The children and adolescents with cancer who are getting remission and becoming long-term survivals are at high risk of impaired fertility. Cyclophosphamide (CP), the most frequently used drug for childhood-cancers causes various types of reproductive toxicity. We aimed at evaluating protective role of chlorophytum borivillianum (CB) extract against pre-pubertal CP exposure-induced testicular toxicity in rats.
Materials and methodsSixty male pre-pubertal SD rats aged postnatal day (PND) 24 were divided into 5 groups. Group-I (control), group-II (CP), and group-III (CB) received normal saline (NS), CP15mg/kg/day and CB200mg/kg/day respectively during PND29-42; group-IV and group-V received CB100mg/kg/day and CB200mg/kg/day respectively along with CP15mg/kg/day for the same period. Half of the rats from each group were sacrificed on PND43 (puberty) to evaluate alterations in oxidative stress parameters and histopathology. Remaining rats were sacrificed on PND63 (young adult age) and sperm analysis (density, motility, viability, and morphology), hormonal (Testosterone, Luteinizing hormone, Follicle stimulating hormone) estimation and histomorphometrical evaluation was done. Co-administration of CB have shown a dose-dependent and significant improvement in anomalies caused by CP as compared to rats received CP only.
ResultsCP treatment led to significant decrease in body weight gain, organ weights, oxidative defense mechanisms, hormone levels, steroidogenesis, spermatogenesis, sperm parameters and increase in oxidative stress, percentage of sperm abnormal morphology as compared to control rats. CP-treated rats have shown severe damage in testicular architecture and development as compared to control rats as evidenced by histopathology and morphometric analysis.
ConclusionCo-administration of CB extract significantly reversed the footprints of these effects in dose-dependent manner. These protective effects of CB may be exploited in improving gonadal function in childhood cancer long-term survivals.
los niños y adolescentes con cáncer que están en remisión y se convierten en supervivientes a largo plazo tienen un alto riesgo de experimentar un deterioro de la fertilidad. La ciclofosfamida (CP), el fármaco más utilizado para los cánceres infantiles, causa varios tipos de toxicidad reproductiva. El objetivo fue evaluar el papel protector del extracto de chlorophytum borivillianum (CB) contra la toxicidad testicular inducida por la exposición prepuberal a la CP en ratas.
Materiales y métodosSesenta ratas SD prepuberales machos de edad posnatal (PND) 24 se dividieron en 5 grupos. El grupo I (control), el grupo II (CP) y el grupo III (CB) recibieron solución salina normal (NS), CP15mg/kg/día y CB200mg/kg/día respectivamente durante PND29-42; el grupo-IV y el grupo-V recibieron CB100mg/kg/día y CB200mg/kg/día respectivamente junto con CP15mg/kg/día durante el mismo período. La mitad de las ratas de cada grupo fueron sacrificadas en PND43 (pubertad) para evaluar las alteraciones en los parámetros de estrés oxidativo y la histopatología. Las ratas restantes se sacrificaron en PND63 (edad adulta joven) y se realizó un análisis de esperma (densidad, motilidad, viabilidad y morfología), estimación hormonal (testosterona, hormona luteinizante, hormona foliculoestimulante) y evaluación histomorfométrica.
Resultadosel tratamiento con PC condujo a una disminución significativa del aumento de peso corporal, el peso de los órganos, los mecanismos de defensa oxidativa, los niveles hormonales, la esteroidogénesis, la espermatogénesis, los parámetros espermáticos y el aumento del estrés oxidativo, y el porcentaje de morfología anormal de los espermatozoides en comparación con las ratas control. Las ratas tratadas con CP han mostrado un daño severo en la arquitectura y el desarrollo testicular en comparación con las ratas de control, como lo demuestra la histopatología y el análisis morfométrico.
Conclusiónla administración conjunta de extracto de CB revirtió significativamente las huellas de estos efectos de manera dependiente de la dosis. Estos efectos protectores del CB pueden explotarse para mejorar la función gonadal en las supervivencias a largo plazo del cáncer infantil.
Cancer is considered the second most leading cause of deaths worldwide and a major cause of non-accidental mortality in children and adolescents. However, due to the introduction of new chemotherapeutic drugs, furtherance in treatment regimens and supportive care, the number of cancer survivors has been increased drastically in recent decades and is expected to improve further in imminent future.1–4 Interestingly, around 80% of children and adolescent receiving cancer treatment are getting remission and becoming long-term survivals.5,6 Despite of immense therapeutic benefits, the cancer therapy cause various adverse effects as they curtail the division of rapidly proliferating non-cancerous cells which affects structural and functional development in this population. It is reported that the testicular development in pre-pubertal age is not quiescent; rather undergo plenty of crucial developmental modifications that are essential to reach puberty. During this pre-pubertal phase, spermatogonial stem cells undergo intense and unremitted proliferation that leads to an increment in the volume of seminiferous tubules and testis by three folds. Further, Leydig cells and Sertoli cells attain functional capabilities which are believed critical for the development of testis and maintaining normal spermatogenesis.7,8 Therefore, any perturbation in development of gonads during pre-pubertal age may have adverse consequences in reproductive outcomes. The cytotoxic anti-cancer drugs, may adversely affect the reproductive outcomes in long-term child-hood survivals.9
Among all the cytotoxic anti-cancer drugs, alkylating agents are most extensively used in management of pediatric cancers and reported to have a high risk of impaired fertility.9 Currently, methods like sperm cryopreservation (for adolescents), testicular tissue cryopreservation (for pre-pubertal and peri-pubertal age) are used to preserve fertility in long-term child-hood survivals.10,11 However, as these methods are expensive and in primordial stage, cannot be used for all the ages of long-term survivals.6 Consequently, it is decisive to develop strategies that can diminish or counter the chemotherapy-induced toxic insult on testis and preserve fertility in long-term childhood survivals.
Cyclophosphamide (CP), an alkylating agent is most extensively used in the treatment of several types of cancers in both children and adults. It is also used as an immunosuppressant in the prevention of graft rejection and autoimmune diseases.12 The metabolites of CP, phosphoramide (with DNA alkylating and cross-linking nature) and acrolein are accountable for its therapeutic and toxic effects. Acrolein is highly reactive and considered as the major contributor to CP-induced oxidative damage and its toxic effects.12 Further, numerous clinical and experimental studies showed that CP treatment causes several structural and functional aberrations in testes thereby impair fertility.12 Though the exact mechanism is unknown, the generation of reactive oxygen species (ROS) is believed to play a critical role in the pathogenesis of CP-induced testicular toxicity.13,14 A plenty of research has been done to characterize CP-induced testicular toxicity and possible interventions in adult experimental animals.12 However, little is known about the effect of pre-pubertal exposure of CP on the male gonadal development and function.
Chlorophytum borivillianum (CB), commonly known as “safed musli” is a traditional medicinal herb belonging to family Liliaceae. It has been comprehensively used in Indian traditional medicine systems like Ayurveda, Unani, and Homeopathy since ancient times.15 CB has profuse therapeutic applications including anti-rheumatic, anticancer, antidiabetic, antioxidant, adaptogenic, anti-stress, rejuvenating, aphrodisiac, spermatogenic agent.15 Due to its phenomenal aphrodisiac effects, it has got widespread acceptance as “alternative Viagra” and extensively used for the treatment of erectile dysfunction, azoospermia, oligospermia and male impotence across the world.15,16 The chief phytochemicals of CB are saponins, alkaloids, flavonoids, and phenolic acids; among all these, saponins are believed to be responsible for its therapeutic potential. Interestingly, saponins of CB possess steroidal structure which is very analogous to testosterone structure.15,16 CB with its outstanding antioxidant properties showed a protective effect against many disease conditions where oxidative stress plays an imperative role in pathogenesis.15,17,18
As the use of natural products in treating many ailments and alleviating drug-induced toxicity is gaining much attention,19 we explored the possible protection from the root extract of CB that is popularly known as “alternative herbal viagra” against CP-induced testicular toxicity in pre-pubertal rats which may be employed to preserve fertility in long-term child-hood survivals treated with chemotherapeutic drugs.
Materials and methodsDrugs and chemicalsCyclophosphamide and dried roots of CB were obtained from Sigma Aldrich, USA and Amrutham Ayurveda Nilayam, Hyderabad respectively. All other reagents (analytical grade) used for the experiment were purchased locally.
Preparation of CB root aqueous extractThe CB root aqueous extract (AE) was prepared using exhaustive extraction method as described elsewhere.20 In brief, the crude powder was added to double-distilled water in 1g:6mL ratio. The mixture was heated to 37°C for 2h with constant stirring and then filtered into a clean round bottom flask using adsorbent cotton wool and muslin. This procedure was repeated for 5-times to ensure maximum yield of water-soluble compounds. The combined extract was concentrated by heating at 37°C in a water bath. Finally, the concentrated AE was dried in rota-evaporator and stored in a dry container at room temperature. The AE of CB root reported to give a high yield of saponins and show powerful antioxidant and aphrodisiac activity at dose levels between 100mg/kg and 500mg/kg compared to alcoholic extracts.15,18,20–22 Therefore, we have selected the AE at two dose levels, i.e. 100 and 200mg/kg/day for current study.
Animals and experimental designThe Institutional Animal Ethics Committee approved the experimental protocol and the experiments were performed in accordance with the CPCSEA (Committee for the Purpose of Control and Supervision of Experimentation on Animals) guidelines. All the animals were housed in controlled environmental conditions (22±2°C temperature, 50±10% humidity and 12 light/12 dark cycle). Animals had unlimited access to Standard laboratory animal feed (purchased from commercial supplier) and water and they were acclimatized to the experimental conditions for one-week prior to the experiments.
The required quantity of CP was dissolved in normal saline. The dried AE of CB roots was re-dissolved in double distilled water just before oral administration. Animals (Male) aged postnatal day (PND) 24 were randomly assigned to five groups with each bearing 12 animals. The Group-I (control), group-II (CP), and group-III (CB) received normal saline (NS), Cyclophosphamide 15mg/kg/day and CB 200mg/kg/day respectively during PND29-42; Group IV and Group V received CB 100mg/kg/day and CB 200mg/kg/day respectively along with CP15mg/kg/day for the same period. NS and CP were administered intraperitonially while CB was given orally. On PND43 (at puberty), six animals from every group were sacrificed, testes were collected and used for oxidative stress and histopathological evaluation. On PND63 (young adult age), remaining animals were sacrificed immediately after collecting the blood; the testes and epididymis were isolated, and used for the sperm analysis (density, motility, viability and morphology) and histomorphometrical assessment. The collected blood was used for hormonal (Testosterone, Luteinizing hormone (LH), and Follicle stimulating hormone (FSH)) estimation.
Evaluation of body weight gain and organ weightsThe animals were weighed weekly once and weight gain during the study period was calculated. During necropsy (PND43 and PND63), testes and epididymis were collected and weighed. As the animals used were in growing stage (juvenile), % body weight gain and relative organ weights were calculated to best portray the treatment effects on body weight and organ weights respectively.
Evaluation of oxidative stressOn PND43, the testes of rats (n=6) were isolated and homogenized using 0.1-M phosphate buffer (pH 7.4) containing 3-mM EDTA. Then, it was centrifuged (700g for 10-min) and the supernatant was used for the determination of oxidative stress markers, including malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione peroxidase (GSH-Px). MDA (Thiobarbituric acid reactive substances -TBARS) levels were measured at 532-nm as described by Ohkawa et al.23 GSH was analyzed at 412-nm according to Ellman's method.24 Catalase activity (the breakdown of hydrogen peroxide) was determined at 610-nm using method described by Sinha et al.25 SOD activity (inhibition of nitrazobluetetrazolium's reduction) was assayed at 560-nm by the method of Kono.26 Total GPx was determined by measuring the rate of oxidation of GSH at 420-nm as described elsewhere27). Protein content was determined at 750-nm by the method of Lowry et al.28 The MDA, GSH, GSH-Px, Catalase, and SOD levels were expressed as μmol/mg protein, μmol/mg protein, units/mg protein, μmol of H2O2/min/mg protein and μmol of H2O2/min/mg protein respectively.
Estimation hormone levelsThe blood samples (approximately 0.8ml) were collected from orbital plexus of young adult rats just before necropsy. The plasma levels of testosterone, LH, and FSH were determined as per the instructions provided in the commercially available kits (IBL International Elisa Kits). The hormone levels were expressed as ng/ml.
Evaluation of sperm count, viability, motility and sperm morphologyThe cauda epididymis was removed after sacrificing the adult animals and placed in a petri-dish containing 2–3ml of Hank's Buffer Saline Solution at room temperature. The epididymis was sliced into small pieces to permit the sperms to swim out and to form a suspension. The above suspension was centrifuged and the obtained supernatant was placed on Neubauer's hemocytometer to determine sperm count using procedure described previously.29 To determine the percentage of sperm with abnormal morphology, the above suspension was incubated for 30min with 2% eosin to stain the sperm. A smear of the above solution was prepared on a glass slide, air-dried and fixed with methanol. From each slide, 200 sperms were scrutinized at 100x magnification (oil immersion lens). Sperm morphology was categorized as normal, quasinormal and grossly abnormal based on the extent of abnormality as described previously.29 Data were shown as % of normal, quasinormal and grossly abnormal sperms. For sperm motility, one ml of above suspension diluted to two ml with Tris buffer solution and pre-warmed (35°C). Then, 2–3 drops this solution was charged on to a slide and examined under microscope visually for sperm motility at 40× magnification from 3 different fields in each sample.30 Bloom and Fawcett method31 was used for determination of sperm viability. In brief, the pre-warmed slides were charged with semen; added with couple of Eosin/Nigrosin stain drops; prepared into uniform smear; air dried; examined under the microscope. The number and % of live sperms (stained) and dead sperm (unstained) were calculated.
Histological evaluation and morphometrical analysisHistological slides were prepared as reported elsewhere.29 Briefly, testicular tissues both pubertal and adult animals were processed in the following order of events: fixed in formalin (10%), dehydrated, embedded in paraffin, sectioned (5μm), mounted on glass slides, dried overnight, deparaffinized, rehydrated, stained (Hematoxylin & Eosin), and examined under the microscope linked with CCD camera and imagining package. The sections from each animal were evaluated for structural changes and morphometric parameters (for young adult rat only). A total of 200 STs were used to calculate mean diameters (Mean of vertical and horizontal diameters) and germinal epithelial height. For tubular density, a total of 20 random focuses (60mm square area) per animal were used. Further, a total of 30 STs from each animal were read to allot Johnsen score based on the structural aspects as per the method described elsewhere.29
Statistical analysisResults were expressed as mean±SEM for each group. Statistical differences between the groups were determined by one-way ANOVA followed by multiple comparisons with Tukey's test using Sigma Stat 2.03 statistical software. The level of statistical significance was set at P<0.05.
Results and discussionBy virtue of abundance in reactive oxygen species (ROS) generating systems, highly unsaturated fatty acids and high rate of cell proliferation, the testis is more susceptible for lipid peroxidation.14 Despite the fact that small amounts of ROS are necessary for normal functioning of sperms, unbalanced levels of ROS can adversely affect the excellence of spermatozoa and thereby their fertilizing capacity.13 In defense against elevated oxidative stress, SOD converts the superoxide radicals to hydrogen peroxide that is then removed by GSH-Px and Catalase. GSH curbs the ROS through the regeneration of other antioxidant enzymes and forming complex with ROS and xenobiotics that elevate ROS. The increase in MDA, an end-product of lipid peroxidation is an indication of elevated oxidative stress and diminished antioxidant defense mechanisms.32
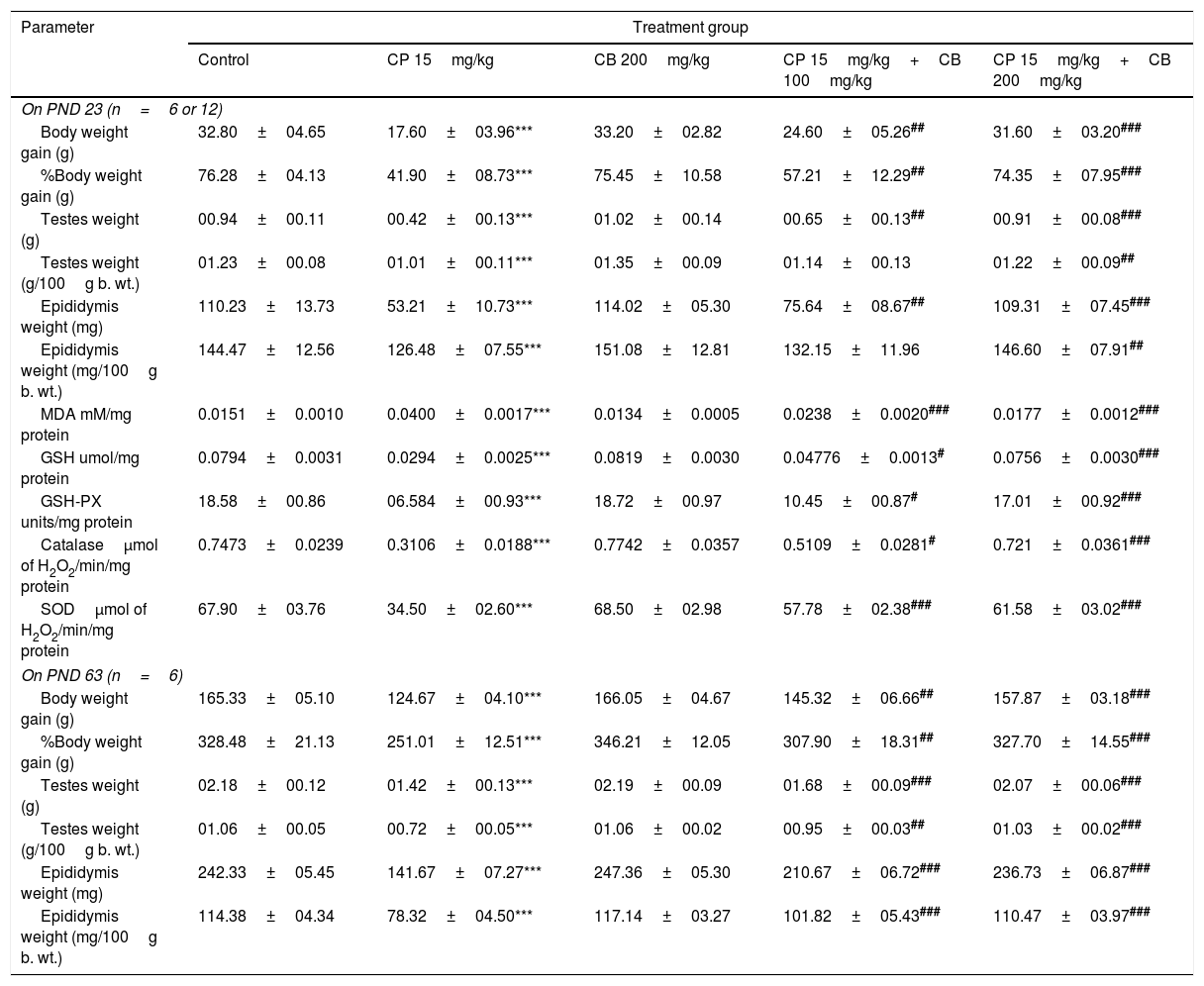
As reported in previous studies in rats,33,34 CP administration in pre-pubertal rats also resulted in decreased GSH, SOD, GPx, CAT levels and increased MDA levels in testis indicating elevated oxidative stress as compared to control rats. The metabolites of CP (mainly phosphoramide mustard) are considered as the culprits for the elevation of oxidative stress.12 Interestingly, co-treatment with CB reversed the CP-induced aberrations in oxidative stress markers in a dose-dependent manner (Table 1). The current results are markedly re-attesting the antioxidant potential of CB extract reported in several previous studies.15
Effect of CP and CB treatments on bodyweight, organ weights (evaluated on both PND43 and PND63) and oxidative stress (evaluated on PND43).
| Parameter | Treatment group | ||||
|---|---|---|---|---|---|
| Control | CP 15mg/kg | CB 200mg/kg | CP 15mg/kg+CB 100mg/kg | CP 15mg/kg+CB 200mg/kg | |
| On PND 23 (n=6 or 12) | |||||
| Body weight gain (g) | 32.80±04.65 | 17.60±03.96*** | 33.20±02.82 | 24.60±05.26## | 31.60±03.20### |
| %Body weight gain (g) | 76.28±04.13 | 41.90±08.73*** | 75.45±10.58 | 57.21±12.29## | 74.35±07.95### |
| Testes weight (g) | 00.94±00.11 | 00.42±00.13*** | 01.02±00.14 | 00.65±00.13## | 00.91±00.08### |
| Testes weight (g/100g b. wt.) | 01.23±00.08 | 01.01±00.11*** | 01.35±00.09 | 01.14±00.13 | 01.22±00.09## |
| Epididymis weight (mg) | 110.23±13.73 | 53.21±10.73*** | 114.02±05.30 | 75.64±08.67## | 109.31±07.45### |
| Epididymis weight (mg/100g b. wt.) | 144.47±12.56 | 126.48±07.55*** | 151.08±12.81 | 132.15±11.96 | 146.60±07.91## |
| MDA mM/mg protein | 0.0151±0.0010 | 0.0400±0.0017*** | 0.0134±0.0005 | 0.0238±0.0020### | 0.0177±0.0012### |
| GSH umol/mg protein | 0.0794±0.0031 | 0.0294±0.0025*** | 0.0819±0.0030 | 0.04776±0.0013# | 0.0756±0.0030### |
| GSH-PX units/mg protein | 18.58±00.86 | 06.584±00.93*** | 18.72±00.97 | 10.45±00.87# | 17.01±00.92### |
| Catalaseμmol of H2O2/min/mg protein | 0.7473±0.0239 | 0.3106±0.0188*** | 0.7742±0.0357 | 0.5109±0.0281# | 0.721±0.0361### |
| SODμmol of H2O2/min/mg protein | 67.90±03.76 | 34.50±02.60*** | 68.50±02.98 | 57.78±02.38### | 61.58±03.02### |
| On PND 63 (n=6) | |||||
| Body weight gain (g) | 165.33±05.10 | 124.67±04.10*** | 166.05±04.67 | 145.32±06.66## | 157.87±03.18### |
| %Body weight gain (g) | 328.48±21.13 | 251.01±12.51*** | 346.21±12.05 | 307.90±18.31## | 327.70±14.55### |
| Testes weight (g) | 02.18±00.12 | 01.42±00.13*** | 02.19±00.09 | 01.68±00.09### | 02.07±00.06### |
| Testes weight (g/100g b. wt.) | 01.06±00.05 | 00.72±00.05*** | 01.06±00.02 | 00.95±00.03## | 01.03±00.02### |
| Epididymis weight (mg) | 242.33±05.45 | 141.67±07.27*** | 247.36±05.30 | 210.67±06.72### | 236.73±06.87### |
| Epididymis weight (mg/100g b. wt.) | 114.38±04.34 | 78.32±04.50*** | 117.14±03.27 | 101.82±05.43### | 110.47±03.97### |
All the values are expressed as mean±SEM, (n=12 or 6), ***P<0.001 vs. Control), #P<0.05 vs. CP15mg/kg, ##P<0.01 vs. CP15mg/kg, ###P<0.001 vs. CP15mg/kg. CP, Cyclophosphamide; CB, Chlorophytum borivillianum; MDA, Malondialdehyde; GSH, Glutathione; GSH-Px, Glutathione peroxidase; SOD, Superoxide dismutase; H2O2, Hydrogen peroxide; n, number of animals (sample size); g, grams; mg, milli grams; b. wt., body weight; mM, milli moles; μmol, micro moles; min, minutes; PND, post natal day.
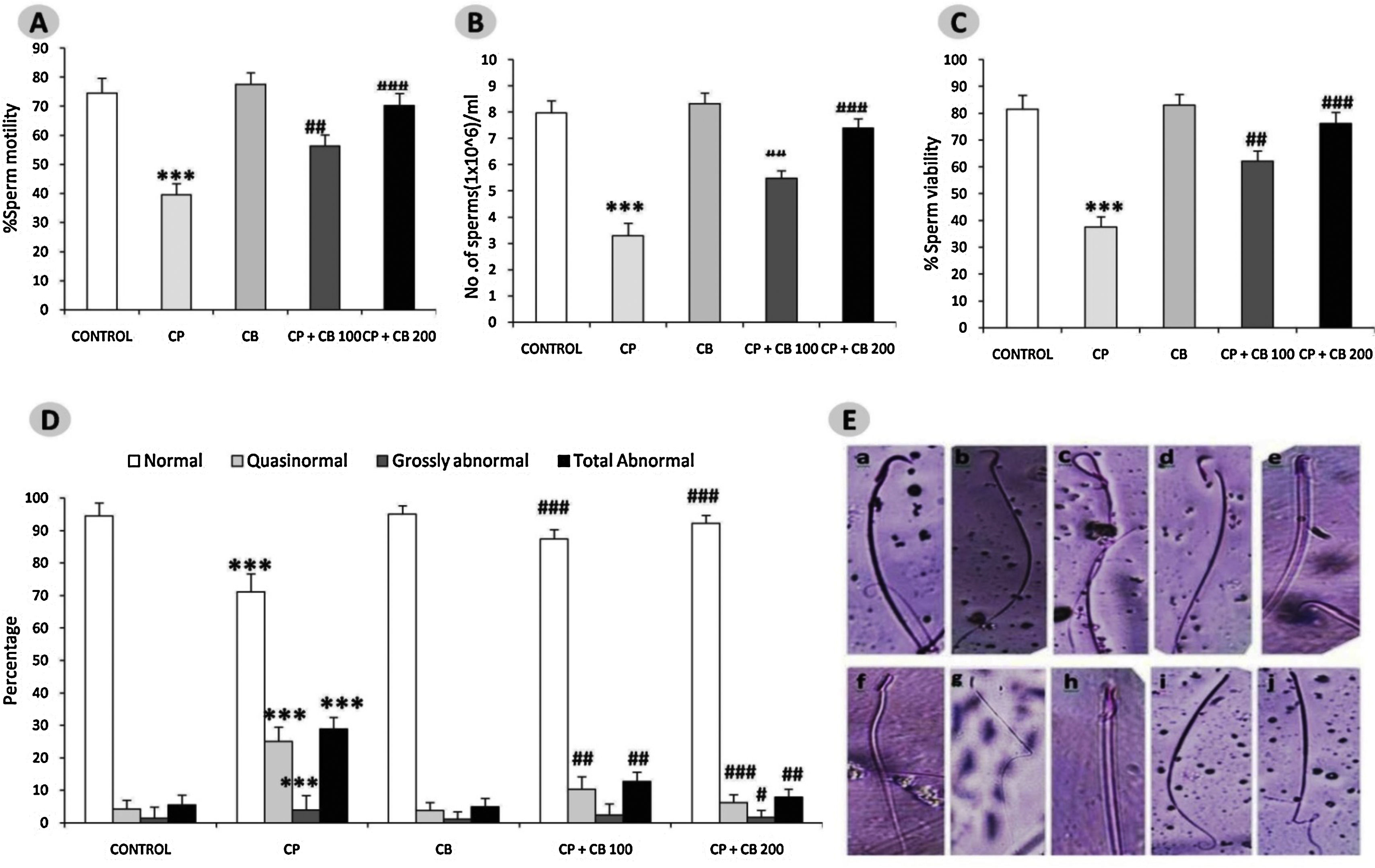
Further, a great body of clinical and experimental research indicating that CP-induced oxidative stress may play a major role in causing various abnormalities in the male reproductive system.12,14 The oxidative stress is considered a major cause for genotoxic damage, qualitative and quantitative impairment in sperm production that may lead to male infertility and have implications for the outcomes in progeny as well.14 In the midst of DNA damage, a eukaryotic cell maintains the genomic stability by arresting or delaying cell cycle which allows for the activation of DNA repair mechanisms. When the DNA damage is irresistible, cell death pathways get activated.35 The footprints of DNA damage in spermatogonial cells can be easily traced out by studying sperm morphology in which the genesis defects (abnormal morphology) are clearly visualized which can be taken as an sign of sperm DNA damage.36 In our study, a high percentage of spermatozoa with head abnormalities in CP-treated rats indicated an elevated degree of DNA fragmentation.
In line with numerous previous reports, our study revealed a significant reduction in the motility, viability, sperm count and number of sperm with normal morphology while a significant increase in sperm with abnormal morphology in all the three categories i.e. quasinormal, grossly normal and total abnormal in CP-treated rats as compared to control rats.12 Reduction in the sperm count could be attributed to impaired testicular development, and spermatogenesis as result of oxidative DNA damage and perturbations in hormonal interplay. However, co-treatment with CB reversed the CP-induced aberrations in sperm parameters in a dose-dependent manner (Fig. 1). The antioxidant and antiapoptotic properties of CB,17,22 may be predicted to lend a helping hand in delivering a healthy and completely matured sperm. The Hypothesis what we have derived for increased motility is with its antioxidant nature CB may protect the mitochondrial membrane which usually gets disturbed in the elevated oxidative stress conditions there by the energy levels in the sperm cell are maintained in a constant manner providing the ability to sperm to swim.37
Effect of CP and CB treatments on Sperm motility (A), Sperm count (B), Sperm viability (C), Sperm morphology (D–E) evaluated on PND6. All the values are expressed as mean±SEM, (n=6), ***P<0.001 vs. control), #P<0.05 vs. CP15mg/kg, ## P<0.01 vs. CP15mg/kg, ###P<0.001 vs.CP15mg/kg. CP, Cyclophosphamide 15mg/kg; CB, Chlorophytum borivillianum 200mg/kg; The section E is clearly indicating the abnormalities (b–j) in sperm morphology in rats treated with CP as compared to the control rats (a). The abnormalities found are banana shaped head (b), bent at cephalocaudal junction(c), detached head with tail (d), hook less (e), amorphous head (f), bent tail (g), bent with projecting filament (h), microcephaly with tail defect (i), and defective tail (j).
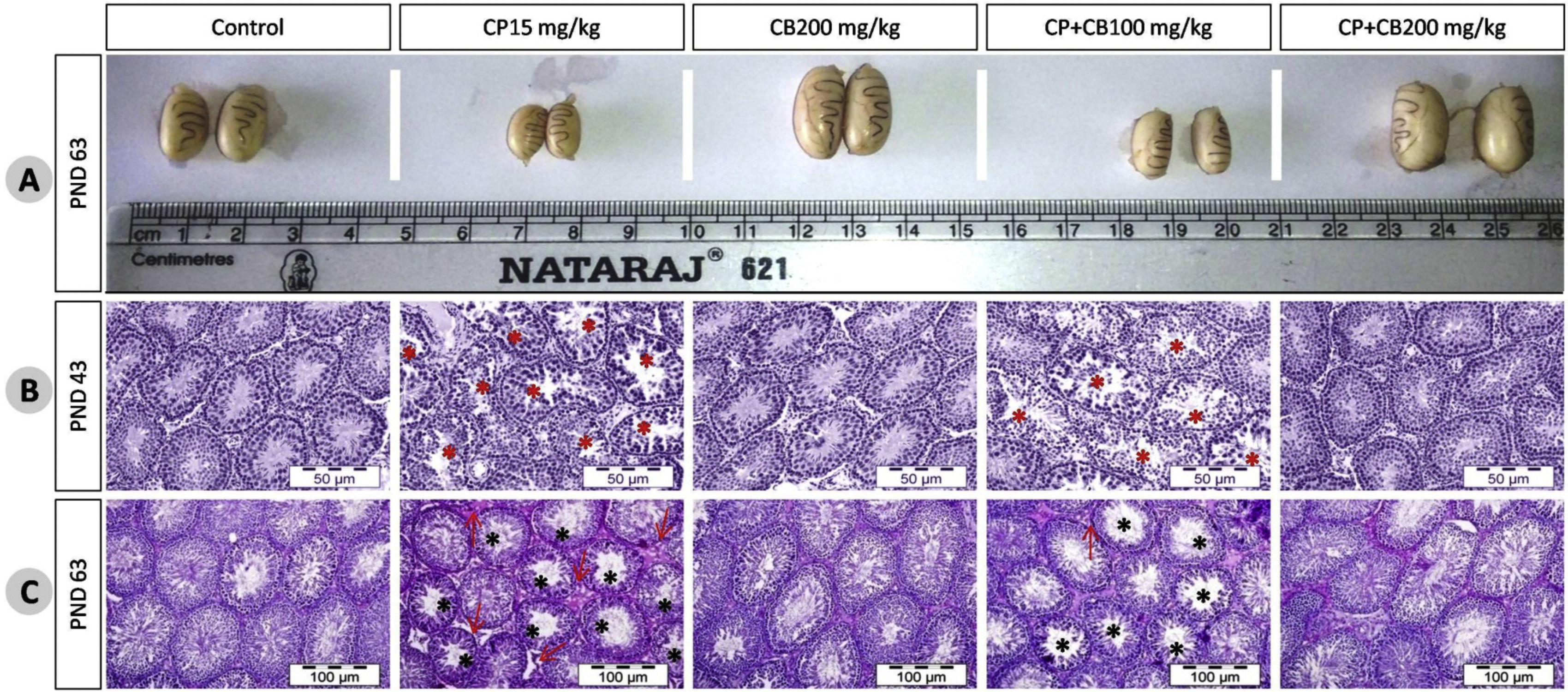
The effect of treatments on body weight, and organ weights at PND43, and PND63are shown in Table 1. The CP-treated rats have shown a significant reduction in body weight gain, % body weight gain, absolute and relative weights of testes and epididymis as compared to control rats at both PND43 and PND63. However, co-treatment with CB resulted in a dose-dependent reversal of CP-induced body, testes and epididymal weight reduction. These reductions were clearly visible in the testicular size (Fig. 2A). This reduction in body weight, testes and accessory organs could be a manifestation of decrease in the testosterone levels as it play a crucial role in development of male reproductive organs.38
Effect of CP and/or CB root aqueous extract on rat testicular size at PND63 (A), histology at PND43 (B) and PND63(C). The representative photomicrographs of testes of control and CB 200mg/kg-alone treated rats at PND43 and PND63 stained with hematoxylin and eosin shows integrated germinal epithelium, spermatozoa at different stages of maturation, STs filled with spermatozoa reflecting normal testicular architecture and development while CP-treated rats have shown atrophied STs with presence of disorganized germinal epithelium, cells sloughing into the lumen, poorly differentiated spermatozoa, marked depletion or absence of spermatozoa, an increase in the number of premature sperm cells in the lumen, depletion of Leydig cells and Sertoli cells conferring severe damage in testicular architecture and development as compared to control rats. The CP-treated rats co-administered with CB aqueous root extract (100 and 200mg/kg) shows improvement in damage in testicular architecture in a dose-dependent manner at both PND43 and PND63. * Mark represents the STs with damage, arrow indicates damaged interstitial area. CP, Cyclophosphamide; CB, Chlorophytum borivillianum; PND, Postnatal day; ST, Seminiferous tubule.
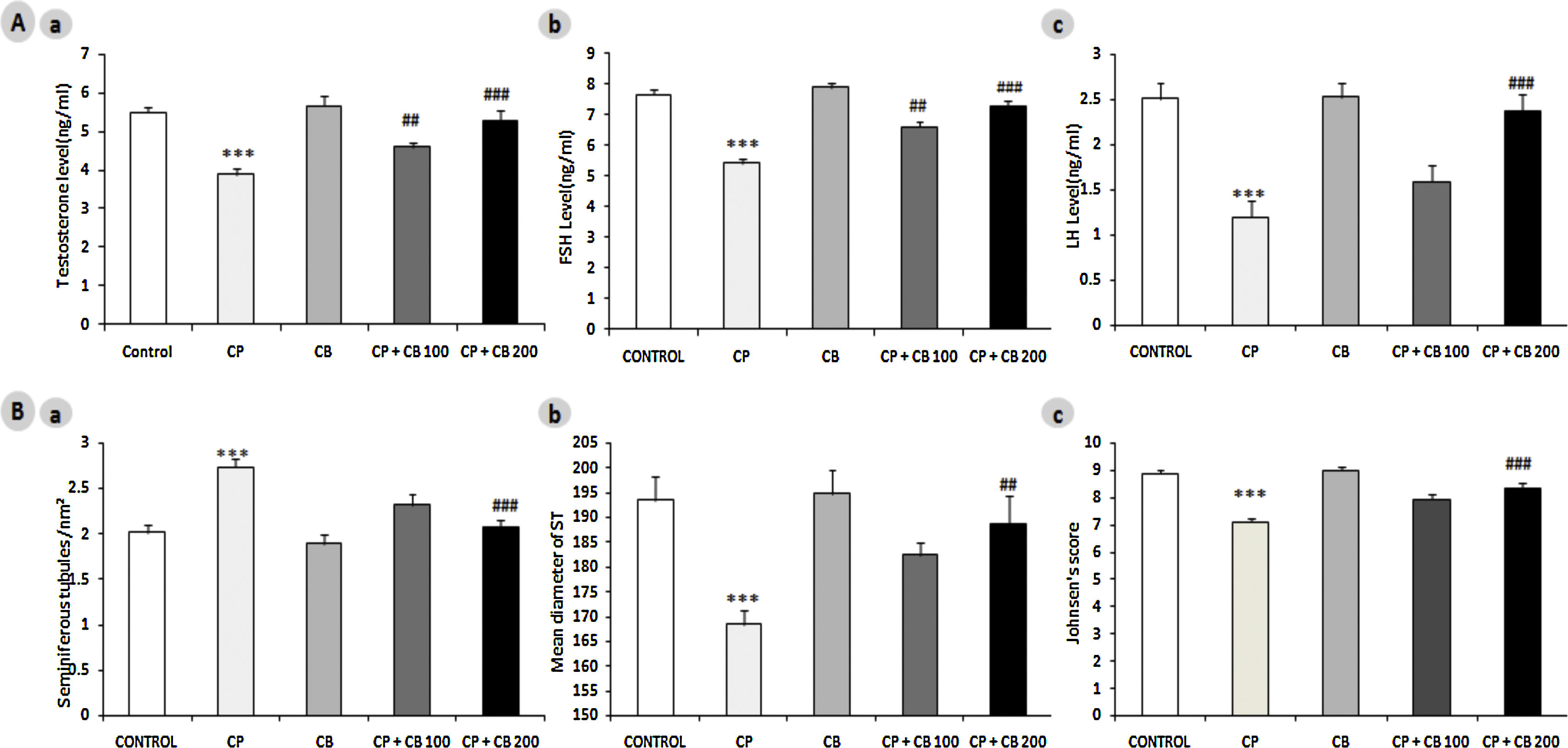
The testes of control and CB 200mg/kg alone-treated rats appear to have integrated germinal epithelium, spermatozoa at different stages of maturation, STs filled with spermatozoa reflects a normal testicular architecture and development (maturation) at both pubertal and young adult age. The CP-treated rats have shown severe damage in testicular architecture and development as compared to control rats at both PND43 and PND63 (Fig. 2B and 2C respectively). There was a coherent evidence of severe and mild vacuolation in STs and testicular interstitium respectively. Further, the STs in CP-treated rats have shown atrophied STs with presence of disorganized germinal epithelium, cells sloughing into the lumen, poorly differentiated spermatozoa, marked depletion or absence of spermatozoa, an increase in the number of premature sperm cells in the lumen. Further, the morphometric analysis of adult rat testes revealed a significant reduction in ST diameter, germinal epithelial height, and johnsen score and increase in tubular density (Fig. 3B). These findings in CP-treated rats are clearly indicating atrophy, impaired spermatogenesis, and development in testes. As reported in previous studies,22,39,40 the co-treatment with CB reversed the CP-induced aberrations in testicular architecture in a dose-dependent manner at both pubertal and adult age.
Effect of CP and CB treatments on plasma levels of A) Hormones:(a) Testosterone, (b) FSH, (c) LH and B) Morphometric parameters: (a) Seminiferous tubules density, (b) Diameter and (c) Johnsens score evaluated on PND63. All the values are expressed as mean±SEM, (n=6), ***P<0.001 vs. control), ## P<0.01 vs. CP15mg/kg, ###P<0.001 vs. CP15mg/kg. CP, Cyclophosphamide 15mg/kg; CB, Chlorophytum borivillianum 200mg/kg; FSH, Follicle stimulating hormone; LH, Luteinizing hormone; PND, Postnatal day.
Further, both FSH (direct regulator of Sertoli cells) and LH (indirect regulator of testosterone production from Leydig cells) play a crucial role in the initiation, maintenance, and maturation of the Spermatogonia.41 Several clinical and experimental studies suggesting that CP treatment perturbs hormonal interplay and gonadal steroidogenesis.12,42 The CP-treated rats have shown a significant elevation in plasma testosterone, FSH and LH levels as compared to control rats at PND63 while co-treatment with CB reversed these aberrations in a dose-dependent manner (Fig. 3A). The FSH levels elevated by CB treatment could have improved the sperm count as FSH play a crucial role in the induction of spermatogonial proliferation thereby maintaining spermatogenesis output.41 In line with other studies,12 CP-induced reduction in testosterone levels in current study indicates toxic insult on interstitial cells and a clear perturbation in endocrine regulatory mechanism. The prediction behind the mechanism of CB in restoring testosterone levels is at least in part due to its ability to scavenge the free radicals which could have prevented the toxic impact of CP on Leydig cells.40 Further, subsequent increase in LH and Leydig cell population after CB administration may be the reason behind increase in the testosterone level. It is irrefutable that the LH, FSH, and testosterone are considered as the hormonal biomarkers of androgenicity. Further, the structures of saponins present in the CB root resemble the structure of testosterone. The androgen-like or aphrodisiac nature of these steroidal saponins in part could have played a role in restoring the CP-induced perturbations in hypothalamic-pituitary-testicular axis and spermatogenesis. Several animal studies indicated that CP has plethora of effects on structure and function of the testes through targeting Leydig cells, Sertoli cells, and germ cells.43 CB reported to correct the perturbations in hypothalamo–pituitary–gonadal axis and cause normal production of LH, FSH, and testosterone.22,40
The decrease in testicular size, weight, tubular diameter, Johnsen score, sperm quality in terms of count, motility, or morphology and increase in density of seminiferous tubule demonstrate impairments in testicular development and spermatogenesis conferring a clear testicular toxicity. However, treatment with CB has alleviated these toxic imprints that may be due to its antioxidant and aphrodisiac properties, which are believed to be offered by its active components like saponins, alkaloids phenols. In addition to steroidal saponins, CB reported to have abundant amounts of zinc, iron, etc. which are considered to be essential for the synthesis of antioxidant enzyme like superoxide dismutase, production of testosterone and sperm production.15 These steroidal saponins gets converted into the male hormone called testosterone thus it helps in maintaining the testosterone levels.44 Several previous studies also postulate that CB promotes biogenesis of steroids, counter testicular damage and rejuvenates of testicular architecture and thereby improves the outcomes of male reproductive system.39 Thus, from the current results it is evident that aqueous extract of CB root can offer protection against CP-induced testicular toxicity by virtue of its unique combination phytochemicals offering antioxidant, steroidogenic, spermatogenic and aphrodisiac effects.
ConclusionThe pre-pubertal exposure to CP resulted in elevated oxidative stress, impaired spermatogenesis, steroidogenesis and testicular architecture in SD rats. Co-administration of CB was able to preserve antioxidant mechanisms, endocrine function and development, and architecture of testes. From the present findings, we conclude that CB by virtue of its exceptional antioxidant and aphrodisiac properties may possibly have therapeutic potential in preserving fertility in pre-pubertal boys undergoing chemotherapy. However, further studies are required to explore in-depth mechanisms involved in fertility preservation and translate these experimental findings to clinical practice.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant animal research ethics committee, CPCSEA (Committee for the Purpose of Control and Supervision of Experimentation on Animals).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflicts of interest.
The authors wish to acknowledge the technical support received from Vivo Bio Tech Ltd, Pregnapur, Telangana.