We performed a long-term evaluation of testicular conservative surgical treatment of benign conditions.
Material and methodsBetween January 2001 and January 2005, a single center perspective clinical study was performed at our Academic Department of Urology. Case files of all patients diagnosed with small testicular mass (less than 1.5cm) and treated with conservative surgery were examined. Patients underwent physical examination, hormone and tumor marker assays, scrotal and abdominal ultrasound, chest X-ray and endocrinological examination. Should a benign disease or a selected malignant condition (Leydig cell tumor) be diagnosed during the frozen section analysis, testicular sparing surgery was performed. Each patient presenting a malignant condition underwent a strict oncological follow up according to the EAU Guidelines.
ResultsFrom January 2001 to January 2005, 80 patients with small testicular mass underwent conservative surgery. Patient mean age was 40.9 years. Mean follow up was 95.78 months. Patients presented either with a palpable testicular nodule (77.5%) or a nodule diagnosed by ultrasound (22.5%). Diagnosis after frozen section examination was Leydig cell tumor in 20 of 80 cases. Mean histological size of the nodule was 0.93cm. Tumor markers were normal before and after surgery. Follow up was conducted for all malignant patients following EAU Guidelines. No local recurrence or metastasis were observed. 100% of patients are still alive.
ConclusionsTesticular Sparing Surgery is feasible in all benign cases. Leydig cell tumors present a favorable long-term follow up when diagnosed early. Conservative surgery proved to be the safer choice.
Llevamos a cabo una evaluación a largo plazo del tratamiento quirúrgico de conservación testicular en situaciones benignas.
Material y métodosEntre enero de 2001 y Enero de 2005, realizamos un estudio clínico perspectivo de un único centro en nuestro Servicio Académico de Urología. Se examinaron las historias clínicas de todos los pacientes diagnosticados de masa testicular pequeña (menor de 1,5cm) tratados con cirugía conservadora. Los pacientes fueron sometidos a exploración física, valoraciones de marcadores hormonales y tumorales, ultrasonidos escrotales y abdominales, radiografías pectorales y evaluación endocrinológica. En los casos de diagnóstico de enfermedad benigna o de situación maligna seleccionada (tumor de células de Leydig) durante el análisis de secciones, se llevó a cabo una cirugía conservadora. Los pacientes que presentaron una situación maligna fueron sometidos a un estricto seguimiento oncológico, conforme a las Pautas de la EAU.
ResultadosDe enero de 2001 a enero de 2005, 80 pacientes con masa testicular pequeña fueron sometidos a cirugía conservadora. La edad media de los pacientes fue de 40,9 años. El seguimiento medio fue de 95,78 meses. Los pacientes presentaron, bien un nódulo testicular palpable (77,5%), bien un nódulo diagnosticado mediante ultrasonidos (22,5%). El diagnóstico tras el examen de secciones congeladas fue de tumor de células de Leydig en 20 de los 80 casos. El tamaño histológico medio del nódulo fue de 0,93cm. Los marcadores tumorales fueron normales antes y después de la cirugía. Se realizó un seguimiento de todos los pacientes con malignidad de acuerdo con las Pautas de la EAU. No se observaron recidivas locales ni metástasis. El 100% de los pacientes sigue con vida.
ConclusionesLa cirugía de conservación testicular es factible en todos los casos benignos. Los tumores de células de Leydig presentan un seguimiento favorable a largo plazo cuando se diagnostican tempranamente. La cirugía conservadora ha demostrado ser la elección más segura.
Testicular sparing surgery (TSS), first described by Richie et al., is becoming more accepted in treating benign disease and malignant ones in selected cases.1 A good reported oncological control and a minimal functional, physical and psychological morbidity have made the procedure more used and known. TSS has also become more commonplace when lesion are suspected of being benign due to the increase in small non palpable lesions detected by ultrasound.2 Nevertheless data in Literature are small and lack in long term follow mainly about malignant conditions also because the natural history is not comprehensively understood and the case series available appear broadly conflicting. Most report a benign prognosis3 but some describe a risk of metastasis in excess of 10%.4,5 Given this TSS has been completely accepted or benign conditions thus standard care or malignant testicular conditions is still radical orchidectomy. Despite this shift, we are mindful of the fact that TSS is highly dependent on the availability of local expertise in intraoperative frozen section examination6 an concern remains about the long-term outcomes or malignant testicular diseases.7 The aim of this study is to understand the role of TSS on diseases of the testis.
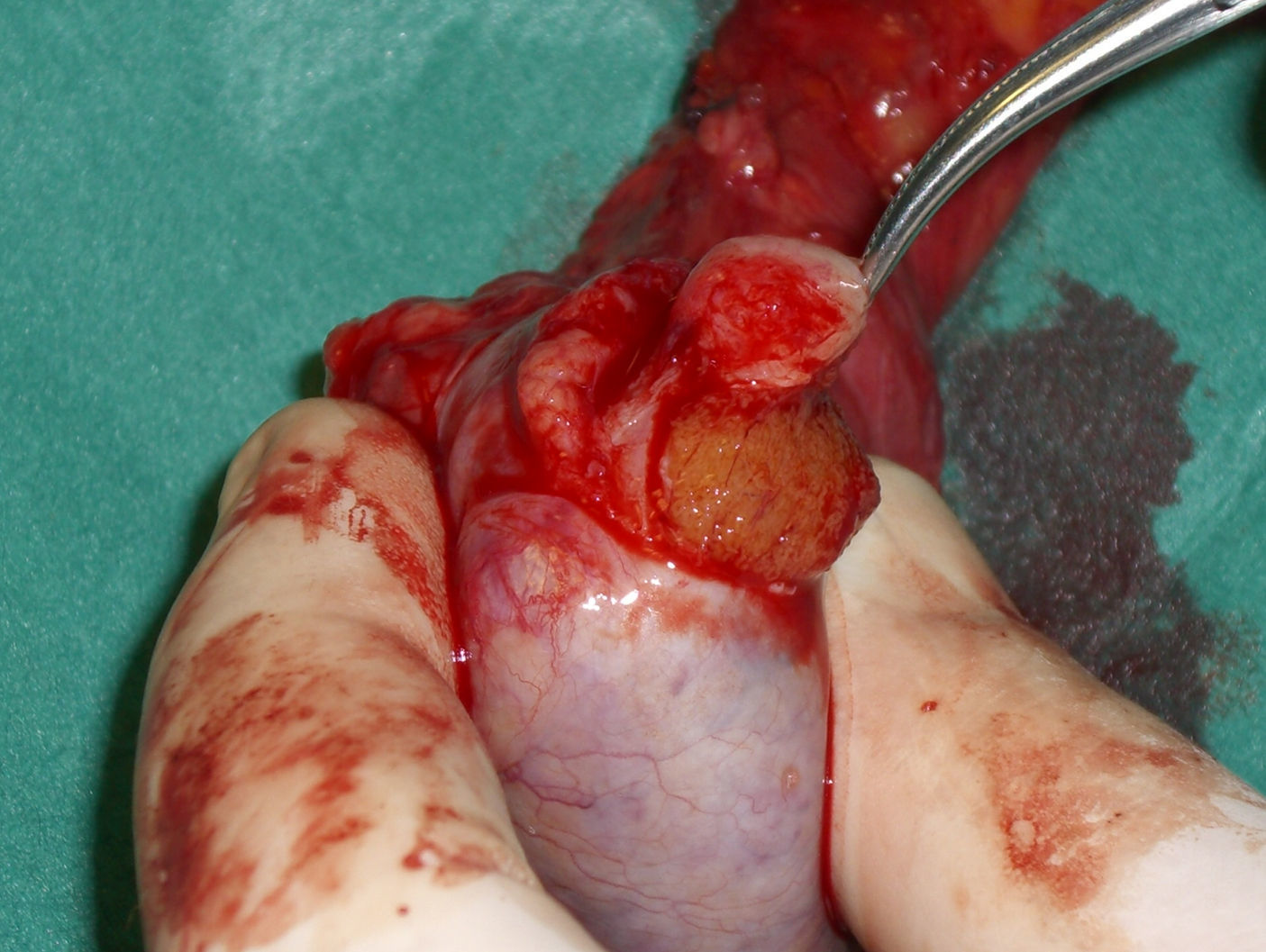
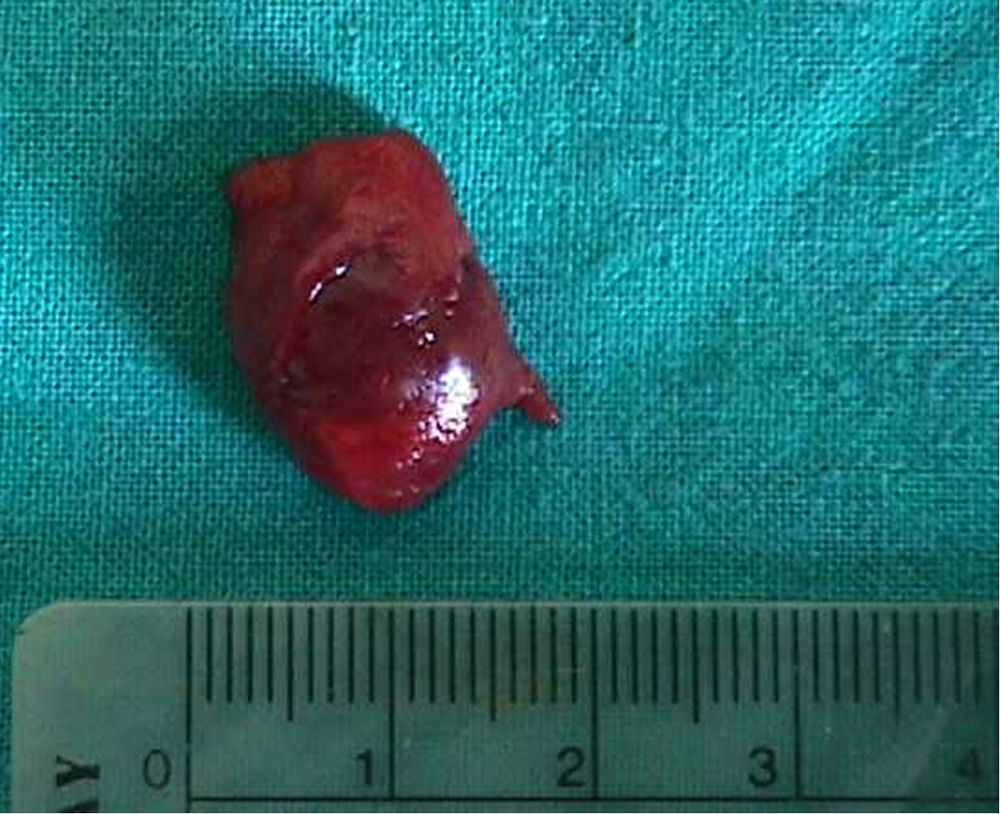
Materials and methodsBetween January 2001 and January 2005, a single center perspective clinical study was performed at our Academic Department of Urology, maintaining all the data with the institutional ethics board approval. Case files of all patients diagnosed with small testicular mass (less than 1.5cm) and treated with conservative surgery were examined. Patients with previous testicular surgery (for a malignant or a benign disease), inflammatory disease of the testis and malignant condition on the controlateral testis were excluded from this study. Preoperative assessment comprised physical examination, urinalysis, routine blood screen including alpha-fetoprotein (AFP), LDH and human chorionic gonadotropin (hCG), and abdominal and scrotal ultrasonography (US). Abdominal computed tomography (CT) and either chest X-ray or thoracic CT screening were performed before or after surgery. The surgical approach was identical in all cases with an inguinal incision, strictly adhering to standard oncologic principles in the treatment of testis tumors. The suspicious lesion was identified by palpation or intraoperative US with a linear 7.5–10ÝMHz probe (ESAOTE MyLab 25). Once the lesion was identified it was completely excised (Fig. 1) and sent for histopathological frozen section examination (FSE) (Fig. 2). Should a benign disease or a selected malignant condition like Leydig cell tumor be diagnosed during the frozen section analysis, TSS was performed. Each patient presenting a malignant condition underwent a strict oncological follow up according to the EAU Guidelines. We analyzed the outcomes in term of testosterone production, recurrence, evidence of metastasis, and disease free survival.
From January 2001 to January 2005, 80 patients with testicular mass smaller than 15mm underwent conservative surgery of the testis after FSE. Patient mean age was 40.9 years (range 20–72). Patients presented either with a palpable testicular nodule (62 patients, 77.5%) or a nodule diagnosed by ultrasound (18 patients, 22.5%). Fifteen lesions were hypoechogenic, 2 were hyperechogenic and one was heterogeneous. There was no evidence of testicular microlithiasis. One patient was monorchid after controlateral orchidectomy for inguinal hernia repair and performed a Testicular Sparing Surgery for immature Teratoma and followed strict oncological follow up. Diagnosis after frozen section examination was Leydig cell tumor (LCT) in 20 of 80 cases (25%); this diagnosis was confirmed at the histopathological definitive examination. 27 patients had a testicular fibrosis, 7 testicular infarction, 2 tuberculosis, 8 adenomatoid tumor and 15 had various conditions such as granulomatosis, spermatocele, mesotelial hyperplasia and nodular orchiditis. No changes in diagnosis were observed comparing the FSE to the definitive report. Mean histological size of the nodule was 0.93cm (range 0.06–1.5cm). Preoperative FSH and LH levels were high in 17 patients. Tumor markers were normal before and after surgery. Testosterone production was normal before and after surgery (range 480–972ng/dl before; 387 to 888ng/dl after 30 days) p<0.005. No postoperative hematomas occurred that required drainage or hospitalization and no infection, or other perioperative or late complication was recorded. Follow up was conducted for all malignant patients following EAU Guidelines with physical examination, tumor markers, scrotal and abdominal ultrasound, chest X-ray. Mean follow up was 95.78 months (range 77–120). No local recurrence or metastasis were observed in patient with previous malignant conditions. 100% of patients are still alive with a 100% free disease survival.
DiscussionTSS represents another branch of minimally invasive management.8 In this case morbidity, functionality, cosmetic and psychological results are decreased. Many small testicular masses discovered today are mainly benign and the malignant ones could be treated with this approach.3 In benign cases radical treatment usually represents overtreatment, contributing to the uptake of TSS. However, concerns remain regarding the risk of compromising disease specific survival and the success of organ sparing to avoid the side effects that prompted the initial effort to preserve apparently normal testicular tissue.9 The most common approach to organ preserving surgery has been TSS but high intensity focused ultrasound, radiation therapy and chemotherapy have been reported. Current European Germ Cell Cancer Consensus group guidelines state the situations in which an organ sparing approach should be considered, including synchronous bilateral testis tumors and a metachronous controlateral (second) testis tumor in a solitary testis. There should be sufficient endocrine function and TSS should be done only at experienced centers.10 Nevertheless data on LCT did not report a long-term follow up. Although LCT is rare among testicular lesions, it is the most common stromal neoplasm of the testis, typically occurring between 30 and 60 years of age. Although these tumors ultimately take a malignant course in 10% of patients, it can be challenging to distinguish between benign and malignant LCT, with metastasis being the only reliable criterion of malignancy. Because of the small number of retrospective case series the current knowledge of the oncologic outcome of patients undergoing Radical Orchidectomy (RO) or TSS for LCT is limited. Because TSS has been reported to be a safe concept, even in select patients with malignant germ cell tumors, it is reasonable to consider its application in a tumor entity that proves to be benign in a majority of cases. Our series shows that TSS is feasible and safe, and provides acceptable cancer control. A significant number of benign lesions were removed, sparing unnecessary morbidity in these patients. No patients had post operative complications nor Testosterone deprivation. This can underline how TSS could play an important role in patient who still want to maintain a good reproductive and sexual functionality. An organ sparing surgical approach is only recommend at our center in select cases and we believe that it should only be performed at centers where there is experience with managing testicular malignant diseases. In these cases radical orchidectomy remains the gold standard and should be discussed as part of informed consent. It is mandatory to highlight the risks of local recurrence and treatment as well as the need for androgen supplementation and fertility risks.11 We routinely measure testosterone preoperatively. In men with a healthy normal controlateral testis we believe that TSS is contraindicated when malignancy is evidenced at the FSE and in case of LCT is greater than 15mm. In these men one should only attempt TSS when it is almost certain that the lesion is benign, i.e. no risk factors for Germ cell Tumor (GCT), small lesion size, negative markers, no history of trauma. Presenting lesion size is important since those greater than 1.5cm are extremely suspicious for malignancy and preserving sufficient parenchyma becomes difficult. Most lesions in our series disclosed a hypoechogenic pattern on US, as had been described in published articles previously. Although a low level of estradiol, a high level of testosterone, gynecomastia, and a hypoechogenic tumor seen in US may be suggestive of an LCT, the management of choice is surgical exploration using an inguinal approach and FSE, especially because usually neither tumor markers (AFP, -hCG) nor hormone status are available at the time of decision-making. Moreover, 5% of germ cell tumors do have concurrent gynecomastia also. Radiologic imaging is mandatory to rule out retroperitoneal or pulmonary metastases. In our practice, staging is identical to that of testis cancer, comprising thoracic and abdominal CT. A reliable FSE is an essential prerequisite of TSS. Several studies reported the sensitivity of FSE to be 81% for benign and 100% for malignant lesions; Connolly et al. calculated a 94.2% positive predictive value of FSE and a 92.6% negative predictive value for malignancy in 80 patients.12 Carmignani et al. reported a sensitivity of 85% for FSE,2 now passed by our results (100%) mainly based on the gained skill by our pathologist more and more involved in these cases. Although it may be difficult to determine the malignant potential of an LCT, the parameters suggested by Kim et al. were widely used.13 Features of malignancy of the primary tumor are marked cellular polymorphism, increased mitotic activity, coagulative tumor necrosis, invasion of lymphatic or blood vessels, extension of the tumor to the spermatic cord, invasion of the capsule, and absence of Reinke's crystals. Within a median follow-up of almost 8 years, no progression (metastasis, local recurrence) was noted. Our observations are in line with most published series of TSS for LCT, in which neither local recurrences nor distant metastasis was described, even if enucleation was performed in young boys for prepubertal LCT or in bilateral LCT of the testis. One series describes a local recurrence of an LCT months after TSS, despite negative surgical margins. Because progressive disease is rarely diagnosed by hormone production and its clinical symptoms, radiographic imaging seems to be the only method to detect metastasis. The extent and diagnostic modalities of follow-up are a matter of controversy. In this study we wanted to highlight the importance of a long follow up period because of the possibility of late-onset metastasis. To date, there is no consensus regarding the treatment of metastatic LCT. Because radiation therapy and various cytotoxic regimens proved to be ineffective in progressive disease, surgical removal seems to be the only chance in treating this tumor.
ConclusionsTo our knowledge this is the larger single center data on TSS including LCT conditions. Testicular Sparing Surgery is feasible in all benign cases. Leydig cell tumors present a favorable long-term follow up when diagnosed early. Conservative surgery proved to be the safer choice in these selected cases, preventing from the risk of iatrogenic hypogonadism. Our data suggest that Leydig cell tumor can be safely regarded as benign and TSS do not alterate gonadal function.
Ethical responsibilitiesRight to privacy and informed consentThe authors state that in this article are not patient data.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Protection of people and animalsThe authors declare that this research experiments have not been done in humans or animals.
Conflict of interestThe authors declare no conflict of interest.
This study was presented at the SIU (Societe Internationale d’Urologie) International meeting held in Berlin from the 16th to 20th of October 2011, and won one of the Awards on poster presentations.