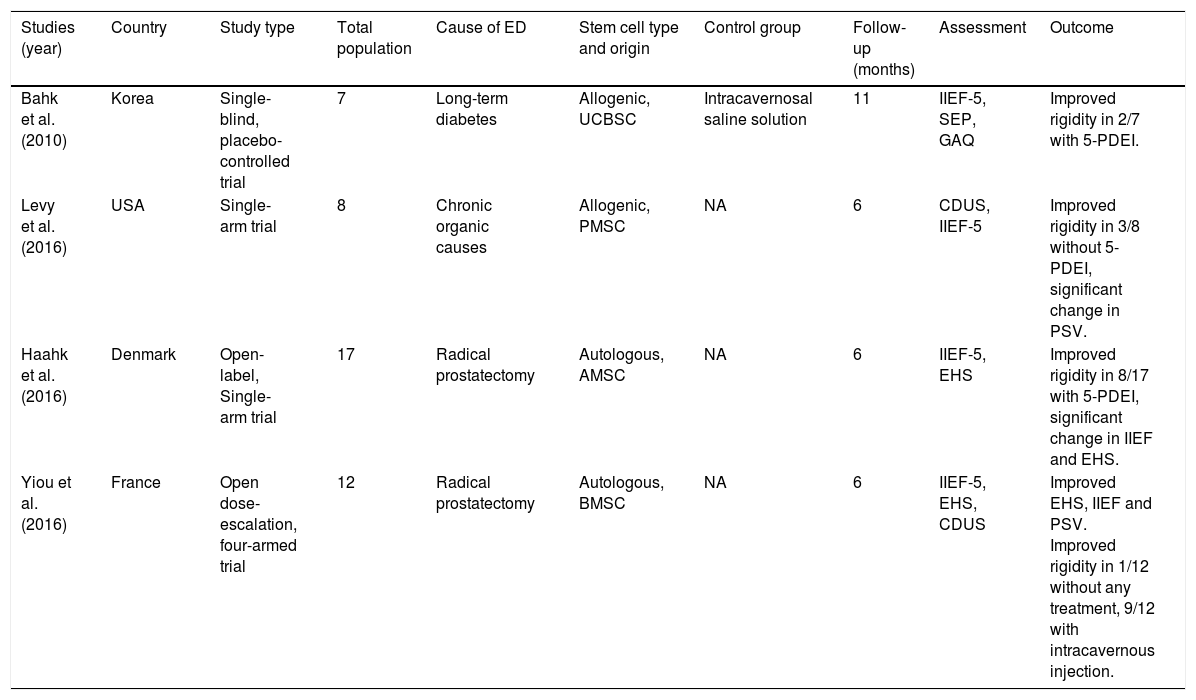
To date, 4 clinical trials using SCs for ED have been published.13 A detailed summary of the studies is described in Table 1.
Clinical trials published on intracavernosal stem cell therapy for erectile dysfunction.
| Studies (year) | Country | Study type | Total population | Cause of ED | Stem cell type and origin | Control group | Follow-up (months) | Assessment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Bahk et al. (2010) | Korea | Single-blind, placebo-controlled trial | 7 | Long-term diabetes | Allogenic, UCBSC | Intracavernosal saline solution | 11 | IIEF-5, SEP, GAQ | Improved rigidity in 2/7 with 5-PDEI. |
| Levy et al. (2016) | USA | Single-arm trial | 8 | Chronic organic causes | Allogenic, PMSC | NA | 6 | CDUS, IIEF-5 | Improved rigidity in 3/8 without 5-PDEI, significant change in PSV. |
| Haahk et al. (2016) | Denmark | Open-label, Single-arm trial | 17 | Radical prostatectomy | Autologous, AMSC | NA | 6 | IIEF-5, EHS | Improved rigidity in 8/17 with 5-PDEI, significant change in IIEF and EHS. |
| Yiou et al. (2016) | France | Open dose-escalation, four-armed trial | 12 | Radical prostatectomy | Autologous, BMSC | NA | 6 | IIEF-5, EHS, CDUS | Improved EHS, IIEF and PSV. Improved rigidity in 1/12 without any treatment, 9/12 with intracavernous injection. |
UCBSC=umbilical cord blood-derived stem cell; PMSC=placental-derived mesenchymal stem cell; AMSC=adipose-derived mesenchymal stem cell; BMSC=bone marrow-mononuclear stem cells; IIEF-5=International Index of Erectile Dysfunction Questionnaire; SEP=Sexual Encounter Profile; GAQ=Global Assessment Question; CDUS=Color Doppler Ultrasound; PSV=Peak Systolic Velocity; NA=not applicable.
Bahk et al. reported a single-blinded study in 7 patients with diabetic-related ED using allogeneic umbilical cord blood SCs injected intracavernousal in a cellular concentration around 1.5×107. SCs were obtained from donors and needed to perform compatibility tests for HLA-A,B,C, and DR. Follow-up assessment included the International Index of Erectile Function Questionnaire of 5 questions (IIEF-5), Sexual Encounter Profile, and Global Assessment Questionnaire. The results obtained in this study were improved rigidity in 2 of 7 patients, able to perform sexual intercourse with 5-PDEI.29
Levy et al. published a study in 8 men with organic causes of ED using placental-derived SCs. Before the SC injection, they apply a intracavernousal solution composed by papaverine, phentolamine and prostaglandin. The assessment was measured with Doppler ultrasound parameters, such as Peak Systolic Velocity (PSV), and the IIEF-5. The outcome obtained was not very encouraging, resulting in improved erection in 3 of 8 patients and no significant change in IIEF-5.30
Haahr et al. studied the use of adipose-derived SCs in 17 patients after radical prostatectomy. The assessment tool used in this study was the IIEF-5 and the results were that 8 of 17 men recovered erection with significant improvement of the IIEF-5. Adipose tissue was obtained during general anesthesia and harvesting was performed with water-jet assisted liposuction. The isolation of the adipose derived cells was performed by an automatic processing system.31
Yiou et al. used BM mononuclear cells in 12 men after 22 months of radical prostatectomy. SC therapy was performed using different doses of mononuclear cells concentrations (2×107, 2×108, 1×109, and 2×109) in a single application. Outcomes were evaluated with the IIEF-15, The Erectile Hardness Score (EHS), and Color Doppler Ultrasound. They reported that 9 of the 12 patients responded to intracavernousal injections, 5-PDEI, or vacuum constriction device. One patient responded with hard erection without any additional therapy.32
A Phase 2 randomized placebo-controlled clinical trial performed by lead researcher, Dr. Martha Haahr, in Odense University Hospital, is currently under development using autologous mesenchymal SCs obtained from adipocytes in a larger group of patients, including 78 men with ED, and a minimum follow-up of 6 months.33
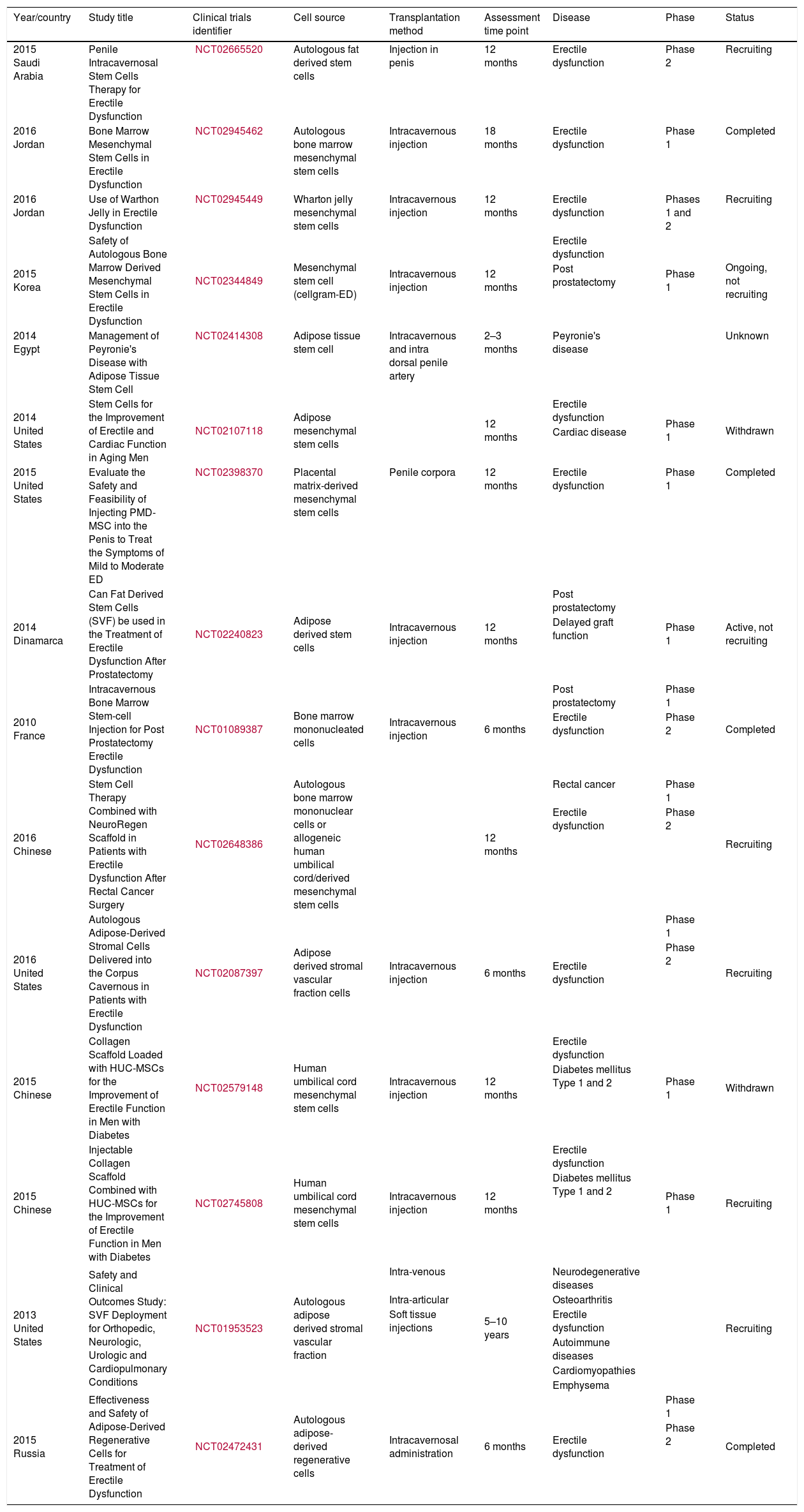
Since 2015, the number of researchers involved in the development of new knowledge about SC therapy for ED increased exponentially. Table 2 summarizes the clinical trials currently developing, as well as some studies that were withdrawn.34
Clinical trials using stem cell therapy for erectile dysfunction registered in the NIH online database.
| Year/country | Study title | Clinical trials identifier | Cell source | Transplantation method | Assessment time point | Disease | Phase | Status |
|---|---|---|---|---|---|---|---|---|
| 2015 Saudi Arabia | Penile Intracavernosal Stem Cells Therapy for Erectile Dysfunction | NCT02665520 | Autologous fat derived stem cells | Injection in penis | 12 months | Erectile dysfunction | Phase 2 | Recruiting |
| 2016 Jordan | Bone Marrow Mesenchymal Stem Cells in Erectile Dysfunction | NCT02945462 | Autologous bone marrow mesenchymal stem cells | Intracavernous injection | 18 months | Erectile dysfunction | Phase 1 | Completed |
| 2016 Jordan | Use of Warthon Jelly in Erectile Dysfunction | NCT02945449 | Wharton jelly mesenchymal stem cells | Intracavernous injection | 12 months | Erectile dysfunction | Phases 1 and 2 | Recruiting |
| 2015 Korea | Safety of Autologous Bone Marrow Derived Mesenchymal Stem Cells in Erectile Dysfunction | NCT02344849 | Mesenchymal stem cell (cellgram-ED) | Intracavernous injection | 12 months | Erectile dysfunction | Phase 1 | Ongoing, not recruiting |
| Post prostatectomy | ||||||||
| 2014 Egypt | Management of Peyronie's Disease with Adipose Tissue Stem Cell | NCT02414308 | Adipose tissue stem cell | Intracavernous and intra dorsal penile artery | 2–3 months | Peyronie's disease | Unknown | |
| 2014 United States | Stem Cells for the Improvement of Erectile and Cardiac Function in Aging Men | NCT02107118 | Adipose mesenchymal stem cells | 12 months | Erectile dysfunction | Phase 1 | Withdrawn | |
| Cardiac disease | ||||||||
| 2015 United States | Evaluate the Safety and Feasibility of Injecting PMD-MSC into the Penis to Treat the Symptoms of Mild to Moderate ED | NCT02398370 | Placental matrix-derived mesenchymal stem cells | Penile corpora | 12 months | Erectile dysfunction | Phase 1 | Completed |
| 2014 Dinamarca | Can Fat Derived Stem Cells (SVF) be used in the Treatment of Erectile Dysfunction After Prostatectomy | NCT02240823 | Adipose derived stem cells | Intracavernous injection | 12 months | Post prostatectomy | Phase 1 | Active, not recruiting |
| Delayed graft function | ||||||||
| 2010 France | Intracavernous Bone Marrow Stem-cell Injection for Post Prostatectomy Erectile Dysfunction | NCT01089387 | Bone marrow mononucleated cells | Intracavernous injection | 6 months | Post prostatectomy | Phase 1 | Completed |
| Erectile dysfunction | Phase 2 | |||||||
| 2016 Chinese | Stem Cell Therapy Combined with NeuroRegen Scaffold in Patients with Erectile Dysfunction After Rectal Cancer Surgery | NCT02648386 | Autologous bone marrow mononuclear cells or allogeneic human umbilical cord/derived mesenchymal stem cells | 12 months | Rectal cancer | Phase 1 | Recruiting | |
| Erectile dysfunction | Phase 2 | |||||||
| 2016 United States | Autologous Adipose-Derived Stromal Cells Delivered into the Corpus Cavernous in Patients with Erectile Dysfunction | NCT02087397 | Adipose derived stromal vascular fraction cells | Intracavernous injection | 6 months | Erectile dysfunction | Phase 1 | Recruiting |
| Phase 2 | ||||||||
| 2015 Chinese | Collagen Scaffold Loaded with HUC-MSCs for the Improvement of Erectile Function in Men with Diabetes | NCT02579148 | Human umbilical cord mesenchymal stem cells | Intracavernous injection | 12 months | Erectile dysfunction | Phase 1 | Withdrawn |
| Diabetes mellitus Type 1 and 2 | ||||||||
| 2015 Chinese | Injectable Collagen Scaffold Combined with HUC-MSCs for the Improvement of Erectile Function in Men with Diabetes | NCT02745808 | Human umbilical cord mesenchymal stem cells | Intracavernous injection | 12 months | Erectile dysfunction | Phase 1 | Recruiting |
| Diabetes mellitus Type 1 and 2 | ||||||||
| 2013 United States | Safety and Clinical Outcomes Study: SVF Deployment for Orthopedic, Neurologic, Urologic and Cardiopulmonary Conditions | NCT01953523 | Autologous adipose derived stromal vascular fraction | Intra-venous | 5–10 years | Neurodegenerative diseases | Recruiting | |
| Intra-articular | Osteoarthritis | |||||||
| Soft tissue injections | Erectile dysfunction | |||||||
| Autoimmune diseases | ||||||||
| Cardiomyopathies | ||||||||
| Emphysema | ||||||||
| 2015 Russia | Effectiveness and Safety of Adipose-Derived Regenerative Cells for Treatment of Erectile Dysfunction | NCT02472431 | Autologous adipose-derived regenerative cells | Intracavernosal administration | 6 months | Erectile dysfunction | Phase 1 | Completed |
| Phase 2 |
Even though there is insufficient evidence that SCs act either by engraftment into the injured tissue with differentiation into specific type of cells, and/or through paracrine effects, arduous research is now developing in order to understand the therapeutic mechanisms and safety of SCs for the management of ED.
To date, no standardized protocols have been published for SC therapy in ED. The interest in this new modality of therapy is pushing science to exhaustive clinical trials with the aim of establishing the most efficient source of SCs, the dosage and frequency that will bring the best and safer clinical outcomes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed conform to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the correspondence author.
Conflict of interestsNo conflicts of interest were declared by the researchers who participated during the development of the study.
The development of this study would not have been possible without the support of many people who were involved in the planning, design, information search and writing of this work. We thank infinitely Dr. med. David Gómez-Almaguer, M.C. Rolando Delgado-Balderas, and Dr. Jhonataan Uribe-montoya.