Several studies analyzed the possible relationships between semen parameters and assisted reproductive technologies (ART) treatments. However, none could establish a threshold to guide in the decision of which treatment technique will be better facing spermiogram values.
ObjectivesA database with spermiogram and clinical parameters was built in order to search for relevant interactions between semen parameters and different ART treatment techniques.
Materials and methodsA general statistical analysis evaluated semen parameters, followed by correlations to study their influence on the pregnancy rate for each type of treatment technique.
ResultsA predominance of teratozoospermia and asthenozoospermia was observed, with strong positive correlations between age and total motility, as well as obvious correlations between concentration and motility. Of the studied population, 41.8% went for ART consultations and presented a mean time of infertility of 2.9 years, a mean male age of 33.4 years and a mean female age of 31.9 years. Of these, 17.1% achieved a spontaneous pregnancy. Of the treatment cycles, 13.1% were by intra-uterine insemination (10.2% of clinical pregnancy rate, CP, with six newborn, NB), 24.9% by in vitro-fertilization (21.7 CP, 18 NB) and 44.9% by intracytoplasmic sperm injection (25.3% CP, 40 NB). From the analysis between semen parameters and the pregnancy rates, per type of ART technique, a significant positive correlation between techniques was found, revealing thresholds associated with the probability of a successful clinical pregnancy.
ConclusionsThe present results suggest that spermiogram reference limits can be developed for each treatment technique that are associated with a higher probability of achieving a clinical pregnancy.
Varios estudios han examinado las posibles relaciones entre los parámetros seminales y los resultados derivados de los tratamientos de infertilidad. Sin embargo, no hemos logrado establecer umbrales que puedan guiar a la hora de la decisión de que técnica de reproducción asistida (ART) se debe elegir.
ObjetivosSe construyó una base de datos con los parámetros seminales y los resultados clínicos de los tratamientos de infertilidad para que se puedan investigar las interacciones relevantes entre ellos.
Materiales y métodosDespués de un análisis estadístico general de los parámetros seminales, se realizó un estudio de correlación entre aquellos y las tasas de embarazo por la técnica de tratamiento.
ResultadosHubo un predominio de teratospermia y astenozoospermia, con fuertes correlaciones entre la edad y la motilidad total, así como correlaciones evidentes entre la concentración y la motilidad. En la población de estudio, el 41,8% ha visitado las consultas de infertilidad, presentando un tiempo medio de 2,9 años de infertilidad, y una edad media de 33,4 (hombres) y 31,9 (mujeres) años. De éstas, el 17,1% logró un embarazo espontáneo. De los tratamientos de ART realizados, 13,1% se debieron a la inseminación intrauterina (tasa de embarazo clínico del 10,2%, GC, con 6 recién nacidos, RN), el 24,9% en la fertilización in vitro (21.7 GC, RN 18) y 44,9% por microinyección intracitoplasmática de espermatozoides (25,3% GC, RN 40). Del análisis entre los parámetros seminales y las tasas de embarazo, para cada tipo de técnica de tratamiento, se observó una correlación significativa entre las técnicas, revelando los umbrales asociados a la probabilidad de lograr con éxito un embarazo clínico.
ConclusionesLos datos sugieren que pueden ser creados los umbrales de referencia de los parámetros seminales para cada técnica de tratamiento, los cuales están más fuertemente asociados con la capacidad de lograr con éxito un embarazo clínico.
In the last decades, a decrease of natality in the industrialized countries has been observed, as couples prefer to defer conception due to professional and financial motives. This led to gonad aging at the age of conception, which is associated with infertility. Besides the delay in conception, other social factors are related to an increase in infertility consultations, such as a higher diffusion of information and the substantial improvement of diagnostic and treatment techniques.1,2
Infertility is defined as the inability of the couple in achieving a full term pregnancy after one year of regular intercourse3 and attains about 10–15% of the population.2 The time of infertility is thus an important diagnostic factor and exhibits a large interval variation with some cases presenting more than 10 years.4,5
Another important determinant is the cause of infertility. The development of new diagnostic tools have changed the incidence of 21% of male factor, 57% of female factor and 4% of mixed causes6 to 51%, 23% and 26%, respectively.5 Idiopathic infertility can also be reduced with the use of more specific and elaborated diagnostics.2,7
The fertile potential of a woman decreases after 30 years of ovary age and dramatically after 35 years, with production of a lower number and quality of the oocytes, with a decrease in implantation and an increase in abortion and chromosomal aberrations.2,4 In males, after 35–45 years, the production and quality of sperm are reduced and attain the volume, number, motility and morphology.8−11 These have been related with an increase in dominant autosomic diseases, schizophrenia, autism, chromosomal aberrations and cancer, all associated with a decrease in clinical pregnancy and an increase in abortion and newborn malformations.10
When evaluating the infertile men several factors are considered, the personal and family history, the physical examination and semen analysis.12,13 Semen analysis is a fundamental tool and the values obtained are a measure to evaluate the potential fertility status.14–16
The most used assisted reproduction techniques (ART) are ovulation induction, intrauterine insemination (IUI), in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Intrauterine insemination is a less invasive procedure for cervical-mucus disorders and anejaculation, in the presence of a normal or near normal ovulation function, or for cases with donor sperm. The term clinical pregnancy rate is about 10–20%.17 In vitro fertilization is designed for tubar obstruction and disorders of ovulation, with normal or near normal semen parameters. The term clinical pregnancy rate is about 27–60%.2 Intracytoplasmic sperm injection is mainly used for severe male factor. After the introduction of ICSI,18–21 couples with severe oligo/astheno/terato/zoospermia and azoospermia22,23 could achieve a full term pregnancy. The selection of the best sperm is critical for the success of the technique,24 with a term clinical pregnancy rate of 27–40%,4 and a slightly lower rate with testicular sperm.23,25
In the present study we developed a database with semen parameters from patients attaining the Department of Clinical Chemistry, Hospital Centre of Porto (CHP), EPE, General Hospital of St. António (HGSA), during 2010–2012. We purpose to evaluate the relationship between the different semen parameters and their influence in the clinical pregnancy rate.
Materials and methodsEthical considerationsA patient database was developed for cases that were referred for semen analysis at the Department of Clinical Chemistry, HGSA-CHP, during 2010–2012. Regarding ART treatments, this Department has access to clinical but not embryological data. These were used under written and informed patient consent according to the National Law of Assisted Medical Procreation (Law n.° 32/2006, of 26 July), and under the requirements of the National Council of Assisted Medical Procreation (CNPMA, 2008).
PatientsWe studied a group of 586 men, with a mean age of 33.75±5.66 (17–60) and a mean of sexual abstinence days of 3.96±1.59. Semen parameters were evaluated according to the Manual of the World Health Organization (WHO) from 2010,15 except for rapid progressive motility, where the confidence interval was that from the Manual of WHO-1999.26
Study designThe relationships between semen parameters were first evaluated. Of the 203 patients that went for ART treatments, the influence of semen parameters on the clinical pregnancy rates after treatment per each ART technique (IUI, IVF and ICSI) was then analyzed.
Semen analysisSemen samples were collected and kept on a thermal plate at 37°C (Thermolyne Corporation, Dubuque Iowa, USA) until total liquefaction (30–60min). The macroscopic (color, odor, aspect, viscosity and volume), biochemical (pH) and microscopic (motility, vitality, concentration, total number of sperm and morphology) parameters were quantified. Microscopic analysis was performed at 400x with a phase contrast microscope (Olympus BX50, Japan). For vitality an eosine-Y solution (Merck, Darmstadt, Germany) was used. Concentration was evaluated in an improved Neubauer chamber (Superior Marienfeld, Karlsruhe, Germany). For morphology, we used the Papanicolau staining at 1000x (Department of Pathology, HGSA-CHP).15,26
Statistical analysisStatistical analysis was performed using the STATISTICA software (version 11, Stat Soft, Tulsa, OK, USA). To ascertain the degree of association between variables of interest a linear correlation analysis was made, determining the Pearson correlation coefficient and the statistical significance (p value) of the associations found. For comparison of means between groups, an analysis of variance with two factors (two-way ANOVA) was implemented, according to a parametric model, followed by the post hoc Tukey's test. In situations with homogeneity of variances (after the Levene test), the nonparametric Kruskal–Wallis test followed by multiple comparisons by the Siegel and Castellan test were performed. Parametric and nonparametric approaches pointed to the same scenario as to the significance of the differences, and it was therefore chosen to present the results of the parametric analysis. In a few occasions, frequencies were compared with the statistical analysis from the IBM SPSS Statistics (version 20), using the chi-square and the Fisher's exact tests. In selected comparisons of pairs of means, the independent-samples T test procedure was conducted. The confidence level adopted in all cases was 95%, considering a significant result when p<0.05.
ResultsRelationships between semen parametersRegarding male age (17–60), the majority had between 30 and 39 (63%) years, with age groups of 1% (3: <20 years), 22% (128: 20–29 years), 34% (201: 30–34 years), 29% (171: 35–39 years) and 14% (83: ≥40 years).
Most of the cases presented normal semen values for abstinence (97% between 2 and 7 days), aspect (97% opalescent), volume (92% ≥1.5ml), pH (97%: ≥7.2), liquefaction (99% complete), concentration (70% ≥15×106ml–1), total number of sperm (TN) (71% ≥39×106), total progressive motility (TPM) (67% ≥32%), total motility (TM) (71%≥40%), teratozoospermia index (92% <1.6) and vitality (VT) (76% ≥58%). However, lower values were found to morphology (63%<4%) and rapid progressive motility (RPM) (52%<25%).
In relation to subgroups, 30% had oligozoospermia, 32% asthenozoospermia, 60% teratozoospermia, 18% oligo-asthenozoospermia, 23% oligo-teratozoospermia, 27% astheno-teratozoospermia, 16% oligo-astheno-teratozoospermia and 4% azoospermia. Cases with normal relative values (normal concentration or TPM or morphology) corresponded to 80% of the patients, whereas cases with normal absolute values (normal concentration and TPM and morphology) corresponded to 30% of the patients.
The finding of a correlation between two variables does not mean causality but indicates that they are related. Thus, the greater the absolute value of a correlation coefficient, the stronger will be the linear relation. It was considered a significant moderate association between variables when “r>0.3” and strong when “r>0.8”.
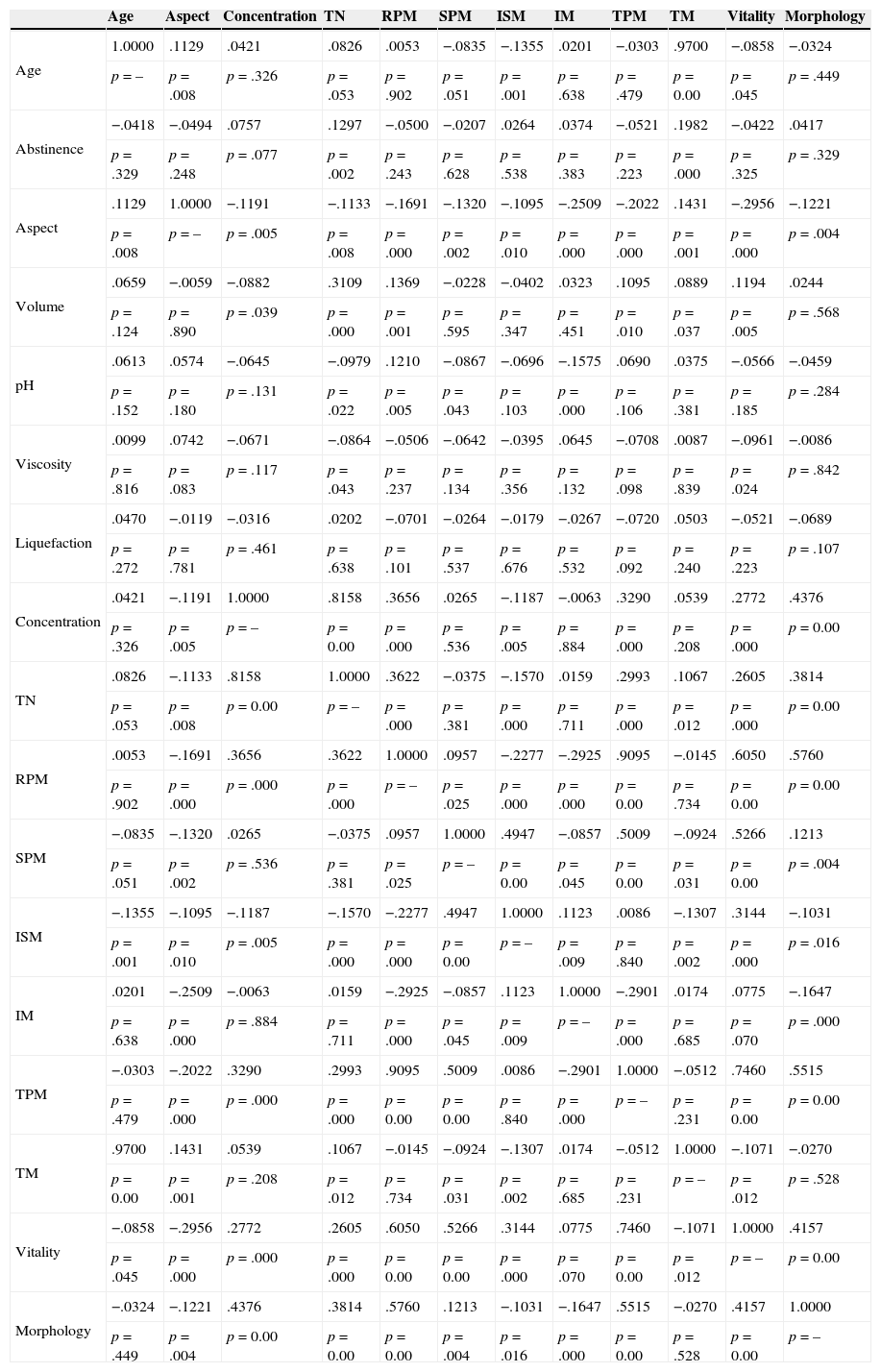
In a first approach we performed a correlation analysis between semen parameters (Table 1), followed by a similar analysis including the different morphological anomalies (Table 2). We observed that the variables age and TM were those with a higher linear association. Other significant positive correlations were also found between concentration and: TN (strong), RPM, TPM and morphology; between the TN and: volume, RPM and morphology; between RPM and: TPM (strong), morphology and VT; between TPM and: morphology and VT; and between morphology and VT (Table 1).
Correlations (r) between semen parameters.
| Age | Aspect | Concentration | TN | RPM | SPM | ISM | IM | TPM | TM | Vitality | Morphology | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.0000 | .1129 | .0421 | .0826 | .0053 | −.0835 | −.1355 | .0201 | −.0303 | .9700 | −.0858 | −.0324 |
| p=– | p=.008 | p=.326 | p=.053 | p=.902 | p=.051 | p=.001 | p=.638 | p=.479 | p=0.00 | p=.045 | p=.449 | |
| Abstinence | −.0418 | −.0494 | .0757 | .1297 | −.0500 | −.0207 | .0264 | .0374 | −.0521 | .1982 | −.0422 | .0417 |
| p=.329 | p=.248 | p=.077 | p=.002 | p=.243 | p=.628 | p=.538 | p=.383 | p=.223 | p=.000 | p=.325 | p=.329 | |
| Aspect | .1129 | 1.0000 | −.1191 | −.1133 | −.1691 | −.1320 | −.1095 | −.2509 | −.2022 | .1431 | −.2956 | −.1221 |
| p=.008 | p=– | p=.005 | p=.008 | p=.000 | p=.002 | p=.010 | p=.000 | p=.000 | p=.001 | p=.000 | p=.004 | |
| Volume | .0659 | −.0059 | −.0882 | .3109 | .1369 | −.0228 | −.0402 | .0323 | .1095 | .0889 | .1194 | .0244 |
| p=.124 | p=.890 | p=.039 | p=.000 | p=.001 | p=.595 | p=.347 | p=.451 | p=.010 | p=.037 | p=.005 | p=.568 | |
| pH | .0613 | .0574 | −.0645 | −.0979 | .1210 | −.0867 | −.0696 | −.1575 | .0690 | .0375 | −.0566 | −.0459 |
| p=.152 | p=.180 | p=.131 | p=.022 | p=.005 | p=.043 | p=.103 | p=.000 | p=.106 | p=.381 | p=.185 | p=.284 | |
| Viscosity | .0099 | .0742 | −.0671 | −.0864 | −.0506 | −.0642 | −.0395 | .0645 | −.0708 | .0087 | −.0961 | −.0086 |
| p=.816 | p=.083 | p=.117 | p=.043 | p=.237 | p=.134 | p=.356 | p=.132 | p=.098 | p=.839 | p=.024 | p=.842 | |
| Liquefaction | .0470 | −.0119 | −.0316 | .0202 | −.0701 | −.0264 | −.0179 | −.0267 | −.0720 | .0503 | −.0521 | −.0689 |
| p=.272 | p=.781 | p=.461 | p=.638 | p=.101 | p=.537 | p=.676 | p=.532 | p=.092 | p=.240 | p=.223 | p=.107 | |
| Concentration | .0421 | −.1191 | 1.0000 | .8158 | .3656 | .0265 | −.1187 | −.0063 | .3290 | .0539 | .2772 | .4376 |
| p=.326 | p=.005 | p=– | p=0.00 | p=.000 | p=.536 | p=.005 | p=.884 | p=.000 | p=.208 | p=.000 | p=0.00 | |
| TN | .0826 | −.1133 | .8158 | 1.0000 | .3622 | −.0375 | −.1570 | .0159 | .2993 | .1067 | .2605 | .3814 |
| p=.053 | p=.008 | p=0.00 | p=– | p=.000 | p=.381 | p=.000 | p=.711 | p=.000 | p=.012 | p=.000 | p=0.00 | |
| RPM | .0053 | −.1691 | .3656 | .3622 | 1.0000 | .0957 | −.2277 | −.2925 | .9095 | −.0145 | .6050 | .5760 |
| p=.902 | p=.000 | p=.000 | p=.000 | p=– | p=.025 | p=.000 | p=.000 | p=0.00 | p=.734 | p=0.00 | p=0.00 | |
| SPM | −.0835 | −.1320 | .0265 | −.0375 | .0957 | 1.0000 | .4947 | −.0857 | .5009 | −.0924 | .5266 | .1213 |
| p=.051 | p=.002 | p=.536 | p=.381 | p=.025 | p=– | p=0.00 | p=.045 | p=0.00 | p=.031 | p=0.00 | p=.004 | |
| ISM | −.1355 | −.1095 | −.1187 | −.1570 | −.2277 | .4947 | 1.0000 | .1123 | .0086 | −.1307 | .3144 | −.1031 |
| p=.001 | p=.010 | p=.005 | p=.000 | p=.000 | p=0.00 | p=– | p=.009 | p=.840 | p=.002 | p=.000 | p=.016 | |
| IM | .0201 | −.2509 | −.0063 | .0159 | −.2925 | −.0857 | .1123 | 1.0000 | −.2901 | .0174 | .0775 | −.1647 |
| p=.638 | p=.000 | p=.884 | p=.711 | p=.000 | p=.045 | p=.009 | p=– | p=.000 | p=.685 | p=.070 | p=.000 | |
| TPM | −.0303 | −.2022 | .3290 | .2993 | .9095 | .5009 | .0086 | −.2901 | 1.0000 | −.0512 | .7460 | .5515 |
| p=.479 | p=.000 | p=.000 | p=.000 | p=0.00 | p=0.00 | p=.840 | p=.000 | p=– | p=.231 | p=0.00 | p=0.00 | |
| TM | .9700 | .1431 | .0539 | .1067 | −.0145 | −.0924 | −.1307 | .0174 | −.0512 | 1.0000 | −.1071 | −.0270 |
| p=0.00 | p=.001 | p=.208 | p=.012 | p=.734 | p=.031 | p=.002 | p=.685 | p=.231 | p=– | p=.012 | p=.528 | |
| Vitality | −.0858 | −.2956 | .2772 | .2605 | .6050 | .5266 | .3144 | .0775 | .7460 | −.1071 | 1.0000 | .4157 |
| p=.045 | p=.000 | p=.000 | p=.000 | p=0.00 | p=0.00 | p=.000 | p=.070 | p=0.00 | p=.012 | p=– | p=0.00 | |
| Morphology | −.0324 | −.1221 | .4376 | .3814 | .5760 | .1213 | −.1031 | −.1647 | .5515 | −.0270 | .4157 | 1.0000 |
| p=.449 | p=.004 | p=0.00 | p=0.00 | p=0.00 | p=.004 | p=.016 | p=.000 | p=0.00 | p=.528 | p=0.00 | p=– |
TN, total number of sperm; RPM, rapid progressive motility; SPM, slow progressive motility; ISM, in situ motility; IM, immotile; TPM, total progressive motility; TM, total motility.
Correlations (r) between semen parameters and the different types of sperm morphology.
| Aspect | RPM | SPM | ISM | IM | TPM | Vitality | Morphology | Head (%) | MP (%) | Tail (%) | CD (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head (%) | −.4068 | .3473 | .4689 | .4315 | .7110 | .4817 | .7216 | .1688 | 1.0000 | .7837 | .4420 | .2991 |
| p=.000 | p=.000 | p=0.00 | p=0.00 | p=0.00 | p=0.00 | p=0.00 | p=.000 | p=– | p=0.00 | p=0.00 | p=.000 | |
| MP (%) | −.2936 | .0223 | .2413 | .3637 | .7437 | .1161 | .4104 | −.2045 | .7837 | 1.0000 | .6381 | .3527 |
| p=.000 | p=.645 | p=.000 | p=.000 | p=0.00 | p=.016 | p=.000 | p=.000 | p=0.00 | p=– | p=0.00 | p=.000 | |
| Tail (%) | −.1572 | −.2397 | .0384 | .2520 | .6013 | −.1864 | .1139 | −.2867 | .4420 | .6381 | 1.0000 | .1787 |
| p=.001 | p=.000 | p=.428 | p=.000 | p=0.00 | p=.000 | p=.018 | p=.000 | p=0.00 | p=0.00 | p=– | p=.000 | |
| CD (%) | −.1326 | .0535 | .2120 | .2484 | .1878 | .1306 | .2413 | −.0739 | .2991 | .3527 | .1787 | 1.0000 |
| p=.006 | p=.269 | p=.000 | p=.000 | p=.000 | p=.007 | p=.000 | p=.127 | p=.000 | p=.000 | p=.000 | p=– |
RPM, rapid progressive motility; SPM, slow progressive motility; ISM, in situ motility; IM, immotile; TPM, total progressive motility; MP, midpiece; CD, cytoplasmic droplets.
In relation to the different components of the sperm, significant moderate positive correlations were found between head anomalies and: anomalies of the midpiece and tail, RPM, TPM and VT; between midpiece anomalies and: anomalies of the head and tail, cytoplasmic droplets and VT; and between tail anomalies and: anomalies of the head and midpiece (Table 2).
Influence of semen parameters and clinical pregnancy per ART techniqueOf the initial studied population (586), 245 (41.8%) couples went for ART consultations at the Center of Assisted Reproduction-CHP. For these, the mean time of infertility was 2.9 (0.67–15 years), the mean male age was 33.4 (20–49 years) and the mean female age was 31.9 (21–40 years). Of these couples, 42 (17.1%) achieved a spontaneous pregnancy. In this group the mean time of infertility was 2.2 (1–7), the mean male age was 32.4 (20–47) and the mean female age was 32.0 (22–40).
Thus, 203 couples performed ART treatment cycles. In this group, the mean time of infertility was 3.1 (0.67–15), the mean male age was 33.3 (23–49) and the mean female age was 32.3 (22–40). The majority of the females had 30–39 (69.5%) years, with age groups of 0% (0: <20 years), 29.6% (60: 20–29 years), 36.9% (75: 30–34 years), 32.5% (66: 35–39 years) and 1.0% (2: 40 years). The mean time of infertility was 3.1 (0.67–15), with the majority (185: 91.1%) presenting more than one year of infertility.
From these 203 couples, 32 (13.1%) performed IUI cycles, 61 (24.9%) IVF cycles and 110 (44.9%) ICSI cycles. In IUI cycles the mean time of infertility was 3.1 (0.67–11), the mean male age was 31.8 (25–40) and the mean female age 31.5 (26–39); in IVF cycles the mean time of infertility was 3.2 (0.67–15), the mean male age was 33.1 (25–44) and the mean female age 32.6 (25–39); and in ICSI cycles the mean time of infertility was 3.1 (0.83–13), the mean male age was 33.9 (23–49) and the mean female age 32.4 (22–40).
In total there were 305 ART treatment cycles. In IUI (49 cycles) there were 6 biochemical pregnancies (BP), 5 (10.2%) clinical pregnancies (CP), 4 single and 1 twin, and 5 ongoing pregnancies (OP), with 5 deliveries and 6 newborn (NB). There was one neonatal death due to prematurity from the twin pregnancy. In the IVF cycles (82), there were 69 embryo transfer cycles (ETC), with 17 BP, 15 (21.7%) CP (10 single, 4 twin) and 14 OP (1 abortion), with 14 deliveries and 18 NB. In the ICSI cycles (174), there were 166 ETC, with 43 BP, 42 (25.3%) CP (30 single, 12 twin) and 35 OP (5 abortions; 2 cases with 1 gestational sac with no embryo), with 35 deliveries and 40 NB. From the 30 single CP, 2 had no embryo in the gestational sac and 4 went in abortion, which gives 24 NB. Of the 12 twin CP, there was 1 abortion, 6 cases with birth of 1 NB and 5 cases with birth of 10 NB, which gives 16 NB.
Analysis revealed a moderate correlation between male and female ages (r=0.6331, p=0.000), but not between the time of infertility and male age (r=0.2534, p=0.001) or between the time of infertility and female age (r=0.1683, p=0.036).
From the analysis of two-factor variance (between semen parameters and the biochemical pregnancy rate per type of ART technique), regarding sperm concentration a significant positive correlation between techniques (F(2, 181)=17.316; p<0.001) was found, with the lower values being found in ICSI. These differences were not observed inside each technique (F(2, 181)=1.290; p=0.278). As in IUI there was a large value dispersion, a new pare-comparison was performed using the T test (p=0.243) followed by the post hoc test of Newman–Keuls (p=0.073), but no significant differences were found (Fig. 1A). The same was observed regarding the TN of sperm (F(2, 181)=14.648; p<0.001) and (F(2, 181=0.095; p=0.910) (Fig. 1B). Similar results were obtained in relation to sperm morphology (F(2, 181)=14.419; p<0.001) and (F(2, 181)=1.067; p=0.346) (Fig. 1C).
Relationships between semen parameters and the different ART techniques to cycles with (⊄) and without (□) biochemical pregnancy. (A) Concentration (106ml−1); (B) total number of sperm (TN: 106/ejaculate); (C) morphology (%); (D) rapid progressive motility (RPM: %); (E) slow progressive motility (SPM: %); (F) total progressive motility (TPM: %). The level of confidence was 95%.
In relation to RPM significant differences were observed between the techniques (F(2, 181)=28.421; p<0.001), with the lower values found in ICSI, and with no significant differences inside each technique (F(2, 181)=0.261; p=0.770) (Fig. 1D). The same was found for TPM (F(2, 181)=21.134; p<0.001) and (F(2, 181)=0.261; p=0.770) (Fig. 1F). For sperm with slow progressive motility there were no significant differences between (F(2, 181)=0.097; p=0.907) or inside (F(2, 181)=0.605; p=0.557) techniques (Fig. 1E).
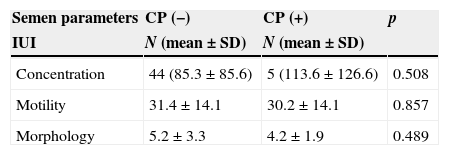
When the means of total concentration, motility and morphology were compared against treatment cycles with negative and positive clinical pregnancy, the same results were observed, with exception of ICSI cycles where cycles with positive clinical pregnancy had a higher mean of morphology (Table 3).
Comparisons between the means of semen parameters and cycles with negative (-) and positive (+) clinical pregnancy (CP) per treatment cycle.
| Semen parameters | CP (−) | CP (+) | p |
|---|---|---|---|
| IUI | N (mean±SD) | N (mean±SD) | |
| Concentration | 44 (85.3±85.6) | 5 (113.6±126.6) | 0.508 |
| Motility | 31.4±14.1 | 30.2±14.1 | 0.857 |
| Morphology | 5.2±3.3 | 4.2±1.9 | 0.489 |
| IVF | N (mean±SD) | N (mean±SD) | |
|---|---|---|---|
| Concentration | 67 (98.9±75.6) | 15 (84.5±52.4) | 0.486 |
| Motility | 28.6±12.8 | 27.6±11.6 | 0.773 |
| Morphology | 4.5±2.6 | 4.8±3.0 | 0.689 |
| ICSI | N (mean±SD) | N (mean±SD) | |
|---|---|---|---|
| Concentration | 132 (27.8±39.8) | 42 (37.3±40.6) | 0.182 |
| Motility | 13.6±11.1 | 12.9±9.2 | 0.709 |
| Morphology | 1.8±2.0 | 2.6±2.6 | 0.042 |
IUI, intra-uterine insemination; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection. Treatment cycles: IUI (49), IVF (82), ICSI (174).
Note: Data in: “n”, mean, and standard deviation (SD).
The high values of SD observed in IUI and ICSI relatively to concentration are due to the high variability of the sample.
Thus for IUI CP (−): confidence interval=25.29, with a range for the true population mean of 60.01–110.59.
Thus for IUI CP (+): confidence interval=110.97, with a range for the true population mean of 2.63–224.57.
Thus for ICSI CP (−): confidence interval=6.79, with a range for the true population mean of 21.01–34.59.
Thus for ICSI CP (+): confidence interval=12.28, with a range for the true population mean of 25.02–49.58.
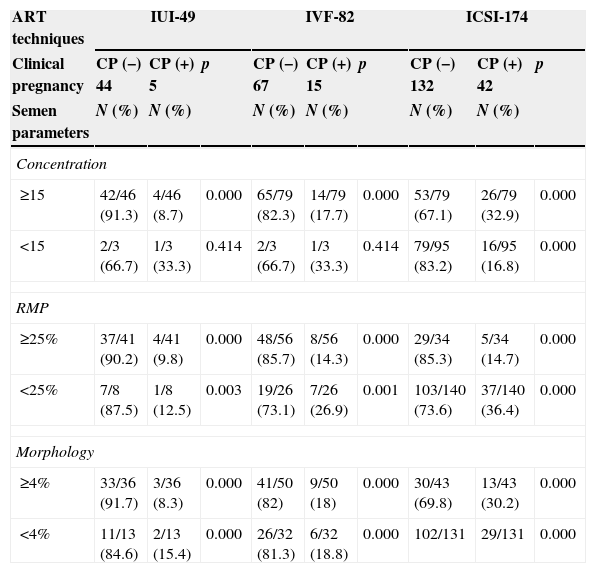
Comparisons between subgroups of concentration, motility and morphology, against treatment cycles with negative and positive clinical pregnancy, showed that for IUI the higher clinical pregnancy rate was obtained when sperm concentration was ≥10×106ml−1 (100% of the cases), the RPM was ≥10% (100%), and normal morphology was ≥2% (100%) or ≥3% (80%). For IVF, the higher pregnancy rate was obtained when sperm concentration was ≥15×106ml−1 (93.3%) or ≥5×106ml−1 (100%), the RPM was ≥10% (100%), and normal morphology was ≥3% (93.3%) or ≥1% (100%). For ICSI the higher pregnancy rate was obtained when sperm concentration was ≥5×106ml−1 (81.0%), the RPM was ≥5% (76.2%) and normal morphology was ≥1% (73.8%) (Tables 4 and 5). Comparisons inside subgroups of concentration, motility and morphology, against treatment cycles with positive clinical pregnancy confirmed these results.
Comparisons between semen parameters and cycles with negative (−) and positive (+) clinical pregnancy (CP) per treatment cycle.
| ART techniques | IUI-49 | IVF-82 | ICSI-174 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical pregnancy | CP (−) 44 | CP (+) 5 | p | CP (−) 67 | CP (+) 15 | p | CP (−) 132 | CP (+) 42 | p |
| Semen parameters | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Concentration | |||||||||
| ≥15 | 42/46 (91.3) | 4/46 (8.7) | 0.000 | 65/79 (82.3) | 14/79 (17.7) | 0.000 | 53/79 (67.1) | 26/79 (32.9) | 0.000 |
| <15 | 2/3 (66.7) | 1/3 (33.3) | 0.414 | 2/3 (66.7) | 1/3 (33.3) | 0.414 | 79/95 (83.2) | 16/95 (16.8) | 0.000 |
| RMP | |||||||||
| ≥25% | 37/41 (90.2) | 4/41 (9.8) | 0.000 | 48/56 (85.7) | 8/56 (14.3) | 0.000 | 29/34 (85.3) | 5/34 (14.7) | 0.000 |
| <25% | 7/8 (87.5) | 1/8 (12.5) | 0.003 | 19/26 (73.1) | 7/26 (26.9) | 0.001 | 103/140 (73.6) | 37/140 (36.4) | 0.000 |
| Morphology | |||||||||
| ≥4% | 33/36 (91.7) | 3/36 (8.3) | 0.000 | 41/50 (82) | 9/50 (18) | 0.000 | 30/43 (69.8) | 13/43 (30.2) | 0.000 |
| <4% | 11/13 (84.6) | 2/13 (15.4) | 0.000 | 26/32 (81.3) | 6/32 (18.8) | 0.000 | 102/131 | 29/131 | 0.000 |
ART, assisted reproduction technology; IUI, intra-uterine insemination; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; concentration, 106sperm/ml; RMP, rapid progressive motility.
Comparisons between semen parameters and cycles with negative (−) and positive (+) clinical pregnancy (CP) per treatment cycle.
| ART technique | IUI-49 | IVF-82 | ICSI-174 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical pregnancy | CP (−) 44 | CP (+) 5 | p | CP (−) 67 | CP (+) 15 | p | CP (−) 132 | CP (+) 42 | p |
| Semen parameters | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Concentration | |||||||||
| ≥15 | 42/46 (91.3) | 4/46 (8.7) | 0.000 | 65/79 (82.3) | 14/79 (17.7) | 0.000 | 53/79 (67.1) | 26/79 (32.9) | 0.000 |
| [10–15[ | 0/1 (0) | 1/1 (100) | 0.157 | 0 | 0 | 22/25 (88) | 3/25 (12) | 0.000 | |
| [5–10[ | 0 | 0 | - | 0 | 1/1 (100) | 0.157 | 19/24 (79.2) | 5/24 (20.8) | 0.000 |
| [1–5[ | 0 | 0 | - | 1/1 (100) | 0 | 0.157 | 22/25 (88) | 3/25 (12) | 0.000 |
| <1 | 2/2 (100) | 0 | 0.046 | 1/1 (100) | 0 | 0.157 | 16/21 (76.2) | 5/21 (23.8) | 0.001 |
| Motility | |||||||||
| ≥25 | 37/41 (90.2) | 4/41 (9.8) | 0.000 | 48/56 (85.7) | 8/56 (14.3) | 0.000 | 29/34 (85.3) | 5/34 (14.7) | 0.000 |
| [10–25[ | 2/3 (66.7) | 1/3 (33.3) | 0.414 | 11/18 (61.1) | 7/18 (38.9) | 0.182 | 45/65 (69.2) | 20/65 (30.8) | 0.000 |
| [5–10[ | 0 | 0 | - | 5/5 (100) | 0 | 0.002 | 17/24 (70.8) | 7/24 (29.2) | 0.004 |
| [1–5[ | 3/3 (100) | 0 | 0.014 | 2/2 (100) | 0 | 0.046 | 29/35 (82.9) | 6/35 (17.1) | 0.000 |
| <1 | 2/2 (100) | 0 | 0.046 | 1/1 (100) | 0 | 0.157 | 12/16 (75) | 4/16 (25) | 0.005 |
| Morphology | |||||||||
| ≥4 | 33/36 (91.7) | 3/36 (8.3) | 0.000 | 41/50 (82) | 9/50 (18) | 0.000 | 30/43 (69.8) | 13/43 (30.2) | 0.000 |
| [3–4[ | 1/2 (50) | 1/2 (50) | 1.000 | 15/20 (75) | 5/20 (25) | 0.002 | 8/12 (66.7) | 4/12 (33.3) | 0.102 |
| [2–3[ | 5/6 (83.3) | 1/6 (16.7) | 0.021 | 7/7 (100) | 0 | 0.000 | 14/20 (70) | 6/20 (30) | 0.011 |
| [1–2[ | 2/2 (100) | 0 | 0.046 | 2/3 (66.7) | 1/3 (33.3) | 0.414 | 33/41 (80.5) | 8/41 (19.5) | 0.000 |
| <1 | 3/3 (100) | 0 | 0.014 | 2/2 (100) | 0 | 0.046 | 47/58 (81) | 11/58 (19) | 0.000 |
ART, assisted reproductive technology; IUI, intra-uterine insemination; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection.
Limits for IIU:
Concentration: higher CP rate when ≥10×106ml−1 (100%).
4+1 (5/5).
Motility: higher CP rate when ≥10% (100%).
4+1 (5/5).
Morphology: higher CP rate when ≥2% (100%); ≥3% (80%).
3+1+1 (5/5); 3+1 (4/5).
Limits for IVF:
Concentration: higher CP rate when ≥5×106ml−1 (100%); ≥15×106ml−1 (93.3%).
14+1 (15/15); 14/15.
Motility: higher CP rate when ≥10% (100%).
8+7 (15/15).
Morphology: higher CP rate when ≥1% (100%); ≥3% (93.3%).
9+5+1 (15/15); 9+5 (14/15).
Limits for ICSI:
Concentration: higher CP rate when ≥5×106ml−1 (81%).
26+3+5 (34/42)
Motility: higher CP rate when ≥5% (76.2%).
5+20+7 (32/42).
Morphology: higher CP rate when ≥1% (73.8%).
13+4+6+8 (31/42).
Semen analysis is a fundamental tool for the research of male infertility. It enables to establish a diagnosis and a prognosis of the capability to conception, as well as to choose the adequate type of ART treatment.15 In the present analysis most of the patients presented normal semen parameters, with the exception of morphology and rapid progressive motility, which explains the predominance of teratozoospermia, asthenozoospermia and astheno-teratozoospermia.
The causes of infertility are also important determinants for the establishment of a successful pregnancy. Our results showed that there was a predominance of male (55%) to female (45%) factors of infertility in total cycles, as well as in IVF/ICSI cycles (44% male factor, 33% female factor and 23% mixed factors), which is in accordance with previous reports that also showed a predominance of male factor.5,6
Correlation analysis revealed a strong positive linear association between age and total motility, but not with rapid progressive motility, total progressive motility or morphology. Although other authors refer the existence of a decline in semen quality with advancing age (≥45 years),8–11 our group of study comprehended only 18 cases above that age, with only one case with 50 years and 3 with 60 years, which is not relevant to observe age effects. Other strong positive correlations were found between the concentration and the total number of sperm, and between rapid progressive motility and total progressive motility, witch were expected findings as they are dependent variables.27 The moderate correlations found of sperm concentration and the total number of sperm with progressive motility and morphology were also expected findings. The correlations found between progressive motility and vitality confirms the importance of membrane integrity for effective sperm motility.28 It is known that sperm morphology reflects the quality of spermatogenesis and the functional competence of sperm27–31 and in accordance, morphology showed significant correlations with concentration, total sperm number, motility and vitality.
Each type of sperm morphologic abnormalities showed positive linear correlations between them and semen parameters. Abnormalities of the sperm head were associated with midpiece and tail abnormalities, thus reinforcing the intimate structural relationship that exists between them, especially with midpiece.27–31 On the contrary, the positive correlations observed with progressive motility and vitality suggest that head abnormalities are not usually associated with motility and membrane integrity, which is in accordance to previous observations.27–31
Midpiece anomalies (place of the centriole and of the beginning of the axoneme, outer dense fibers and mitochondrial sheath) were positively correlated with abnormalities of the sperm head and tail (place of the axoneme, outer dense fibers, fibrous sheath and glycolysis), further reinforcing the structural relationship between these sperm components.27–31 The absence of correlations with progressive motility, suggest that motility is only affected by specific abnormalities. The association with cytoplasmic droplets is a consequence of defective spermatogenesis,31 whereas the positive correlation with vitality suggests that they are independent variables.
For tail abnormalities the observed positive correlations were with head and midpiece abnormalities, which is an expected finding as they are structurally connected.29,30 Although as expected a negative significant difference was observed between tail abnormalities and progressive motility, this difference did not reach correlation significance. This suggests that only specific tail abnormalities are associated with sperm immotility.29,30
The techniques employed in ART treatments intend to give a solution for couple infertility.2 In the present work we evaluated the influence of semen parameters on the biochemical pregnancy rate after treatment with IUI, IVF and ICSI. Comparisons revealed significant differences between the techniques but not inside each one. The clinical pregnancy rates obtained were similar to those reported for Portugal and Europe, 10.2% in IUI vs. 10.1%,17 21.7% in IVF vs. 35.7%/28.925 and 25.3% for ICSI vs. 28.6%/28.7%,25 respectively. ICSI was the technique most used and the clinical pregnancy rate was just slightly higher but not significant to that obtained with IVF, and both were significantly higher than that obtained by IUI. As expected,4,32 ICSI was associated with the worst evaluated semen parameters.
Comparisons between subgroups of concentration, motility and morphology, against treatment cycles with negative and positive clinical pregnancy, showed that there are thresholds for the achievement of a higher clinical pregnancy rate. For IUI: when spermiogram values presented a sperm concentration of ≥10×106/ml, ≥10% of motility and ≥2% of morphology; for IVF: when sperm concentration was ≥5×106ml−1, ≥10% of motility and ≥1% of morphology; and for ICSI: when sperm concentration was ≥5×106ml−1, ≥5% of motility and ≥1% of morphology.
In conclusion, as we could find a positive correlation between the different degrees of severity regarding sperm concentration, rapid progressive motility and morphology, it was possible to undercover the lower thresholds for each of these semen parameters to achieve a higher clinical pregnancy rate using IUI, IVF or ICSI. These thresholds will help to decide the best technique to use in cases of male infertility.
FundingUMIB is funded by National Funds through FCT-Foundation for Science and Technology, under the Pest-OE/SAU/UI0215/2014.
Conflict of interestThe authors declare to have no conflict of interest.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
UMIB is funded by National Funds through FCT-Foundation for Science and Technology, under the Pest-OE/SAU/UI0215/2014.