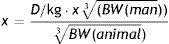Extract of Pygeum africanum (PAE) is commonly used herbal medication in the treatment of benign prostatic hyperplasia. In Montenegro and neighboring countries, PAE is primarily advertised as dietary supplement in the treatment of erectile dysfunction. The purpose of this study was to broaden the current cognition concerning its safety profile.
Material and methodsTwenty-four adult male Wistar rats were used. The first control group (O) received water and second control group (OO) received olive oil for 30 days. The third and fourth groups (PA5 and PA10) were treated with PAE dissolved in olive oil (50 and 100mg/kg p.o. daily). The behavior of animals was observed continuously, bodyweight gain (BWG) was calculated weekly and the weight of selected organs was measured at the end of experiment. Total protein and glutathione content of the liver were analyzed. Standard biochemical analyses were also performed.
ResultsBWG was higher in PA5 compared to both controls at all measuring intervals. Liver weight/body weight ratio was significantly higher in PA10 in comparison with O. Prostate weight/body weight ratio was lower in both PA5 and PA10 compared to OO, achieving statistical significance in PA5. The value of creatinine was higher in PA5 and PA10 compared to both control groups, but achieving statistical significance in PA10 only. LDH was also increased in PA5 and PA10 compared to both controls.
ConclusionsBoth dosage regimens of PAE, particularly PA10, caused some toxicological effects in Wistar rats after one month of application. Kidney, skeletal muscle and/or myocardium are suspected as target sites of PA toxicity most likely. In order to provide more reliable conclusion it is necessary to conduct an additional research on the basis of these findings.
El extracto de Pygeum africanum (PAE) es un producto de origen vegetal que frecuentemente se utiliza en el tratamiento de la hiperplasia benigna de próstata. En Montenegro y en los países limítrofes, el PAE se anuncia principalmente como suplemento dietético en el tratamiento de la disfunción eréctil. El propósito de este estudio fue ampliar el conocimiento actual respecto a su perfil de seguridad.
Material y métodosSe utilizaron 24 ratas macho adultas de raza Wistar. El primer grupo de control (O) recibió agua y el segundo grupo de control (OO) recibió aceite de oliva durante 30 días. El tercer y cuarto grupos (PA5 y PA10) se trataron con PAE disuelto en aceite de oliva (50 y 100mg/kg vo diariamente). Se observó continuamente el comportamiento de los animales, semanalmente se calculó el incremento del peso corporal (IPC), y el peso de los órganos seleccionados se midió al final del experimento. Se analizaron el contenido total de proteína y glutatión del hígado. También se realizaron análisis bioquímicos habituales.
ResultadosEl IPC fue mayor en PA5 que en los 2 grupos control en todos los intervalos de medición. La relación entre peso del hígado y peso corporal fue considerablemente mayor en PA10 que en O. La relación entre peso de la próstata y peso corporal de la próstata fue menor tanto en PA5 como en PA10 en comparación con OO, por lo que se obtuvo significación estadística en PA5. El valor de la creatinina fue más elevado en PA5 y PA10 en comparación con ambos grupos de control, pero alcanzó significación estadística solo en PA10. La LDH también se incrementó en PA5 y PA10 en comparación con los 2 grupos control.
ConclusionesLas 2 pautas de dosificación de PAE, sobre todo en PA10, provocaron algunos efectos toxicológicos en ratas de raza Wistar después de un mes de aplicación. Se sospecha que muy probablemente el riñón, el musculoesquelético o el miocardio sean lugares diana de los efectos tóxicos de PAE. Para ofrecer una conclusión más fiable, es necesario llevar a cabo más investigación sobre la base de estos hallazgos.
According to the latest European Association of Urology guidelines,1 pharmacological management of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH) includes several groups of drugs: α1-adrenoceptor antagonists, 5α-reductase inhibitors, muscarinic receptor antagonists, phosphodiesterase-5 inhibitors and different plant extracts – phytotherapy. In addition, vasopressin analog (desmopressin) and beta-3 agonists (mirabegron) are also recommended for special conditions.
The use of herbal medicines is increasing in many areas of therapy and in this sense prostate disease is no exception.2 Despite conflicting results concerning their efficacy, plant based extracts as medicines or health-promoting agents are often used as first-line treatment for bothersome male LUTS.3Pygeum africanum (PA) is among the most widely used plants for the treatment of LUTS. Extract is prepared from the bark of the African plum tree, an evergreen member of the rosaceae family native to the montane regions of Sub-Saharan Africa and the island of Madagascar. In a recent study of Kadu et al.,4 different bioactive constituents derived from bark extracts from 20 tree populations sampled throughout the species’ natural range have been found. In average, concentration of ursolic acid, β-sitosterol and β-sitostenone were prevalent, followed by significantly lower concentration of ferulic acid, n-docosanol, myristic acid and lauric acid.
Although precise mechanism of action of Pygeum africanum extract (PAE) remains unclear, both in vivo and in vitro it improves bladder contractile function,5,6 exerts antiproliferative and apoptotic effects on proliferative prostate fibroblasts and myofibroblasts by TGFB1 downregulation and inhibition of FGF2 specific signaling,7 exhibits androgen antagonistic activity and inhibits LNCaP and C4-2 prostate cancer cell growth and cellular invasiveness.8,9 Twenty years ago, anti-inflammatory and phyto-estrogenic effects were found.10,11
So far there are limited data on the pharmacokinetics of PA. Larre et al.12 concluded that oral intake of PA can result in sufficient serum level of active substances to induce a transcriptome modification and inhibition of prostatic myofibroblast growth. Data related to other pharmacokinetic properties and possible interactions with other drugs and/or herbal remedies is missing.
As regards tolerability and toxicity, aqueous extract of PA led to a moderate increase in serum ALT and CPK, mainly when it was administered to the rats at a dose of 1000mg/kgBW.13 Chloroform extract of PA caused marked clinical signs, organ damage and a 50% mortality rate at a dose of 3.3g/kg for 6 days.14 The main lesions that were observed at this dose were centrilobular hepatocellular degeneration and necrosis, diffuse nephrosis, myocardial degeneration, lymphocytic necrosis and neuronal degeneration. Based on available bibliographic data and on decades of clinical use, there can be concluded satisfactory safety profile of PA.
In general, preceding clinical studies of PA were of short duration and small in size, with varied dose, high placebo response and the outcomes using standardized validated measures of efficacy were rarely reported.12 In addition, because of product heterogeneity, limited regulatory framework and methodological limitations of the published trials and meta-analyses, the latest EAU Guidelines1 did not make any specific recommendation on phytotherapy of male LUTS in general, including PA.
For several years, products that come from PA have been forcefully advertised in Montenegro, but also in neighboring countries. As dietary supplements these preparations are primarily proposed for the treatment of erectile dysfunction that is not consistent with the evidence-based medicine and current recommendations. Possible risks and negative consequences cannot be excluded.
The objective of this study was to broaden the current cognition concerning the safety profile of PAE.
Material and methodsStudy designTwenty-four sexually mature male Wistar rats, weighing 250–300g, were randomly distributed into 4 groups of 6 animals. The first control group (O) received water (1ml/kg BW p.o.) and second control group (OO) received olive oil (1ml/kg BW p.o.). The third and fourth groups of rats (PA5 and PA10) were treated with PA dissolved in OO.
According to the Committee on Herbal Medicinal Products of the European Medicines Agency (EMA), human recommended dose of PA is a capsule of 50mg twice daily.15
Pharmacological dose of PAE for rats in relation to human recommended dose was calculated according to the Clark's formula:
x – dose for animalBW – bodyweight
D/kg – human recommended dose
In order to investigate the toxicological profile of PAE, obtained value (10mg/kgBW) was multiplied with five (50mg/kgBW/day, PA5) and ten (100mg/kgBW/day, PA10).
Solutions were prepared daily and were applied every morning for 30 days. Animals received an equal volume of solvent (OO) per kg/BW in which determined dose of PAE was dissolved. Bodyweight was measured weekly, so the next dose was adjusted to the metered BW.
Substances and dosesFollowing substances were used: extract of P. africanum (Pigenil®, soft capsules 50mg, PHARMAFAR S.r.l., Torino, Italy); olive oil (bottle 50ml); urethane solution 25%; sulphosalicylic acid 4%; Elman's reagent (0.2mmol/dm3 DTNB in 0.1mol/dm3 K3PO4, pH=8); Biuret reagent (0.15% CuSO45H2O; 0.6% K,Na-tartrate, 0.1% KJ dissolved in 0.85mmol/dm3 NaOH).
Each soft capsule of Pigenil® contains:
- -
P. africanum extract (equivalent to 10g of dry bark powder) containing 0.5% of total n-docosanol in 50mg (active substance);
- -
peanut oil, hydrogenated coconut oil, beeswax and lecithin (excipients) and
- -
gelatin, glycerin, titanium dioxide (E171), copper chlorophyll (E141), ethyl p-hydroxybenzoate sodium salt and propyl p-hydroxybenzoate sodium salt (capsule ingredients).
Sexually mature male Wistar rats were bred in the vivarium at Department of Pharmacology, Toxicology and Clinical Pharmacology, Medical Faculty of the University in Novi Sad, Serbia. Animals were kept in plexiglass cages (six per cage) at standard laboratory conditions (light period of 12h/day, constant room temperature of 21±1°C and humidity 55%±1.5%). They were fed by standard food for laboratory rats, with free access to food and water. Animal care and all experimental procedures were carried out in compliance with the Animal Care Committee regulations of Medical Faculty in Novi Sad, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals edited by Commission of Life Sciences, National Research Council (USA).
Experimental proceduresAfter 30 days of treatment the animals were measured, then anesthetized with 25% urethane solution (2.5ml/kg intraperitoneally). Blood was collected in order to prepare serum for biochemical analyses. The liver was measured, and then a portion of 1mg was removed for the preparation of homogenate which was used for the determination of total protein content (TPC) of the liver and glutathione content (GC) in homogeneous suspension of hepatocytes. Left kidney, prostate and left testis were also separated and measured.
Toxicological profile of PAE was estimated on the basis of daily monitoring of behavior and bodyweight gain (BWG) at weekly intervals and at the end of treatment. Additional parameters were liver weight/body weight (LW/BW) ratio, kidney weight/body weight (KW/BW) ratio, prostate weight/body weight (PW/BW) ratio and testis weight/body weight ratio (TW/BW). The value of urea, creatinine, electrolytes, transaminases (ALT and AST) and lactate dehydrogenase (LDH) was also measured using standard methodology.
For determination of GC in hepatocyte suspension, we used a method that was applied by Kapetanovic et al.16 (modified Elman's method).
TPC was measured by biuretic method, modified by Gornall.17
Statistical analysisAll data are expressed as a mean±standard deviation (SD). The level of significance between the groups was assessed by analysis of variance (ANOVA) for multiple comparisons, followed by Tukey's test where appropriate. The accepted level of significance was p<0.05.
ResultsBy continual monitoring of experimental animals, there was not noticed any change in their behavior. None of the animals died during treatment. As regards appearance, mobility, the intake of food and water, experimental animals did not show any difference in relation to the control groups. BWG in rats that were treated with PAE in two doses compared to control groups, in intervals of seven days, is shown in Table 1.
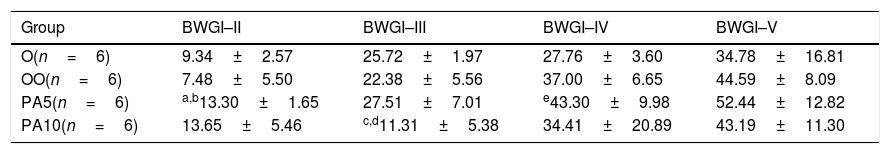
BWG in rats treated with Pygeum africanum extract (PA5 and PA10) compared to control substances (O and OO), in intervals of seven days, in percentages (%) (mean±SD).
| Group | BWGI–II | BWGI–III | BWGI–IV | BWGI–V |
|---|---|---|---|---|
| O(n=6) | 9.34±2.57 | 25.72±1.97 | 27.76±3.60 | 34.78±16.81 |
| OO(n=6) | 7.48±5.50 | 22.38±5.56 | 37.00±6.65 | 44.59±8.09 |
| PA5(n=6) | a,b13.30±1.65 | 27.51±7.01 | e43.30±9.98 | 52.44±12.82 |
| PA10(n=6) | 13.65±5.46 | c,d11.31±5.38 | 34.41±20.89 | 43.19±11.30 |
Legend: BWG – bodyweight gain; O – first control group who received water (1ml/kg BW p.o.); OO – second control group who received olive oil (1ml/kg BW p.o.); PA5 – first experimental group who received a lower dose of Pygeum africanum extract (50mg/kgBW/day p.o.); PA10 – second experimental group who received a higher dose of Pygeum africanum extract (100mg/kgBW/day p.o.).
BWG was increased in PA5 at all measuring intervals, achiving statitical significance compared to both controls on the second, and compared to only first control group on the fourth measurement. After an initial increase of BWG after one week of treatment, the higher dose of PAE significantlly decreased the BWG after two weeks in comparison to both controls.
The influence of PAE in two doses on LW/BW, KW/BW, PW/BW and TW/BW ratio is shown in Table 2.
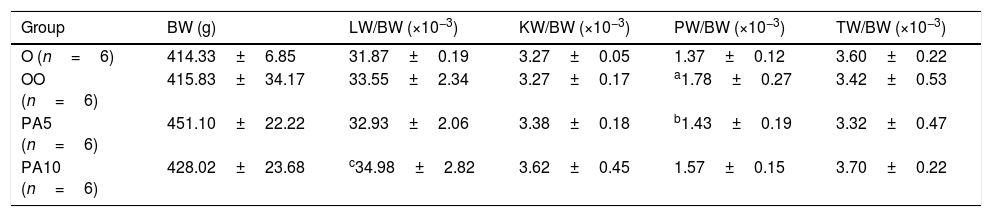
BW at the end of treatment, LW/BW, KW/BW, PW/BW and TW/BW ratio in rats that were treated with PAE (50 and 100mg/kgBW/day p.o.) compared with control groups (means±SD).
| Group | BW (g) | LW/BW (×10−3) | KW/BW (×10−3) | PW/BW (×10−3) | TW/BW (×10−3) |
|---|---|---|---|---|---|
| O (n=6) | 414.33±6.85 | 31.87±0.19 | 3.27±0.05 | 1.37±0.12 | 3.60±0.22 |
| OO (n=6) | 415.83±34.17 | 33.55±2.34 | 3.27±0.17 | a1.78±0.27 | 3.42±0.53 |
| PA5 (n=6) | 451.10±22.22 | 32.93±2.06 | 3.38±0.18 | b1.43±0.19 | 3.32±0.47 |
| PA10 (n=6) | 428.02±23.68 | c34.98±2.82 | 3.62±0.45 | 1.57±0.15 | 3.70±0.22 |
Legend: O – first control group who received water (1ml/kg BW p.o.); OO – second control group who received olive oil (1ml/kg BW p.o.); PA5 – first experimental group who received a lower dose of Pygeum africanum extract (50mg/kgBW/day p.o.); PA10 – second experimental group who received a higher dose of Pygeum africanum extract (100mg/kgBW/day p.o.); BW – bodyweight; LW – liver weight; KW – kidney weight; PW – prostate weight; TW – testis weight.
There have to be observed statistically significant increase of the PW/BW ratio in the group of animals who were treated with OO, in comparison to animals who received water only. One-month treatment with lower dose of PAE decreased the PW/BW ratio compared to group that received OO and higher dose of PAE significantly increased the LW/BW ratio compared with the control group O, but not with OO. KW/BW ratio was higher in PA5 and PA10 compared to both control groups after one-month treatment, but without statistically significant difference.
PAE in two doses also caused some alterations of biochemical parameters that were analyzed (Table 3).
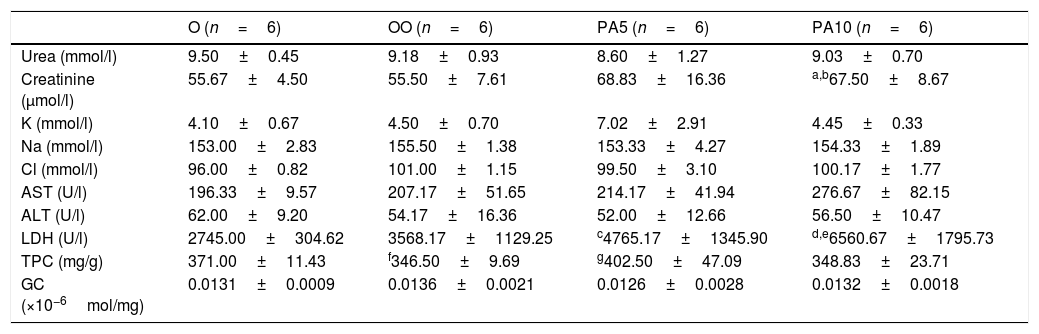
Urea, creatinine, potassium, sodium, chloride, AST, ALT, LDH, TPC and GC in Wistar rats who were treated with PAE (50 and 100mg/kgBW/day) for 30 days and control groups (means+SD).
| O (n=6) | OO (n=6) | PA5 (n=6) | PA10 (n=6) | |
|---|---|---|---|---|
| Urea (mmol/l) | 9.50±0.45 | 9.18±0.93 | 8.60±1.27 | 9.03±0.70 |
| Creatinine (μmol/l) | 55.67±4.50 | 55.50±7.61 | 68.83±16.36 | a,b67.50±8.67 |
| K (mmol/l) | 4.10±0.67 | 4.50±0.70 | 7.02±2.91 | 4.45±0.33 |
| Na (mmol/l) | 153.00±2.83 | 155.50±1.38 | 153.33±4.27 | 154.33±1.89 |
| Cl (mmol/l) | 96.00±0.82 | 101.00±1.15 | 99.50±3.10 | 100.17±1.77 |
| AST (U/l) | 196.33±9.57 | 207.17±51.65 | 214.17±41.94 | 276.67±82.15 |
| ALT (U/l) | 62.00±9.20 | 54.17±16.36 | 52.00±12.66 | 56.50±10.47 |
| LDH (U/l) | 2745.00±304.62 | 3568.17±1129.25 | c4765.17±1345.90 | d,e6560.67±1795.73 |
| TPC (mg/g) | 371.00±11.43 | f346.50±9.69 | g402.50±47.09 | 348.83±23.71 |
| GC (×10−6mol/mg) | 0.0131±0.0009 | 0.0136±0.0021 | 0.0126±0.0028 | 0.0132±0.0018 |
Legend: O – first control group who received water (1ml/kg BW p.o.); OO – second control group who received olive oil (1ml/kg BW p.o.); PA5 – first experimental group who received a lower dose of Pygeum africanum extract (50mg/kgBW/day p.o.); PA10 – second experimental group who received a higher dose of Pygeum africanum extract (100mg/kgBW/day p.o.); TPC – total protein content of the liver; GC – glutathione content in homogeneous suspension of hepatocytes.
Although average values of serum creatinine were elevated in both PA5 and PA10, only higher dose of PAE caused statistically significant increase compared to both control groups.
One-month treatment of Wistar rats with lower dose of PAE significantly increased the value of LDH in comparison to control O. Higher dose of PAE significantly increased the value of LDH compared to both O and OO.
When it comes to the TPC, it must be noted from Table 3 that one-month treatment of Wistar rats with OO significantly reduced TPC compared to water. PAE in five-fold higher daily dose significantly increased the TPC in the liver as compared to the group treated with OO.
As shown in Table 3, one-month treatment of experimental animals with PAE in both doses did not significantly affect the value of urea, electrolytes, transaminases and GC in hepatocyte suspension.
DiscussionThirty-day treatment of sexually mature male Wistar rats with PAE in five and ten times higher dose compared to human, experimental animals tolerated well and none died during the treatment. By daily observation of the animal's behavior, there was not remarked any change that could be attributed to the influence of applied extracts. If we take into account that the recommended human daily dose of PAE is 100mg (approximately 1.4mg/kgBW), it can be concluded a good tolerability of investigated preparation.
Experimental group that received a lower dose of extract (PA5) had a bigger increase in body weight in all the measurements, compared with controls. On the basis of these results it can be assumed the possible positive influence on food intake and appetite, but other mechanisms that cause BWG cannot be excluded.
PAE in higher applied dose significantly increased the LW/BW ratio in comparison to the first control group, but not to the group which received OO. Since the one-month administration of OO also increased the LW/BW ratio to some extent compared with the group that received water, the obtained results cannot be attributed only to the influence of PAE in applied dose.
Both PAE reduced the PW/BW ratio in relation to the control which received OO, but the difference with lower applied dose reached statistical significance. These results confirm the previous findings that PAE cannot be considered as an indifferent substance in terms of the influence on the structure and/or function of prostate. Whether it comes to the possible anti-androgenic effect of atraric acid that was found in some studies18 or altered cell kinetics and apoptosis as the discovery of others,19 or perhaps a combination of both mechanisms, it cannot be concluded on the basis of our results. It cannot be excluded the inhibitory effect of PAE on the proliferation of fibroblasts and myofibroblasts of the prostatic stroma, that was confirmed by the Boulbes et al.20
Regarding biochemical analyses, two results are remarkable: an increase of serum LDH and creatinine levels in both groups of animals that were receiving PAE. The deviation from the control groups is significantly more pronounced in animals that received higher dose of PAE. Five types of LDH isoenzymes were found in different human tissues such as heart, red blood cells, reticuloendothelial system, liver, striated muscle, kidneys, lungs, placenta and pancreas.21 Because it is released during tissue damage, elevated serum level of LDH in serum is a marker of common injuries and disease such as heart failure, anemia, and lung or liver disease.22 It also may be used as a tumor marker.23 In rats, LDH was found in high concentration in myocardium, kidney, liver and skeletal muscle.24 Without precise data of the type and level of different LDH isoenzyme, it can be assumed that PAE could cause structural/functional changes in all of these tissues. It is unlikely that PAE could exhibit a carcinogenic effect at any place after one month of application. Key argument that eliminates the liver as possible target site of PA toxicity in applied doses is the obtained value for urea, AST, ALT and GC which do not differ significantly in comparison to the values obtained in the control groups of animals. These results probably support the absence of oxidative damage and/or reduction of the functional capacity of the liver under the influence of applied PAE. Gathumbi et al.13 found a moderate increase in serum ALT in rats with dose of PAE that was ten times higher than ours (1000mg/kgBW). Elevated LDH levels in both experimental groups specify kidney, skeletal muscle and/or myocardium as potential target sites of PA toxicity in applied doses most likely. An additional argument that supports potential nephrotoxicity of PAE in applied doses is an elevated value of creatinine as the main indicator of the kidney filtration capacity in both experimental groups of animals. Creatinine is the major waste product of creatine metabolism in muscle. In the kidney, it is filtered by the glomerulus and actively excreted by the tubules.24 On the basis of applied methodology, it is not possible to estimate exactly the type and severity of tissue damage after one-month exposure to PAE in determined doses.
An interesting result is that the lower dose of PAE significantly increased the TPC as compared to the OO group, while this value did not differ in group PA10. Neither here it cannot be eliminated the effect of OO. Significant reduction of TPC as the result of one-month treatment with OO, could be explained as the consequence of the increased hepatic fat accumulation and fatty infiltration of the liver.25,26 In a similar experimental model Richter et al. found the existence of moderate, non-degenerative fatty infiltration of the liver which started in periportal spaces.27 These changes were attributed to the effects of monounsaturated oleic acid. Possible conclusion could be that the lower applied dose of PAE essentially acted as the protective factor against fatty infiltration of the liver caused by OO?! Ten times higher dose caused the same effect as the solvent. These results indicate the need for further investigation.
ConclusionsThe results of this study expanded the current state of knowledge regarding the toxicological profile of PAE, in particular of Italian brand Pigenil®. PAE in applied doses increased the value of serum creatinine and LDH in Wistar rats that could support possible nephrotoxicity, skeletal and/or cardiac muscle damage. Olive oil as the solvent was shown as the limiting factor in the precise interpretation of obtained results, because it demonstrated specific effects and probably influenced the toxicity of PAE to some extent. In order to accurately explain obtained results and make more precise conclusion regarding PA safety profile, it is necessary to conduct additional research on the basis of obtained findings.
Ethical statementAnimal care and all experimental procedures were carried out in compliance with the Animal Care Committee regulations of Medical School in Novi Sad, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals edited by Commission of Life Sciences, National Research Council (USA).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.