Chronic exposure to fluoride causes tissue damage induced by oxidative imbalance, Cyperus esculentus (CE) possess anti-inflammatory and immunostimulatory properties. This study focused on Salutary role of Cyperus esculentus in sodium fluoride (NaF) induced testicular degeneration and sperm quality deteriorations.
MethodsSexually mature male Sprague-Dawley rats were randomly divided into four groups (n=6). Animals in control group received 2 mls of normal saline per day; CE group received 500mg/kg bw of CE; NaF group received 5mg/kg bw of NaF; NaF+CE group received 500mg/kg bw of CE (for 14 days pre-treatment) and NaF co-treatment till 56 days via gastric gavage. Parameters tested include: testicular histology, sperm parameters, sex hormone, fertility test, malondialdehyde (MDA), superoxide dismutase (SOD), reduced glutathione, glutathione peroxidase (GPX), catalase (CAT), testicular fluoride and testicular cholesterol.
ResultsSodium fluoride significantly (p<.05) decrease testicular antioxidant (SOD, CAT, GSH and GPx), sperm quality, hormone profiles (TT, FSH, LH, estrogen levels), testicular cholesterol, morphometric parameters, Johnsen's Score and number of implantations in female rats with corresponding (p<.05) increase in oxidative stress makers and abnormal sperm morphology. Also depleted seminiferous epithelium and degenerate spermatogenic cells. Pretreatment with 500mg/kg bw of CE lowered NaF toxicity by significantly reducing the lipid peroxidation products, fluoride accumulation in the testis, histopathological changes of the testes and spermatozoa abnormalities and reverted observed NaF-induced inhibition in antioxidant parameters and weight of accessory sex organs.
ConclusionsCyperus esculentus attenuated NaF-induced testicular injuries and protected the seminiferous epithelium, reduced oxidative stress and promoted spermatogenesis.
La exposición crónica al fluoruro causa daño tisular inducido por un desequilibrio oxidativo. Cyperus esculentus (CE) tiene propiedades antiinflamatorias e inmunoestimulatorias. Este estudio se centró en el papel beneficioso de Cyperus esculentus en la degeneración testicular inducida por fluoruro de sodio (NaF), y en el deterioro de la calidad del esperma.
MétodosSe dividieron aleatoriamente ratas macho Sprague Dawly® sexualmente maduras en 4 grupos (n=6). Los animales del grupo control recibieron 2ml de solución salina normal al día; el grupo CE recibió 500mg/kg/día; el grupo NaF recibió 5mg/kg de NaF; el grupo NaF + CE recibió 500mg/kg de CE (durante 14 días previos al tratamiento) y co-tratamiento de NaF hasta el día 56.° mediante sonda gástrica. Los parámetros testados fueron: histología testicular, parámetros espermáticos, hormonas sexuales, prueba de fertilidad, malondialdehído (MDA), superóxido dismutasa (SOD), glutatión reducido, glutatión peroxidasa (GPX), catalasa (CAT), fluoruro testicular y colesterol testicular.
ResultadosEl fluoruro sódico redujo significativamente (p<0,05) el antioxidante testicular (SOD, CAT, GSH y GPX), la calidad del esperma, los perfiles hormonales (TT, FSH, LH, niveles de estrógenos), colesterol testicular, parámetros morfométricos, puntuación de Johnsen y número de implantaciones en ratas hembra, con el incremento correspondiente (p<0,05) de los marcadores de estrés oxidativo y morfología espermática anormal. También se redujo el epitelio seminífero y se degeneraron las células espermatogénicas. El tratamiento previo con 500mg/kg de CE disminuyó la toxicidad de NaF, reduciendo significativamente los productos de peroxidación lipídica, la acumulación de fluoruro en los testículos, los cambios histopatológicos testiculares y las anomalías en espermatozoides, revirtiendo la inhibición observada inducida por NaF en los parámetros antioxidantes y el peso de los órganos sexuales accesorios.
ConclusionesCE atenuó los daños testiculares inducidos por NaF y protegió el epitelio seminífero, redujo el estrés oxidativo y promovió la espermatogénesis.
Fluoride is a pervasive natural pollutant and an important trace element to human body; it has been employed for prevention of dental caries.1 Fluoride enter into human body system through drinking water, food, industrial pollution, drugs and cosmetics.2 Fluoride is a nonmetallic halogen that are naturally available in the soil, rocks.3 Small fluoride concentrations have therapeutically action against dental caries, exposure to high doses from water ingestion and use of fluoride toothpastes or fluoride-rich diets increases the body burden of fluoride.4 Excessive fluoride exposure induced toxicity to the soft tissues such as, brain, heart, liver, kidney and testis due to changes in enzymatic metabolism and breakdown of redox balance.5 Fluoride contamination of drinking water can disrupt male gametogenesis and steroidogenesis and induce testicular oxidative stress.6 Fluoride act as an enzyme inhibitor, due to its strong electronegativity and forms ions in solution.7
Cyperus esculentus L. (Tiger nut) is a perennial plant species that belongs to the Cyperaceae family and grows abundantly in the Mediterranean region.8 Tubers of this plant are considered one of the earliest food sources known to humanity, where they have been documented to be cultivated by ancient Egyptians since 5000 BC.9 These tubers are commonly known by several names such as chufa sedge, nut grass, yellow nutsedge, tiger nut sedge, earth almond and Northern nut grass10Cyperus esculentus is a potentially valuable food source for humans and animals due to its rich nutritional contents of fat, carbohydrates, and minerals.11Cyperus esculentus has been reportedly used for production of soap and oil in the cosmetic industry12 and in Africa in the production of the popular drink called “kunu”, because of its rich milky taste it is consumed as snack and delicacy13 and rich sugar content, vitamin B1, C and E, minerals such as calcium, magnesium and iron,14 as well as digestive enzymes such as catalase, peroxidase, lipase and among others.15 Medically, it has used in cases of flatulence, dysentery, debility, indigestion and diabetes,16 it has been claimed that treatment with Cyperus esculentus extract improves sperm count and motility in male rats, which is associated with increased gonadotropins and testosterone serum levels.17 Based on the previous report, this study focused on salutary role of Cyperus esculentus tubers (tiger nut) extract on fluoride-induced testicular degeneration and sperm quality deteriorations in animal model
Materials and methodsChemicalsFluoride in the form of sodium fluoride (NaF) was purchased from PASCAL Scientific (London, United Kingdom) (Product no.MW76613), thiobarbituric acid and reduced glutathione were purchased from Sigma–Aldrich Corp. (St. Louis, MO, USA). All other reagents used were of analytical grades.
Plant materialPlant materials Cyperus esculentus (CE) were purchased from Shasha market Akure and taken to the Centre for research and development (CERAD), Federal University of Technology, Akure (FUTA), Ondo State, Nigeria for proper identification and authentication. The samples of the Cyperus esculentus nut were identified and authenticated by Mr. Omomoh Bernard and sample of the plant voucher FUTA/0196 deposited for reference purpose
Extraction of plant materialThe fresh Cyperus esculentus nut were thoroughly washed in sterile water and air dried under shade for 6 weeks to a constant weight in the laboratory. The air-dried nuts were weighed using CAMRY (EK5055, Indian) electronic weighing balance and were milled with automatic electrical Blender (model FS-323, China) to powdered form. Seven hundred grams of the milled plant sample was later soaked in 1500ml of phosphate-buffered saline (PBS) for 2h at room temperature, and was later filtered through cheese cloth and then through Whatman #1 filter paper, the filtrate was then bottled in clean screw-cap bottles and stored in a refrigerator until use.
Phytochemical screeningQualitative phytochemical analysis of the aqueous extract of Cyperus esculentus nuts was done in accordance with Soni and Sosa.18 While modifications on the report by Grindberg and Williams19 on high performance liquid chromatography was adopted to quantify the vitamins.
AnimalsSexually mature male Sprague Dawley rats (Rattus norvegicus), weighing 180±20g, were obtained from Federal University of Technology, Akure research Farm. The animals were housed in polypropylene cages, each cage housing six animals and allowed to acclimatize for two weeks before the commencement of the experiment. The rats were maintained under standard natural photoperiodic condition of 12h of darkness and 12h of lightness (D:L; 12:12h dark/light cycle) at room temperature (25–32°C) and humidity of 50–55%.20 Their cages were cleaned every day. The rats were fed with standard rat chow and drinking water was supplied ad libitum.
Acute toxicity study of the extractThe test performed according to OECD guidelines 423. Swiss albino mice were administered with aqueous extract of Cyperus esculentus up to 2000mg/kg. Animals were observed for gross behavior changes as well as for motility for 14 days.21
TreatmentsSexually mature male Sprague-Dawley rats were randomly divided into four groups of six rats each (n=6). Animals in control group was given 2 mls of normal saline per day. Animals in Cyperus esculentus group received 500mg/kg body weight of Cyperus esculentus, fluoride group received 5mg/kg body weight of sodium fluoride, fluoride+Cyperus esculentus group received 500mg/kg body weight of CE for 14 consecutive days before administration and continued up to 56 days simultaneously with NaF treatment through gastric gavage. The procedure lasted for 8 weeks (Duration of spermatogenesis in rat being 51.6–56 days.22
Sample collectionAt the end of the experimental period, blood was drawn from the animals by puncturing retro-orbital venous sinus. Whole blood was used for the determination of hydroperoxide level, while separated plasma was used for hormone analysis. After collection of blood samples, the animals were autopsied under light ether anesthesia. Subsequently, testes, vas deferens, epididymis, prostate glands and seminal vesicles were excised from surrounding tissues and placed into tube. Thus, organs were dried between two sheets of filter paper and their wet weight was determined. Next, the relative organ weight was calculated by use of the formula: organ weight/body weight×100. Left epididymis was weighed, while right epididymis was rinsed in warm phosphate buffered saline (PBS) and incubated at 37°C for the evaluation of sperm quality. Whereas, left testes were processed for histological study, right testes were processed for determination of MDA, GSH, SOD, CAT, GPx and cholesterol.
Sperm analysisCollection and incubation of epididymal spermSpermatozo were obtained from the fresh right epididymis of adult rat described by Narayana et al.23 Briefly, epididymis was cut into small pieces with a sharp razor blade and dispersed in 3 ml of phosphate buffered saline (pH 7.2) to obtain a suspension with gentle stirring. Dispersed sperm samples were kept in an incubator.
Epididymis sperm count, viability and motilityThe spermatozoa from the cauda epididymis were obtained by cutting into 2ml of medium {Hams F10} containing 0.5% bovine serum albumin.24 After 5min of incubation at 37°C (with 5% CO2), the cauda epididymis sperm reserves were determined using a hemocytometer. Sperm motility, viability (live/death ratio) and morphology (normal and abnormal [head defect]) were analyzed with a microscope (Leica DM750) and reported as the mean percent of motile sperm according to the method developed by the World Health Organization.25
Sex hormone and pituitary gonadotropins determinationThe serum levels of Testosterone (TT), Follicule stimulating hormone (FSH), Leutenizing hormone (LH) and estrogen were measured using commercially available enzyme-linked immunoassay kits (Diagnostic automation Inc, CA) obtained from Randox Laboratories Ltd., Admore Diamond Road, Crumlin, Co., Antrim, United Kingdom, according to the manufacturer's instructions.
Fertility of male ratsFertility was estimated in adult male rats treated with sodium fluoride, Cyperus esculentus nut and control male counterparts. Each male rat was placed in an individual cage with two virgin untreated females rats of the same strain. They were left together for 10 days during which two oestrone cycles had elapsed in female rats. And after 10 days the exposed male rats are removed, pregnant females were killed by cervical dislocation under light ether anesthesia and the number of pregnant rats,implantation sites, and the number of fetuses was recorded.26
Determination of lipid peroxide levelsBlood hydroperoxide level was evaluated using an analytical system. The test is a colorimetric test that takes advantage of the ability of hydroperoxide to generate free radicals after reacting with transitional metals, when buffered chromogenic substance is added; a colored complex appears. This complex was measured spectrophotometrically. Lipid peroxidation level in the testis was measured by a method27 using thiobarbituric acid reactive substances (TBARS) with some modifications as previously described by Aboul-Soud et al.28 Testis was homogenized in ice cold 0.15M KCl (10%) and the concentration of TBARS was expressed as nmol of MDA per mg protein using 1,1,3,3-tetramethoxypropane as standard. The absorbance was read at 532nm.
Determination of testicular fluorideTotal fluoride concentration was determined in testes using a previously reported protocol.29 The samples were heated with 7ml nitric acid and per chloric acid (2v/1v) in boiling water bath until evaporation. After digestion, the samples were diluted by definite volume of distilled water and filtered. The fluoride concentration was measured in samples by use of atomic absorption, Model RHB-4 (Japan). Data are presented as total fluoride (mg/g of tissue dry).
Determination of testicular reduced glutathione (GSH)Reduced form of glutathione was determined using Ellman's reagent 5-5-dithio-bis (2-nitrobenzoic acid) (DTNB) as a coloring reagent.30 The absorbance was read at 412nm by spectrophotometer. GSH concentration was calculated from a standard curve.
Determination of testicular superoxide dismutase (SOD)Testicular superoxide dismutase was assayed by the method of Asada et al.,31 which involves the inhibition of photochemical reduction of nitro blue tetrazolium (NBT) at pH 8.0. A single unit of enzyme is defined as the quantity of superoxide dismutase required to produce 50% inhibition of photochemical reduction of NBT. The absorbance was read at 580nm against a blank using UV–Vis spectrophotometer. The activity was expressed as U/mg protein.
Determination of testicular catalase (CAT)Catalase activity was estimated in testis homogenate by the method reported by Aebi.32 The specific activity of catalase has been expressed as mmoles of H2O2 consumed/min/mg protein. The difference in absorbance at 240nm per unit time is a measure of catalase activity.
Determination of testicular glutathione peroxidase (GPX)The activity of the antioxidant enzyme glutathione peroxidase was determined using glutathione reductase and NADPH. This method is based on the oxidation of NADPH at 25uC, which is indicated by the decrease in absorbance at 340nm.33 Results are expressed in U/mg protein.
Determination of testicular cholesterolThe estimation of testicular cholesterol was carried out by the method of Zlatki.34 To test tubes containing 5ml of working FeCl3 solution, 0.2ml of testis homogenate prepared in glacial acetic acid was added. The contents were mixed and 3ml of concentrated H2SO4. The optical density after color development was read at 540nm on a spectrophotometer and expressed as mg/100mg tissue wt.
Histological preparation of tissueThe testis was harvested and fixed in Bouin‘s fluid for 24h and dehydrated by passed through ascending grades of alcohol (70%, 80%, 90% and absolute 100%). Cleaned with xylene to remove the alcohol and embedded in paraffin. Sections of tissue (3–5μm thick) were prepared by using a rotary microtome and stained with hematoxylin and eosin and in neutral deparafinated xylene (DPX) medium for microscopic observations. Photomicrographs were taken at a magnification of x400. Histopathological findings were investigated with Johnsen scoring system.35 Five sections per animal and 10 seminiferous tubules per section were assessed using a score of 1–10 under ×40 magnification. For histo-morphometric evaluation, the parameters namely seminiferous tubule diameter (STD), thickness of the germinal epithelium of the ST (from the basement membrane to lumen), spermatogonia (Sg), preleptotene, spermatocytes (PLSc), pachytene spermatocytes (PSc), and spermatid cells (Sd) observed in 10 tubules per testicular section and 10 section per groups were measured at ×40 magnifications by using calibrated OLYSIA Soft Imaging System GmbH, version 3.2 (Japan).36
Ethical considerationsAll experimental procedures followed the recommendations provided in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and Published by the National Institute of Health.37
Data presentation and statistical analysisData were expressed as Mean±SEM. Statistical differences between the groups were evaluated by one-way ANOVA, followed by Newman–Keuls Multiple Comparison Test. Differences yielding p<0.05 were considered statistically significant. Statistical analyses of data were performed using Graph Pad Prism 5 Windows (Graph Pad Software, San Diego, CA, USA).
ResultsAcute toxicity studiesFor aqueous extracts, no toxicity was found up to 2000mg/kg. Hence extract was selected as lower dose, intermediate dose and higher dose, i.e., 200mg/kg, 400mg/kg & 600mg/kg-p.o, of body weight, respectively.
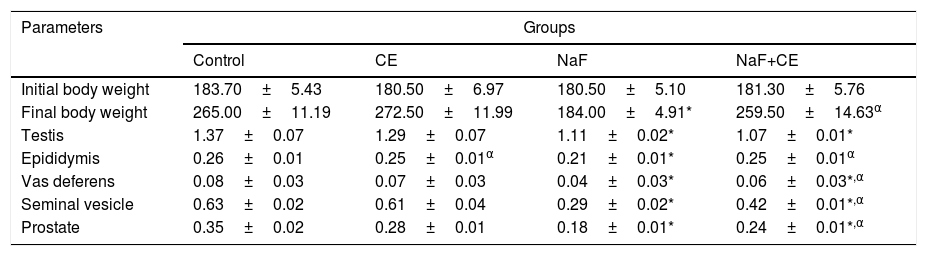
Weights of reproductive organThe mean value of body weight in sodium fluoride treated group significantly (p<0.05) reduced in comparison with the control group and NaF+CE groups increased when compared with the control and significantly (p<0.05) increased in comparison with that of NaF group. The mean value of weights of testes and accessory sex organs decreased significantly (p<0.05) in both NaF treated and NaF+CE groups. However, the mean values of accessory sex organs of NaF+CE group were significantly higher than those of NaF treated group (Table 1).
Effect of Cyperus esculentus nut extract on body weight, reproductive organ weights (g) relative to body weight in experimental NaF-exposed rats.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | CE | NaF | NaF+CE | |
| Initial body weight | 183.70±5.43 | 180.50±6.97 | 180.50±5.10 | 181.30±5.76 |
| Final body weight | 265.00±11.19 | 272.50±11.99 | 184.00±4.91* | 259.50±14.63α |
| Testis | 1.37±0.07 | 1.29±0.07 | 1.11±0.02* | 1.07±0.01* |
| Epididymis | 0.26±0.01 | 0.25±0.01α | 0.21±0.01* | 0.25±0.01α |
| Vas deferens | 0.08±0.03 | 0.07±0.03 | 0.04±0.03* | 0.06±0.03*,α |
| Seminal vesicle | 0.63±0.02 | 0.61±0.04 | 0.29±0.02* | 0.42±0.01*,α |
| Prostate | 0.35±0.02 | 0.28±0.01 | 0.18±0.01* | 0.24±0.01*,α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA.
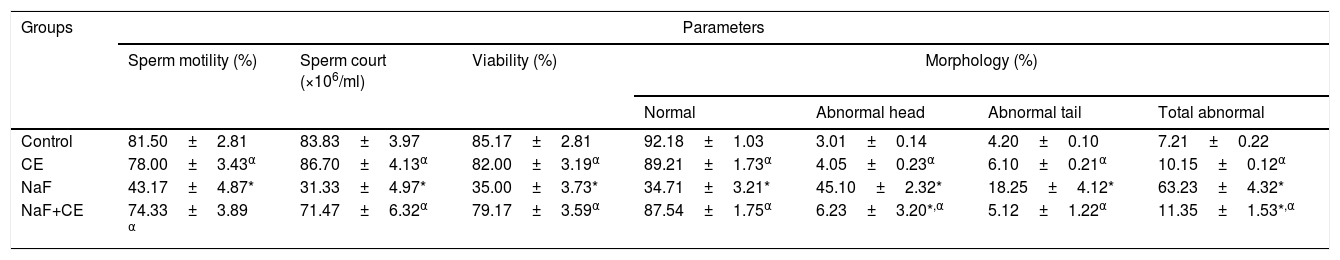
There was significant (p<0.05) decreased in mean value of sperm motility, count, viability and normal morphology of sodium fluoride (NaF) exposed rats and resulted in a significant (p<0.05) increase in head, tail and total sperm abnormalities when compared with the values of control group. The administration of NaF+CE in combination increased sperm motility, count, viability and normal morphology and decrease abnormal sperm rates as compared to NaF group. Also, the extract caused significant (p<0.05) increases in sperm motility, sperm count and viability of extract treated rats. Furthermore, the percentages of abnormal sperm cells (morphology) in Cyperus esculentus nut treatment groups were not significantly different from the control but significant (p<0.05) dissimilar from the NaF treated group (Table 2).
Effect of Cyperus esculentus nut extract on sperm parameters in experimental NaF treated rats.
| Groups | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Sperm motility (%) | Sperm court (×106/ml) | Viability (%) | Morphology (%) | ||||
| Normal | Abnormal head | Abnormal tail | Total abnormal | ||||
| Control | 81.50±2.81 | 83.83±3.97 | 85.17±2.81 | 92.18±1.03 | 3.01±0.14 | 4.20±0.10 | 7.21±0.22 |
| CE | 78.00±3.43α | 86.70±4.13α | 82.00±3.19α | 89.21±1.73α | 4.05±0.23α | 6.10±0.21α | 10.15±0.12α |
| NaF | 43.17±4.87* | 31.33±4.97* | 35.00±3.73* | 34.71±3.21* | 45.10±2.32* | 18.25±4.12* | 63.23±4.32* |
| NaF+CE | 74.33±3.89 α | 71.47±6.32α | 79.17±3.59α | 87.54±1.75α | 6.23±3.20*,α | 5.12±1.22α | 11.35±1.53*,α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA.
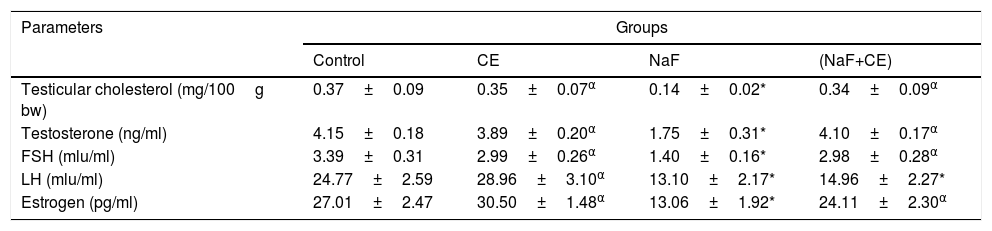
Mean value of testicular cholesterol significantly (p<0.05) decreased in both NaF-treated and NaF+CE groups respectively in comparison with the control groups, mean value of TT, LH, Estrogen and FSH level of rats in the sodium fluoride treated group significantly (p<0.05) reduced. In the NaF+CE and CE group, testosterone levels, Estrogen and FSH level were not significantly different from the values of control but LH levels was significantly (p<0.05) different from the values of control (Table 3).
Effect of Cyperus esculentus nut extract on testicular cholesterol, plasma testosterone level, FSH, LH and Estrogen in experimental NaF-treated rats.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | CE | NaF | (NaF+CE) | |
| Testicular cholesterol (mg/100g bw) | 0.37±0.09 | 0.35±0.07α | 0.14±0.02* | 0.34±0.09α |
| Testosterone (ng/ml) | 4.15±0.18 | 3.89±0.20α | 1.75±0.31* | 4.10±0.17α |
| FSH (mlu/ml) | 3.39±0.31 | 2.99±0.26α | 1.40±0.16* | 2.98±0.28α |
| LH (mlu/ml) | 24.77±2.59 | 28.96±3.10α | 13.10±2.17* | 14.96±2.27* |
| Estrogen (pg/ml) | 27.01±2.47 | 30.50±1.48α | 13.06±1.92* | 24.11±2.30α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA.
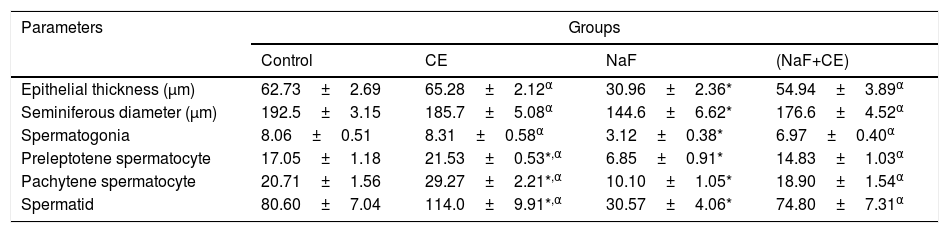
Morphometric results in NaF treated group showed decreased seminiferous epithelial thickness and seminiferous tubules diameter in the majority of tubules as compared to control (p<0.05). tiger nut treatment increased the mean germinal epithelium thickness and tubular diameter in NaF treated rats. Reduction in thickness of the epithelium and diameter of seminiferous tubules were statistically significant compared with NaF group (p<0.05). There was observed significant (p<0.05) decrease in mean value of the spermatocytes and spermatids cell in NaF treated rats when compared to that of control group. However, there was significant increase in mean value of spermatocyte cells, spermatogenic cells and spermatid cell counts in the CE and NaF+CE treated groups in comparison with the NaF group which indicate ameliorative effect of tiger nut extract on spermatogenesis in rats (Table 4).
Effects of Cyperus esculentus nut extract on histomorphometry of Epithelial thickness and seminiferous tubules of experimental NaF-treated male rats.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | CE | NaF | (NaF+CE) | |
| Epithelial thickness (μm) | 62.73±2.69 | 65.28±2.12α | 30.96±2.36* | 54.94±3.89α |
| Seminiferous diameter (μm) | 192.5±3.15 | 185.7±5.08α | 144.6±6.62* | 176.6±4.52α |
| Spermatogonia | 8.06±0.51 | 8.31±0.58α | 3.12±0.38* | 6.97±0.40α |
| Preleptotene spermatocyte | 17.05±1.18 | 21.53±0.53*,α | 6.85±0.91* | 14.83±1.03α |
| Pachytene spermatocyte | 20.71±1.56 | 29.27±2.21*,α | 10.10±1.05* | 18.90±1.54α |
| Spermatid | 80.60±7.04 | 114.0±9.91*,α | 30.57±4.06* | 74.80±7.31α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA.
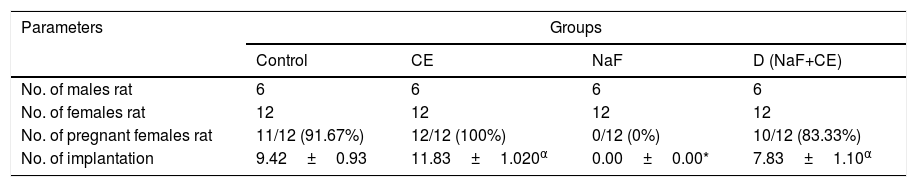
In fluoride treated group, the fluoride has adverse effect on fertility potentials of treated male rats since the female rats copulated with the treated male rats did not pregnant proven infertility. However, in group co-treated with sodium fluoride and tiger nut extract observed to have positive impact on fertility potentials of treated male rats since there was significant (p<0.05) increase in number of pregnant female and implantation. There was increase in number of pregnant female and implantation in group treated with tiger nut extract when compared with the control and NaF+CE treated group and significant (p<0.05) increase in comparison with the fluoride treated group (Table 5).
Effects of Cyperus esculentus nut extract on the fertility of experimental NaF-treated male rats.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | CE | NaF | D (NaF+CE) | |
| No. of males rat | 6 | 6 | 6 | 6 |
| No. of females rat | 12 | 12 | 12 | 12 |
| No. of pregnant females rat | 11/12 (91.67%) | 12/12 (100%) | 0/12 (0%) | 10/12 (83.33%) |
| No. of implantation | 9.42±0.93 | 11.83±1.020α | 0.00±0.00* | 7.83±1.10α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA.
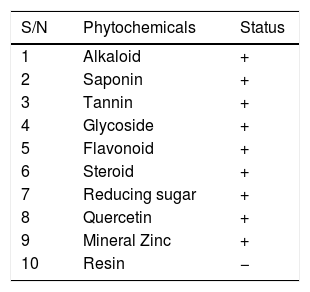
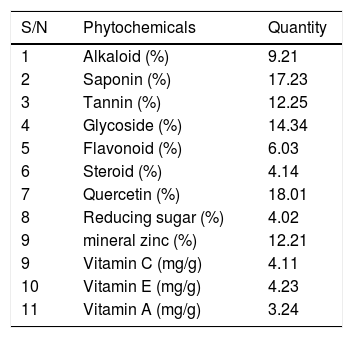
Qualitative analysis of Cyperus esculentus nut shows the presence of alkaloid, saponin, tannin, glycoside, flavonoid, steroid, quercetin and reducing sugar (Table 6). After the quantitative analysis, there was observed high values of vitamins A, C and E. Total alkaloid, total tannin, total glycoside, total saponins, total quercetin and total flavonoids had higher values compared to total steroid and total reducing sugar present (Table 7).
Quantitative phytochemical analysis of aqueous extract of Cyperus esculentus nut.
| S/N | Phytochemicals | Quantity |
|---|---|---|
| 1 | Alkaloid (%) | 9.21 |
| 2 | Saponin (%) | 17.23 |
| 3 | Tannin (%) | 12.25 |
| 4 | Glycoside (%) | 14.34 |
| 5 | Flavonoid (%) | 6.03 |
| 6 | Steroid (%) | 4.14 |
| 7 | Quercetin (%) | 18.01 |
| 8 | Reducing sugar (%) | 4.02 |
| 9 | mineral zinc (%) | 12.21 |
| 9 | Vitamin C (mg/g) | 4.11 |
| 10 | Vitamin E (mg/g) | 4.23 |
| 11 | Vitamin A (mg/g) | 3.24 |
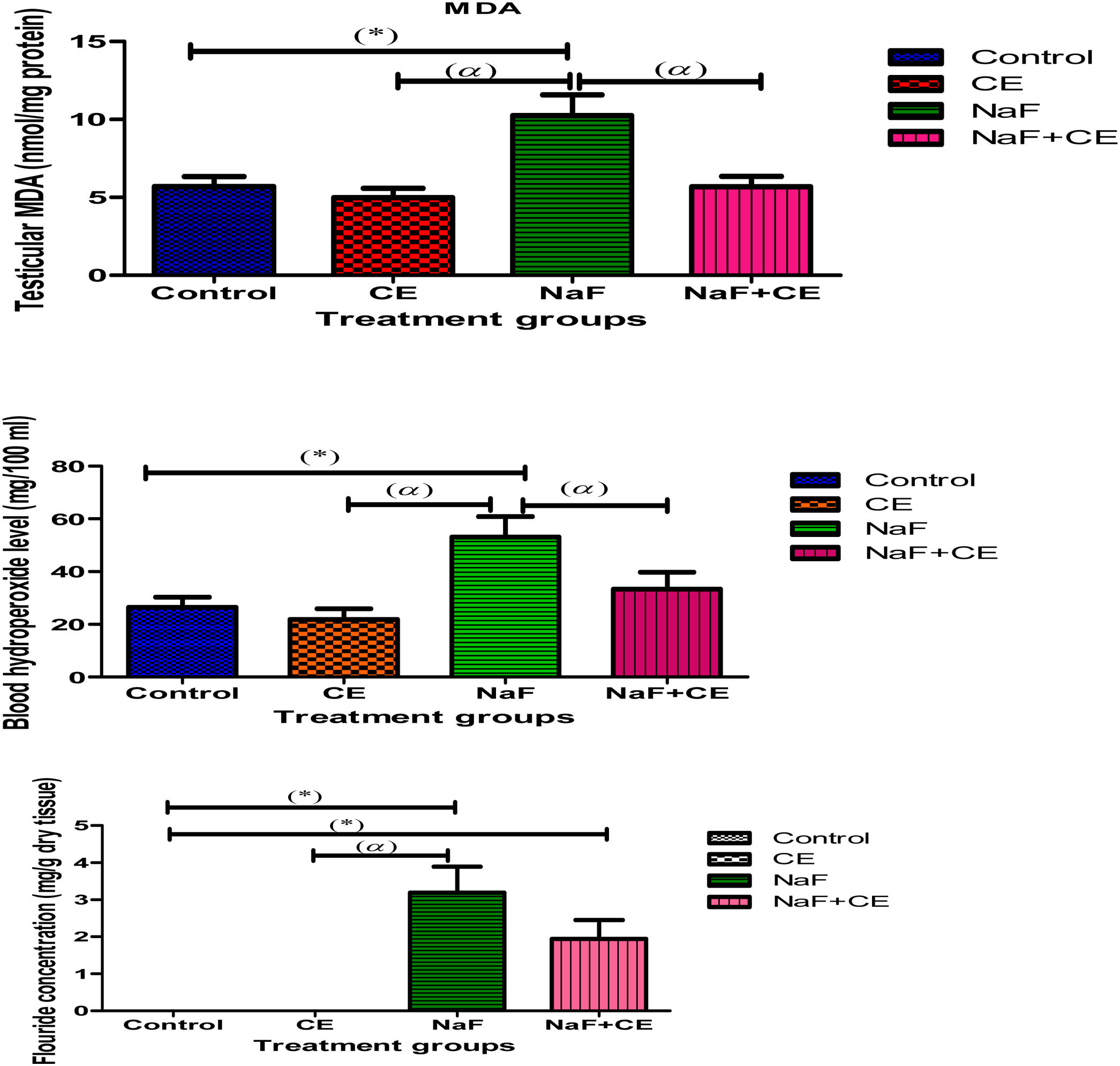
There was a statistically significant (p<0.05) decrease in the level of blood hydroperoxide in Tiger nut-treated animals whereas reduction in tMDA level was not significant. Sodium fluoride (NaF) administration resulted in a significant (p<0.05) elevation in the levels of blood hydroperoxide and testicular tMDA in comparison with values in the control group. The blood hydroperoxide level also increased significantly (p<0.05) in the combined treatment of Cyperus esculentus extract with NaF while testicular tMDA did not change significantly compared to the control values. Moreover, the hydroperoxide or tMDA level of NaF+CE group was significantly (p<0.05) lower than that of NaF treated group. Although, NaF level in testis exhibited a significant elevation (p<0.05) in both NaF and NaF+CE groups (Fig. 1).
The histogram shows effect of Cyperus esculentus nut extract on lipid peroxidation products, blood hydroperoxide level and testicular fluoride concentration in experimental NaF treated rats. Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA. Key: MDA, malondialdehyde; CE, Cyperus esculentus; NaF, Sodium fluoride.
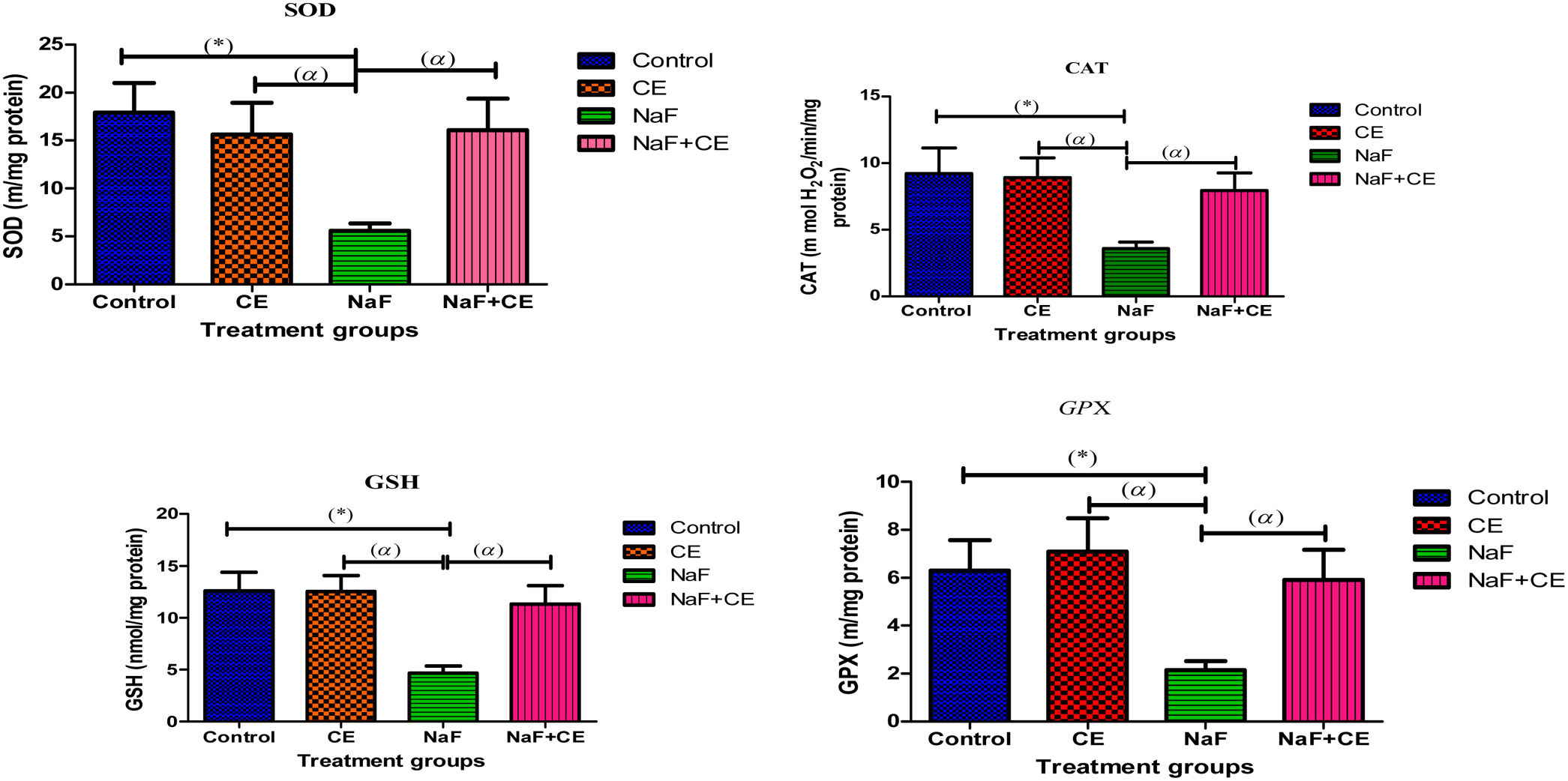
There was observed increase in testicular reduced glutathione (tGSH) and (tGPx) in Cyperus esculentus group and significant (p<0.05) decrease in the level of testicular GSH and activity levels of testicular antioxidant enzymes (SOD, CAT and GPx,) in NaF treated group when compared to the control group, co administration of tiger nut extract and NaF prevented the observed decrease in testicular SOD, CAT, GSH and GPx (Fig. 2).
The histogram shows effect of Cyperus esculentus nut extract on testicular glutathione content and activities of antioxidant biomarker enzymes in experimental NaF treated rats. Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA. Key: SOD, superoxide dismutase; CAT, catalase; GSH, glutathione; GPX, glutathione peroxidase; CE, Cyperus esculentus; NaF, Sodium fluoride.
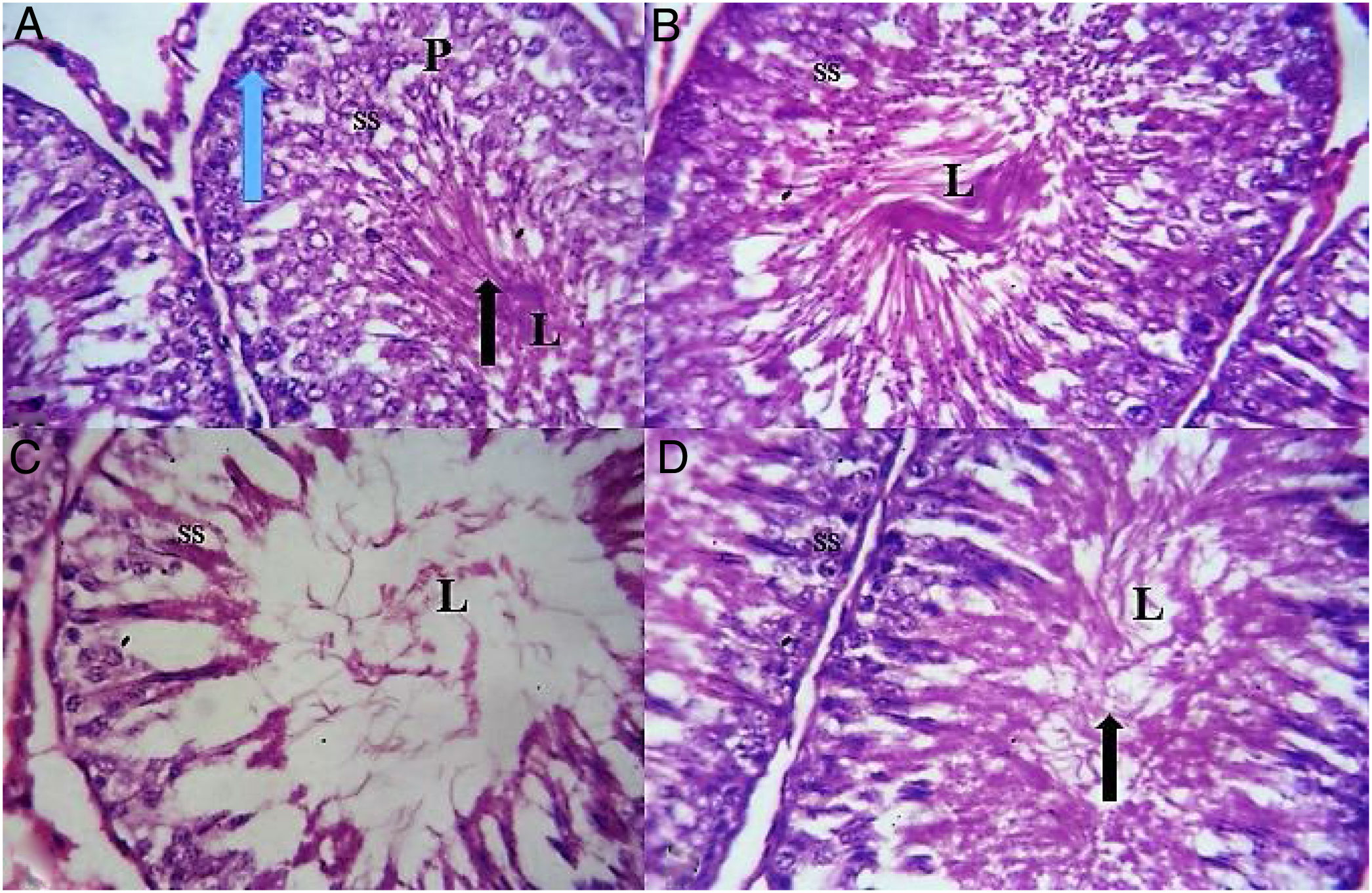
Cross section of the testis of the animal revealed normal spermatogenic cells, sertoli and leydig cells and precise spermatogenesis with abundant sperm cells in the lumen in the control and TN group. However, animal in NaF treated group showed a marked of testicular lesions in seminiferous tubules with decrease luminal spermatozoa, irregular basement membrane, disorganization, necrosis and degeneration of spermatogenic cells and hemorrhage in interstitial tissues. Co administration of NaF and CE showed normal cellular composition with no obvious aberrations compared to NaF exposed group that showed marked depletion of spermatogenic cells with a widened and a hypocellular interstitium (Fig. 3).
(A) Testicular section of control rat showing seminiferous tubules containing cells of spermatogenic series (SS) and lumen (L) containing spermatozoa; blue arrow-spermatogonium; p-primary spermatocytes; black arrow-spermatids and spermatozoa. (B) Testicular section of CE group rat shows normal spermatogenesis and cell arrangement in the seminiferous tubules. (C) Testicular sections of NaF-treated group showing hypocellularity, reduction in cells of spermatogenic series (SS) due to degeneration, sloughing and shorting of seminiferous epithelium with a single layer of basal spermatogonia and empty lumen (L). (D) Testicular sections of NaF+CE-treated group showing appearance of normal structure of testicular seminiferous tubules with abundant sperm cell (black arrow) in the lumen (L) (H&E; ×400).
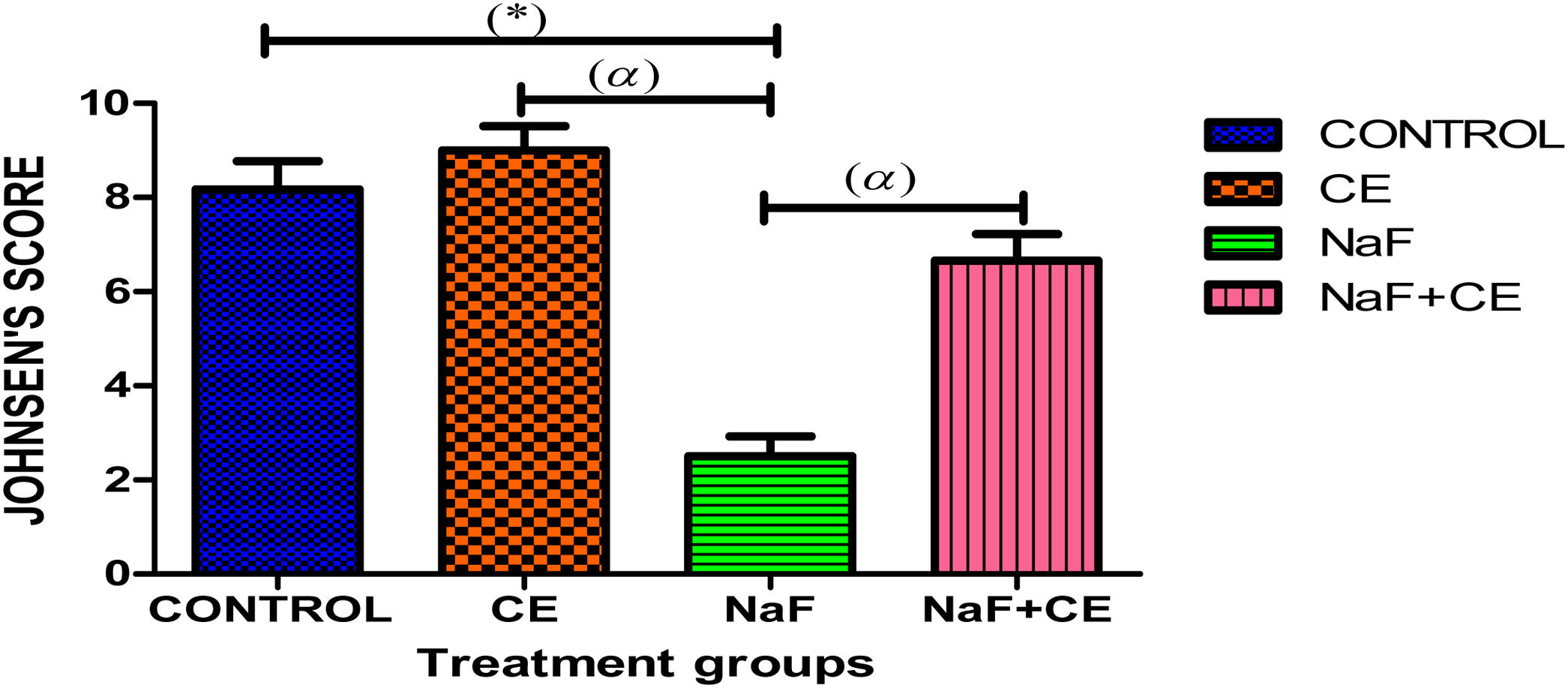
Sodium chloride (NaF) treated group showed significant (p<0.05) decreased in mean Johnsen's score in comparison with the control, CE and NaF+CE treated group. There was increase in mean Johnsen's score in CE treated group when compared with the control group. CE and NaF+CE treated group shows significant (p<0.05) increase in mean Johnsen's score in comparison with the NaF treated group (Fig. 4).
The histogram shows Johnsen's score of testicular tissue in the all groups. Values are expressed as Mean±S.E.M, n=6 in each group, *: represent significant different from control, α: represent significant different from NaF at p<0.05, One-Way ANOVA. Keys: CE, Cyperus esculentus; NaF, Sodium fluoride.
Oxidative damage has been reported as main mechanisms by which fluoride induces toxic effects and elevation of production of reactive oxygen species (ROS) which lead to cell death.38
In this study, sodium fluoride elevates oxidative stress and antioxidant biomarkers level in the testis of rats by reduced the levels of GSH along with antioxidant enzymes (SOD, CAT, and GPx) and increase testicular malondialdehyde (MDA) and blood hydroperoxide. It has been reported that MDA is one of the major products of peroxidized polyunsaturated fatty acids and increased MDA content is an important indicator of lipid peroxidation.39 Antioxidants are known to counter the effects of oxidants, and Cyperus esculentus nut is rich in antioxidant phytochemicals.40 It is therefore acceptable that phytochemicals constituent of Cyperus esculentus nut are capable of attenuating oxidative stress induced by NaF. This prove that Cyperus esculentus nut extract have a beneficial effect in reducing the toxic effect of NaF on testis by reducing the accumulation of fluoride in the testis. In this study, fluoride consumption reduced GSH content in testicular tissues thereby, exposed the spermatogenic cells to oxidative damage however, some bioactive component of Cyperus esculentus nut neutralizes ROS that accumulates in the testis as a result of GSH depletion by NaF, thereby complementing the endogenous antioxidants. This is constant with the report of Molina et al.,41 that GSH is known to induced higher concentrations of antioxidant enzymes such as SOD.
In the current investigation, in comparison with the control group, NaF administration significantly decreases sperm count, sperm viability, sperm motility, normal sperm morphology and increased abnormal sperm morphology by subjecting the spermatozoa to increased oxidative stress and DNA damage because their plasma membranes contain large quantities of polyunsaturated fatty acids.42 Increased formation of ROS has been correlated with a reduction of sperm motility.43 The role of fluoride toxicity on spermatogenesis could be due to reductions of testosterone levels by NaF and by reducing the testicular zinc levels, thereby impairs angiotensin-converting enzyme (ACE) activity and thus causes inhibition of spermatogenesis.44 The mechanism by which fluoride affects sperm motility has not been clearly elucidated. However, it has been postulated that fluoride could act directly on the motile apparatus without affecting other metabolic systems.45 Intervention of Cyperus esculentus nut extract increase sperm quality. Improvement of sperm quality may be correlated to the antioxidant components of Cyperus esculentus nut, such as flavonoids, saponins, vitamin E, vitamin C) and vitamin A that improve testicular functions and sperm quality.46 Therefore, it is plausible deducing that these rich antioxidant constituent of Cyperus esculentus nut boosted the testicular non-enzymatic and enzymatic antioxidants to effectively scavenge the free radicals preventing lipid peroxidation.
In this present study, administration of NaF affect the normal function of the steroli and leydig cells by significantly reduces LH, FSH and testosterone levels. It has been reported that in male, reduction of testosterone level may impair spermatogenesis and cause male infertility which is evidence in this study due to reduction of testosterone in NaF exposed group. Also, there was observed significant decrease in the estrogen level. Furthermore, a fall in the plasma testosterone level in the animals treated with NaF seems to be due to a reduction in the activity of enzymes involved in the biosynthesis of testosterone47 or due to the decrease in testicular cholesterol, a precursor of testosterone synthesis. Administration of Cyperus esculentus nut extract significantly elevate the levels of LH, FSH, estrogen and testosterone as a result of antioxidant present in the Cyperus esculentus nut. Increase in estrogen level might be due to the conversion of testosterone to estrogen.48 Elevation of decrease testosterone, FSH, LH and estrogen by Cyperus esculentus nut extract shows that Cyperus esculentus nut extract may have positive libido effect because it increases the serum LH and testosterone levels in this study which is constant with the report of Mohammed et al.49 The significant increase in testosterone level in CE and NaF+CE group when compare with NaF group concur with the find of Ekaluo et al.,50 who reported increased effect of the aqueous extract of Cyperus esculentus nut on the level of testosterone. The decreased levels of sperm count, weight of reproductive organs, serum hormonal levels and number of implantations in female rats reveals the antifertility activity of fluoride when compared with control group. However, Cyperus esculentus nut extract administration increased the levels of sperm count, weight of reproductive organs, serum hormonal levels and number of implantations in female rats reveals profertility potential of Cyperus esculentus nut.
In the present study, there was observe decreased in body, testis and accessory organs weight in NaF exposed group in comparison with the control group since growth of accessory sex glands require testosterone.51 However, administration of Cyperus esculentus nut extract increases the body weight, testis and accessory organs weight, effect of CE on increasing weight of testes and epididymis agrees with the report of Amaal and Essraa.52 This could be attributed to its rich vitamin and protective role of particularly vitamin C present in CE nut against oxidative stress and morphological changes of the testicular tissues.53 Increase in testicular and epididymal weights have been observed to be an indication of higher sperm production,54 which could be due to increased androgen biosynthesis as evidenced by a significant increase in testosterone level.55
Histological examination of NaF exposed group revealed histopathological changes in the seminiferous tubules, such as disorganization in the germinal epithelium layer of seminiferous tubules and depleted spermatogenic cells. We can therefore deduce from our findings that fluoride significantly inhibited the proliferative activity of the spermatogonia in all stages of the seminiferous tubules cycle, it significantly decreased the seminiferous tubules diameter, degenerated germ cells and decreased the number of Leydig cells. For decades, it has been proved that testosterone which is produced by the interstitial cells of Leydig is a necessary prerequisite for the maintenance of established spermatogenesis.56 The reduced cellularity of interstitium in testis of animals treated with only NaF would consequently lead to decrease in testosterone resulting in the poor spermatogenesis observed. Cyperus esculentus nut aqueous extract maintained histoarchitecture of the testis, increased the proliferative activity of spermatogonia compared to the control animals. Cyperus esculentus nut has been reported to be rich in quercetin is a dietary flavonoid that exhibits strong antioxidant activity reduces lipid peroxidation.57 From our observation, concomitant administration of aqueous extract of Cyperus esculentus nut with NaF protected the testis from the pernicious effects of fluoride. This protective nature of Cyperus esculentus nut is enhanced by some of its phytochemical constituents: the presence of ascorbic acid which is known for its protection on cell membranes and its scavenging effects on free radicals.58
ConclusionIn conclusion NaF was found to induced reproductive dysfunction by alter spermatogenesis, induced testicular toxicity and cause infertility, intervention of Cyperus esculentus nut extract protected the cyto-architecture of the testis from the damaging effects of fluoride therefore Cyperus esculentus nut augments spermatogenesis, attenuates fluoride-induced oxidative stress through an antioxidant system of activities and promote fertility.
Ethical disclosuresThe experimental procedures were conducted in accordance with the NIH guidelines for the care and use of laboratory animals and in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Ethical considerationsAll experimental procedures followed the recommendations provided in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and Published by the National Institute of Health
Conflict of interestsThe authors declare that they have no competing interests.
Authors are grateful to Dr. K.D. Ileke of Department of Biological Science Federal University of Technology Akure for identification of plant material, Mr. Ige of Histology Laboratory Department of Anatomy and cell Biology, Obafemi Awolowo University, Ife, Nigeria for preparation of histology slide and Dr. Ijomone Neuro Laboratory, Department of Human Anatomy, Federal University of Technology Akure Nigeria for production of macrograph