Diagnostic tests for vascular erectile dysfunction (ED) depend on cavernous smooth muscles (CSM) relaxation following an intracorporal injection (ICI). Enhanced sympathetic tone, which is not uncommon during performance of these tests, can bias its results. Also, CSM diseases can cause veno-occlusive diseases (VOD) ED. Corpus cavernosum electromyography (CC-EMG) potentials’ amplitudes represent the integrated sympathetic activity of healthy CSM. Stem-cells and gene-therapy are potential therapeutic options for impaired CSM.
ObjectiveTo utilize CC-EMG, as a new diagnostic technique that can confirm the integrity of CSM, and to identify patients with impaired CSM activity, among those diagnosed as vascular ED per Color Duplex Doppler Ultrasonography (CDDU).
Patient and methodsGroup 1 included 24 patients with ED and negative response to ICI. Group 2 included 10 men without ED. Patients included in group 1 had penile CDDU examination and all participants had spontaneous CC-EMG recordings.
ResultsAccording to CDDU parameters, group 1 was sub-grouped as nine arterial, ten VOD and five mixed type. CC-EMG potentials’ amplitudes ranged 223–320, 179–237, 103–250 and 83–200μV for group 2 and arterial, mixed and VOD subgroups respectively. The widest ranges of potentials’ amplitudes were recorded in the subgroups of patients with an element of VOD. Four patients with ED, within these subgroups, had CC-EMG potentials’ amplitudes ranged 200–250μV that exceeded/approached the lowest value recorded from men in group 2.
ConclusionCC-EMG recordings elicited marked differences of CSM activity among patients diagnosed with an element of VOD ED per CDDU. This finding highlighted the need to utilize CC-EMG to assess the integrity of CSM. Identifying patients with impaired CSM activity may modify the chosen methods for therapeutic interventions.
Las pruebas diagnósticas para la disfunción eréctil (DE) vascular dependen de la relajación de los músculos cavernosos lisos (MCL) después de una inyección intracorporal (IIC). El tono simpático mejorado, que es frecuente durante la realización de estas pruebas, puede sesgar los resultados. Además, las enfermedades de los MCL pueden provocar DE con enfermedades venooclusivas (EVO). Las amplitudes de los potenciales de la electromiografía de los cuerpos cavernosos (CC-EMG) representan la actividad simpática integrada de los MCL sanos. Las células madre y la terapia génica son opciones terapéuticas potenciales para los MCL con discapacidad.
ObjetivoUtilizar CC-EMG como una nueva técnica de diagnóstico que pueda confirmar la integridad de los MCL e identificar a los pacientes con actividad de MCL deteriorada entre los diagnosticados con DE vascular por ecografía Doppler color dúplex (CDDU, por sus siglas en inglés).
Paciente y métodosEl grupo 1 incluyó a 24 pacientes con DE con una respuesta negativa a la IIC. El grupo 2 incluía a 10 varones sin DE. En los pacientes incluidos en el grupo 1 se realizó CDDU en el pene y todos los participantes presentaron grabaciones de la CC-EMG espontáneas.
ResultadosDe acuerdo con los parámetros CDDU, el grupo 1 se dividió en subgrupos, como 9 arteriales, 10 VOD y 5 tipos mixtos. Las amplitudes de los potenciales de la CC-EMG variaron entre 223-320, 179-237, 103-250 y 83-200μV para el grupo 2 y los subgrupos arteriales, mixtos y VOD, respectivamente. Los amplios rangos de amplitudes de los potenciales se registraron en los subgrupos de pacientes con un elemento de VOD. Cuatro pacientes dentro de estos subgrupos tenían amplitudes de potencial de la CC-EMG de 200-250μV que excedían/acercaban el valor más bajo para varones en el grupo 2.
ConclusiónLas grabaciones de la CC-EMG provocaron marcadas diferencias de actividad de los MCL entre los pacientes diagnosticados con un elemento de VOD DE por CDDU. Este hallazgo destacó la necesidad de utilizar CC-EMG para evaluar la integridad de los MCL. La identificación de pacientes con actividad de CSM afectada puede modificar los métodos elegidos para las intervenciones terapéuticas.
Penile erection and flaccidity are two autonomic events related to the state of the cavernous smooth muscles (CSM).1 Penile erection is a neurovascular response that induces CSM relaxation accompanied with increased blood flow to the penis. The common organic causes of erectile dysfunction (ED) include vascular impairment, neurological factor(s) and/or endocrinal disease(s).2,3
The currently used diagnostic tests of vascular ED, namely intracorporal injection (ICI), Color Duplex Doppler Ultrasonography (CDDU), cavernostometry and caversonography, are dependent on a key mechanism of CSM relaxation following an ICI.4–6 Enhanced sympathetic tone, which is not uncommon during performance of these tests, can affect its accuracy.4–6 Also, CSM diseases can cause VOD ED but the integrity of CSM is not currently confirmed by the available diagnostic tests.
During periods of penile flaccidity, the penis is under a continuous spontaneous sympathetic activity that induces CSM contraction and the penile flaccidity. Electric activity of corpus cavernosum, corpus cavernosum electromyography (CC-EMG), was first recorded in 1989.7 CC-EMG potentials are recorded during periods of penile flaccidity but disappear on inducing erections.1,7,8 It was evidenced that these potentials represent the spontaneous sympathetic activity of the CSM.8 CC-EMG potentials used to be recorded though deep penetrating electrodes9,10 but nowadays using surface electrodes is the preferred method.8,11,12 The recorded potentials’ amplitude, using surface electrodes, represents the integrated activity of healthy CSM. In patients with ED, it was evidenced that CC-EMG potentials’ amplitude is not related to the patient's age.13
Testosterone plays an important role in the integrity and maintenance of the penile tunica, CSM, vascular endothelium and autonomic innervation to the penis.14,15 Testosterone is necessary for healthy erectile function and its deficiency may result in ED.14,16
Hypothesis: Men without ED have healthy CSM. CSM disease is one of the etiological factors that can cause veno-occlusive disease (VOD) ED. Current available diagnostic tests do not identify patients with impaired CSM activity. Spontaneous CC-EMG records the integrated electrical activity of CSMs; it can be applied to assess integrity of CSM.
This work aimed to apply CC-EMG, as a diagnostic test, to assess the integrity of CSM and to identify patients with impaired CSM activity among ED patients with a negative response to ICI.
Patients and methodsThis work was designed to study the recorded spontaneous cavernous electrical activity, in men with ED, using surface electrodes. The study included 2 groups: group 1 included patients presented with ED with a negative response to ICI and group 2 included men with normal erectile function.
Participants gave histories, undergone physical and genital examinations, provided blood samples, for total testosterone estimation, and completed the Arabic Translation of International Index of Erectile Function (IIEF-5) questionnaire.17 ICI and penile CDDU were done for participants in group 1. All participants had spontaneous CC-EMG recordings. The recorded CC-EMG from the group of men without ED (group 2) represented a base line spontaneous electrical activity of the healthy CSMs.
The study protocol was approved by 2 Board Committees, at Departments of Andrology and Neurophysiology, and the Ethics Committee, Faculty of Medicine, Cairo University. During the period from September 2014 to January 2016, men presented to the Andrology outpatient clinic at the University hospital and were willing to participate in the study, gave informed consents.
History taking included personal, sexual, medical and surgical histories. Examination included general, neurological and genital examinations. Genital examination focused on penile size, presence of any congenital anomalies as hypospadias and/or chordae and presence of penile fibrosis as Peyronie's disease.
Escalating doses of intracorporal injection (ICI) of prostaglandin E1 (PGE1); 5, 10 and 20μg were given to evaluate the ED.
CDDU machine with 10megahertz linear array transducer with a color flow mapping was used for CDDU. The corporal bodies were scanned to detect echogenicity. A peak systolic velocity (PSV)<30cm/s and end diastolic velocity (EDV)>5cm/s were considered as arterial disease and VOD respectively. In the presence of both finding, the patient was considered to have a mixed type. According to CDDU data, group 1 was sub-grouped into arterial, VOD and mixed subgroups.
Total testosterone was estimated using radio-immune assay at Chemical Pathology Department of the University Hospital from 8 to 10AM. The normal testosterone reference ranged 2.5–12ng/ml and 1.9–12ng/ml for participants less and more than 50 years old respectively.
Included participants in group 1 fulfilled the following criteria: inability to attain or maintain a penile erection sufficient for successful vaginal intercourse, IIEF-5 score less than 17, an ICI response of E0–E3, vascular ED and normal total testosterone level. Inclusion criteria for group 2 were: married persons with normal erectile function, normal coital activity (IIEF-5 scores range from 22 points to 25 points) and normal total testosterone without any associated diseases. Exclusion criteria were: presence of neurological or psychotic disease(s), intake of medication(s) affecting the central nervous system, presence of penile lesion(s) on genital examination and none homogenous corpora cavenosa on CDDU.
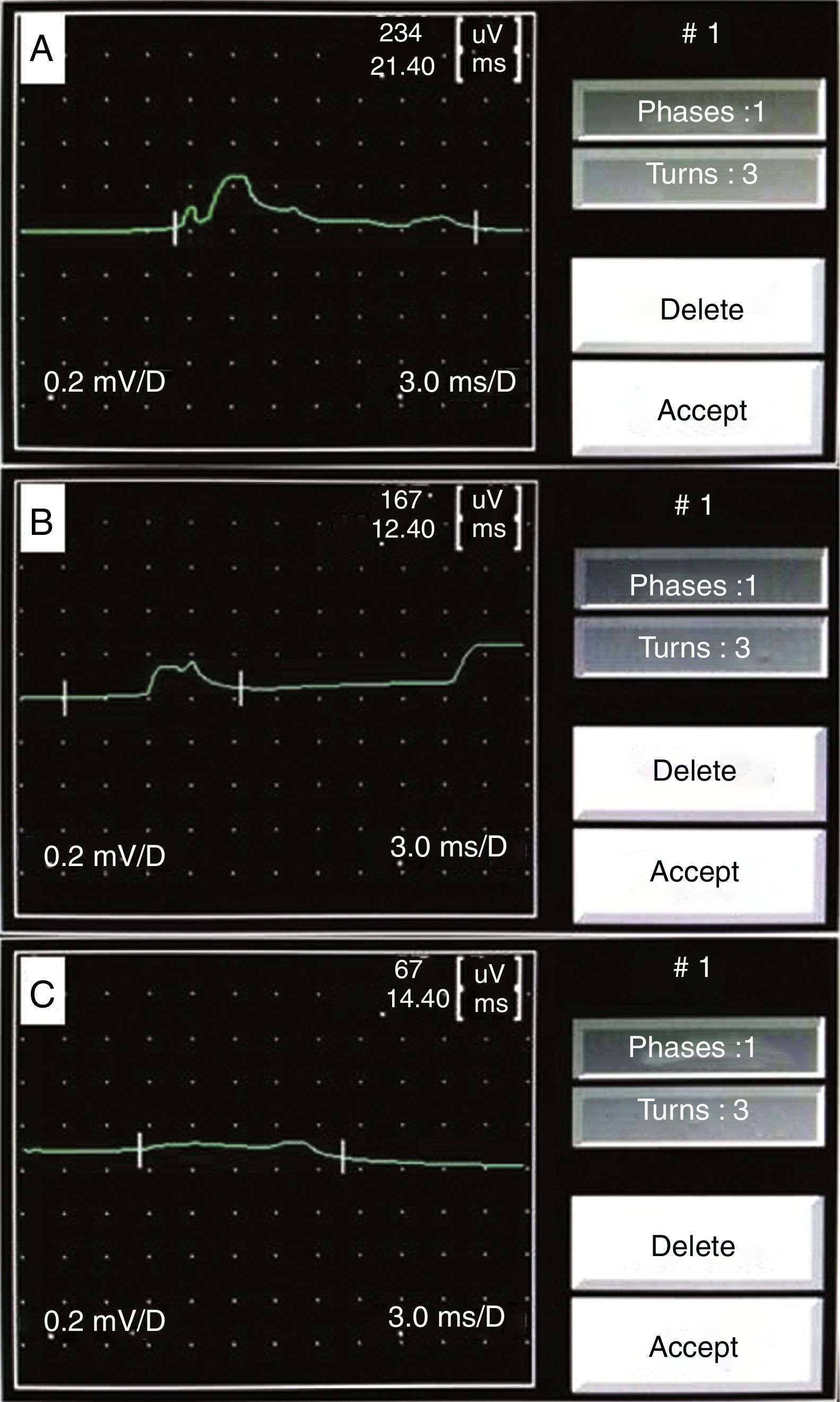
Dantec Keypoint system, with mono-channel entry and two bipolar electrodes and one grounding electrode, connected to a notebook computer with quantitative software was used to record spontaneous CC-EMG potentials. Recording was done in the supine position, ambient temperature, and quiet and well lit examination room. Participants were informed of the relative noninvasiveness of the test. The penis was uncovered to allow the examiner to observe subtle changes in penile volume. Two Beckman's silver-silver chloride surface electrodes were placed on the right lateral aspect of the penile shaft, about 2cm apart from each other (bipolar electrodes recording), and a grounding electrode was placed on the right kneecap. After electrode application, a 20-min period was allowed before the start of CC-EMG recording to diminish the patient's anxiety. Subjects were asked to relax but not to sleep during the recording. Band pass filters of 0.1Hz as a low-frequency filter and leaving the high-frequency filter open were used to process the recorded signals. While the penis was in the flaccid state, recordings of spontaneous CC-EMG were performed for 30min (Fig. 1A–C). The potential's amplitude for each participant represented the mean of all his recorded potentials’ amplitudes, on the CC-EMG, during the 30min period test.
Data were reviewed and analyzed using Statistical Package for the Social Science version 21 (SPSS Inc., Chicago, IL, USA). Numerical data are described in terms of means and standard deviations (SD) and categorical data are presented as numbers and percentages (%). Comparative study of categorical data used Chi square test. Statistical differences among groups were tested using independent Student T test for 2 groups and one way Analysis Of Variance test (ANOVA) for more than 2 groups followed by post hoc comparison with Tukey HSD test. Pearson's correlation was used to test the strength and direction of the correlation between variables. Regression analysis was done to find the main predictors for CC-EMG amplitude and frequency. P value ≤0.05 was set as significant.
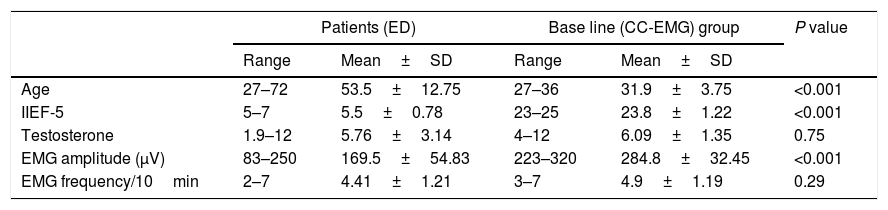
ResultsThis study included 34 participants: 24 patients complained of ED (group 1) and 10 men with normal erectile function (group 2). Twenty (58%) participants were smokers, 15 (62.5%) in group 1 and 5 (50%) in group 2. In group one, 11 (45.8%) were diabetics, 4 (16.6%) were hypertensive and 2 (8.3%) had hyperlipidemia. The mean CC-EMG potentials’ amplitudes were 169.5±54.83 and 284.8±32.45 for groups 1 and 2 respectively with significant difference (P<0.001). The mean CC-EMG potentials’ frequencies were 4.41±1.21 and 4.9±1.19 for groups 1 and 2 respectively with insignificant difference (P=0.29). Comparisons between included groups regarding age, IIEF-5, testosterone level and EMG amplitudes and frequencies are presented in Table 1.
Comparisons between patients with erectile dysfunction (ED) and base line (CC-EMG) group regarding age, IIEF-5, testosterone level and EMG amplitude and frequency.
| Patients (ED) | Base line (CC-EMG) group | P value | |||
|---|---|---|---|---|---|
| Range | Mean±SD | Range | Mean±SD | ||
| Age | 27–72 | 53.5±12.75 | 27–36 | 31.9±3.75 | <0.001 |
| IIEF-5 | 5–7 | 5.5±0.78 | 23–25 | 23.8±1.22 | <0.001 |
| Testosterone | 1.9–12 | 5.76±3.14 | 4–12 | 6.09±1.35 | 0.75 |
| EMG amplitude (μV) | 83–250 | 169.5±54.83 | 223–320 | 284.8±32.45 | <0.001 |
| EMG frequency/10min | 2–7 | 4.41±1.21 | 3–7 | 4.9±1.19 | 0.29 |
Eighteen (75%) of group 1 had E3 response to ICI but 6 (25%) had E2 response. The mean diameter of right cavernosal arteries, before and after ICI, was 0.55±0.08mm and 0.95±0.16mm respectively. The mean diameter of left cavernosal arteries, before and after ICI, was 0.55±0.09mm and 0.97±0.17mm respectively. PSV in right and left cavernosal arteries ranged 11.8–53.6cm/s and 13–50.1cm/s with a mean of 29.7±12.1cm/s and 28.2±10.5cm/s respectively. EDV in right and left cavernosal arteries ranged 0–12.7cm/s and 0–12.9cm/s with a mean of 5.4±3.6cm/s and 5.5±3.6cm/s respectively. According to CDDU parameters, group 1 was sub-grouped as nine arterial, ten VOD and five mixed type.
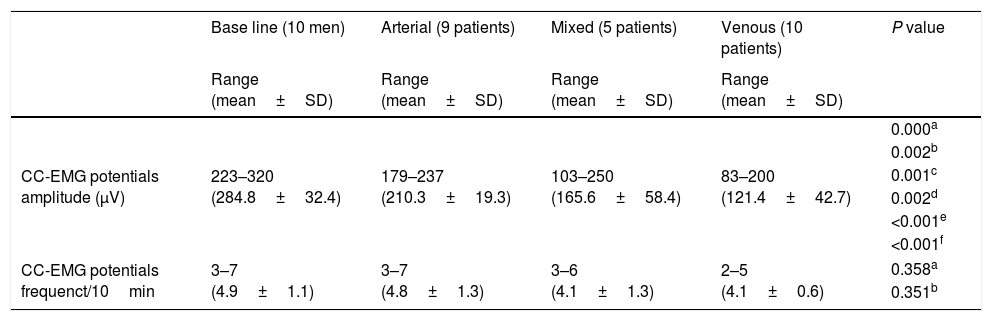
CC-EMG potentials’ amplitudes (Fig. 1) ranged 223–320, 179–237, 103–250 and 83–200μV with a mean 284.8±32.4, 210.3±19.3, 165.6±58.4 and 121.4±42.7μV for group 2 and arterial, mixed and VOD subgroups respectively with significant differences (Table 2). Means of the potential frequencies were statistically insignificant between group 2 and the subgroups (Table 2). Comparisons of the base line group and ED subgroups, regarding CC-EMG amplitudes and frequencies, are presented in Table 2. Three cases in the mixed subgroup had CC-EMG potentials’ amplitudes of 250 and 230 and 202μV and one case in the VOD subgroup had CC-EMG potentials’ amplitude of 200μV.
Comparisons between the base line group and subgroups of patients with erectile dysfunction (ED) regarding CC-EMG amplitude and frequency.
| Base line (10 men) | Arterial (9 patients) | Mixed (5 patients) | Venous (10 patients) | P value | |
|---|---|---|---|---|---|
| Range (mean±SD) | Range (mean±SD) | Range (mean±SD) | Range (mean±SD) | ||
| CC-EMG potentials amplitude (μV) | 223–320 (284.8±32.4) | 179–237 (210.3±19.3) | 103–250 (165.6±58.4) | 83–200 (121.4±42.7) | 0.000a |
| 0.002b | |||||
| 0.001c | |||||
| 0.002d | |||||
| <0.001e | |||||
| <0.001f | |||||
| CC-EMG potentials frequenct/10min | 3–7 (4.9±1.1) | 3–7 (4.8±1.3) | 3–6 (4.1±1.3) | 2–5 (4.1±0.6) | 0.358a |
| 0.351b | |||||
CC-EMG amplitude was significantly positively correlated to IIEF-5 (r=0.74, P=<0.001) but was not correlated to ED duration (P=0.38). Neither IIEF-5 nor ED duration was correlated to CC-EMG frequency (P=0.20 and 0.56 respectively).
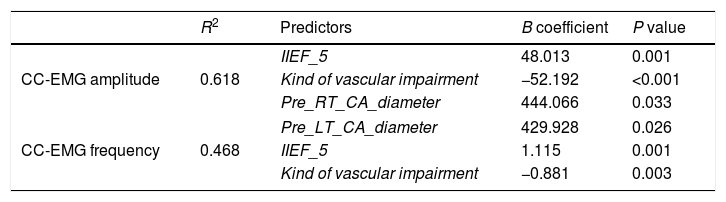
Linear regression analysis clarified that the main predictors for CC-EMG amplitude were IIEF-5, kind of vascular impairment and pre right and left cavernosal arteries diameters (Table 3). These predictors represent 61.8% of the predictors (R2=0.618). Whereas the main predictors of EMG frequency were IIEF-5 and kind of vascular impairment that represented 46% of the predictors (R2=0.468). Table 3 presents multiple regression models for CC-EMG amplitude and frequency.
Linear regression analysis: predictors of CC-EMG amplitude and frequency.
| R2 | Predictors | B coefficient | P value | |
|---|---|---|---|---|
| CC-EMG amplitude | 0.618 | IIEF_5 | 48.013 | 0.001 |
| Kind of vascular impairment | −52.192 | <0.001 | ||
| Pre_RT_CA_diameter | 444.066 | 0.033 | ||
| CC-EMG frequency | 0.468 | Pre_LT_CA_diameter | 429.928 | 0.026 |
| IIEF_5 | 1.115 | 0.001 | ||
| Kind of vascular impairment | −0.881 | 0.003 | ||
In the present study the means of potentials’ amplitudes (and frequencies), recorded from potent men, were comparable to that reported in previous studies used surface electrodes.8,12 These reproducible results pointed to the reliability of CC-EMG as a diagnostic test that can confirm the integrity of CSM. Although in previous studies,9,10 that used coaxial needle electrodes; they reported much higher potentials’ amplitudes (more than 500μv), these differences can be attributed to the different techniques used to record the potentials.
CC-EMG potentials’ amplitude decreases with primary and/or secondary degenerative changes affecting CSM. The secondary degenerative type may follow a penile vascular pathology.
In the present study the mean potentials’ amplitudes were significantly the highest in the group of potent men; but the mean potentials’ amplitudes were significantly lowest in the subgroups with an element of VOD per CDDU; they had the lowest integrated CSMs’ electrical activity. This finding agreed with previous studies; a decreased smooth muscle fibers content and impaired smooth muscle contractility and nerve evoked relaxation were recorded in corpora cavernosa of cases diagnosed as VOD ED.18–20 In the present work, the means of the potentials’ frequencies among included groups and subgroups had insignificant differences; because the frequency represents the numbers of neurological impulses conveyed to the penis and it is not related to the integrated CSM activity.
In the present study, the most impressive finding was the wide range of potentials’ amplitudes recorded in the subgroups with an element of VOD. Furthermore, in the same subgroups, only four of the participants had potentials’ amplitudes that approached/exceeded the lowest normal value recorded from men without ED. These findings elicited the marked differences, of CSM integrity, among patients who had the same diagnosis per CDDU.
The currently available diagnostic tests, for vascular ED, neither diagnose patients with enhanced sympathetic tone nor identify those with impaired CSM integrity. It is interesting to discuss results of previous studies that may help to clarify this matter. In a previous study,21 the CC-EMG recorded increased electrical activity of CSM, following papaverine ICC, in 14 (31%) out of 45 patients diagnosed as VOD ED. In these patients a poor response to the ICI and greater venous leakage were observed. In our opinion, this increased electrical activity most likely represents enhanced sympathetic tone that impaired CSM relaxation. In another previous study, the group of ED patients with significantly lowest PSV, on CDDU, responded to ICI by insufficient erections associated with reduction/suppression of CC-EMG potentials’ amplitudes.22 Authors reported that this association increased the specificity of CDDU to 92.9% as a diagnostic tool for arterial ED.22 Our explanation of these findings is that the mentioned association excluded patients with impaired CSM and/or enhanced sympathetic activity and this increased the test specificity. We mean that reduction/suppression of CC-EMG potentials’ amplitudes, following an ICI, is a clue for responsive CSM with absence of enhanced sympathetic tone.
In the present work the main predictors of CC-EMG potentials’ amplitude were IIEF5, kind of vascular impairment and pre right and left cavernosal arteries diameters. All of these variables are dependent on the integrity of CSMs and vascular smooth muscles. Furthermore, a significant positive correlation of potentials’ amplitudes to IIEF-5 scores was found. It is expected that men without ED had healthy CSMs with higher integrated electrical activity that can explain these findings.
It is essential to confirm CSM integrity before commencing therapy. CC-EMG has the potential to identify cases with CSM diseases to avoid unnecessary surgeries. Moreover, CC-EMG may be useful in determining, in a non-invasive fashion, ED patients who are likely to fail first and second lines medical therapies. In the future, stem cells and gene therapy might have roles as therapeutic options for ED due to impaired CSM activity.23–27 CC-EMG would be valuable when these therapeutic options would be available.
ConclusionCC-EMG recordings elicited marked differences of CSM activity among patients diagnosed with an element of VOD ED per CDDU. This finding highlighted the need to utilize CC-EMG to assess the integrity of CSM. Identifying patients with impaired CSM activity may modify the chosen methods for therapeutic interventions.
Limitations of the studyFirst limitation: it is a small sample size. Second limitation: although, the recorded CC-EMG potentials’ amplitude is not related to the patient's age in men with ED,13 the mismatch of age between the base line group and ED group is considered a limitation of the study. Third limitation: the baseline CC-EMG potentials’ amplitude, below which a patient is considered having an impaired CSM activity, remains to be defined by further studies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interest.
This research is not funded.