In our study, we sought answers to many questions about male infertility from a different perspective. The first step in male infertility is anamnesis, physical examination and sperm count. The European Academy of Andrology recommends examination of genetic causes in individuals with fewer than 5million/ml semen counts. The American Urological Association and American Society for Reproductive Medicine have guidelines recommending performing karyotype and AZF subgroup deletion testing in azoospermia and fewer than 5 million sperm total count. Klinefelter syndrome and Y chromosome microdeletions are still very important in male infertility. Based on patients with Klinefelter syndrome or Y microdeletion, we sought answers to many questions in male infertility.
Materials and methodsIn the presented study 327 male patients with having fewer than 15millionsperm/ml detected in at least two consecutive sperm analysis were examined. Patients were divided into sub-groups according to the presence of semen count, chromosomal anomaly and Y microdeletion. In addition, FSH, LH and testosterone levels were analyzed.
ResultsNumerical chromosomal anomalies were observed in 34 (10.4%) of 327 patients, and all of these anomalies were found as 47, XXY. Individuals with no AZF microdeletion constituted 95.1% (n=311) of the study group. The overall frequency of AZF microdeletions was 4.9% (16/327). No AZF microdeletions were detected for the patients who have sperm counts above 2million/ml. FSH, LH and testosterone levels were found significantly different between the groups.
DiscussionThe results of our study provide another layer of evidence to demonstrate the controversial threshold value of the EAA. In light of our data and current literature, we recommend to set the threshold value at 2million/ml for semen analysis. Further studies conducted in different ethnic groups and larger patient groups would contribute to clarify what exact value should be used to apply genetic tests.
En nuestro estudio, buscamos respuestas a muchas preguntas relativas a la infertilidad masculina, desde una perspectiva diferente. El primer paso en la infertilidad masculina es la anamnesis, el examen físico y el recuento seminal. La Academia Europea de Andrología recomienda el examen de las causas genéticas en individuos con menos de 5millones/ml de recuento seminal. La Asociación Americana de Urología y la Sociedad Americana de Medicina Reproductiva cuentan con directrices que recomiendan la realización de pruebas de cariotipos y deleción del subgrupo AZF en los casos de azoospermia y un recuento seminal total inferior a 5millones/ml. El síndrome de Klinefelter y las micro-deleciones del cromosoma Y siguen siendo muy importantes en la infertilidad masculina. Basándonos en los pacientes con síndrome de Klinefelter o micro-deleción del cromosoma Y, buscamos respuestas a muchas cuestiones de la infertilidad masculina.
Materiales y métodosEn el presente estudio examinamos 327 varones con valores inferiores a 15 millones de esperma/ml detectados en al menos 2 análisis seminales consecutivos. Dividimos a los pacientes en subgrupos con arreglo a la presencia de recuento seminal, anomalía cromosómica y micro-deleción del cromosoma Y. Además, analizamos los niveles de FSH, LH y testosterona.
ResultadosSe observaron anomalías cromosómicas numéricas en 34 (10,4%) de los 327 pacientes, encontrándose dichas anomalías como 47, XXY. Los individuos sin micro-deleción AZF constituyeron el 95,1% (n=311) del grupo de estudio. La frecuencia general de las micro-deleciones AZF fue del 4,9% (16/327). No se detectaron micro-deleciones AZF para los pacientes con recuentos seminales superiores a 2millones/ml. Los niveles de FSH, LH y testosterona fueron significativamente diferentes entre los grupos.
DiscusiónLos resultados de nuestro estudio aportan otra evidencia para demostrar el controvertido valor umbral de EAA. A la luz de nuestros datos y de la literatura actual, recomendamos establecer el valor umbral en 2millones/ml para el análisis seminal. Los futuros estudios a realizar en diferentes grupos étnicos y muestras de mayor tamaño de pacientes contribuirían a clarificar qué valor exacto debería utilizarse para solicitar pruebas genéticas.
Infertility, a reproductive system disease, is defined as the failure to obtain clinical pregnancy despite 1 year or more of unprotected regular coitus.1 It is a global health problem affecting more than 50 million couples in the world. Female factors account for about the 50% of infertility problems. 25% of the cases is associated to male factors and both male and female factors contribute to the remaining 25%. The causes of infertility are quite diverse, including: endocrine dysfunction, gametogenesis problems, infections, cancer, anatomical reasons and psychological factors. Nevertheless, approximately half of infertility cases cannot be diagnosed clearly. The most important etiological factor for the undiagnosed patient group is thought to be the genetic alterations. Since the mid-20th century, genetic changes have been known to have a role in the etiology of infertility, and, recent studies continued to identify new genetic factors which is an indication of sophisticated notion of infertility.2,3 Male infertility has come to the fore due to ease of working on sperm cells. In the last decade, many genes on autosomal and gonosomal chromosomes, particularly spermatogenic failure genes [Testis Expressed 11 (TEX11), Ubiquitin Specific Peptidase 9 Y-Linked (USP9Y) and Cation Channel Sperm Associated 1 (CATSPER1)] have been associated with male infertility.4–6 However, these newly identified changes were detected in a limited number of patients and it is currently not well ingrained in the clinical algorithm. Klinefelter syndrome (KS) defined in 1942 and azoospermia factor (AZF) deletions as defined in 1976 are still the most important genetic causes of male infertility.2,7
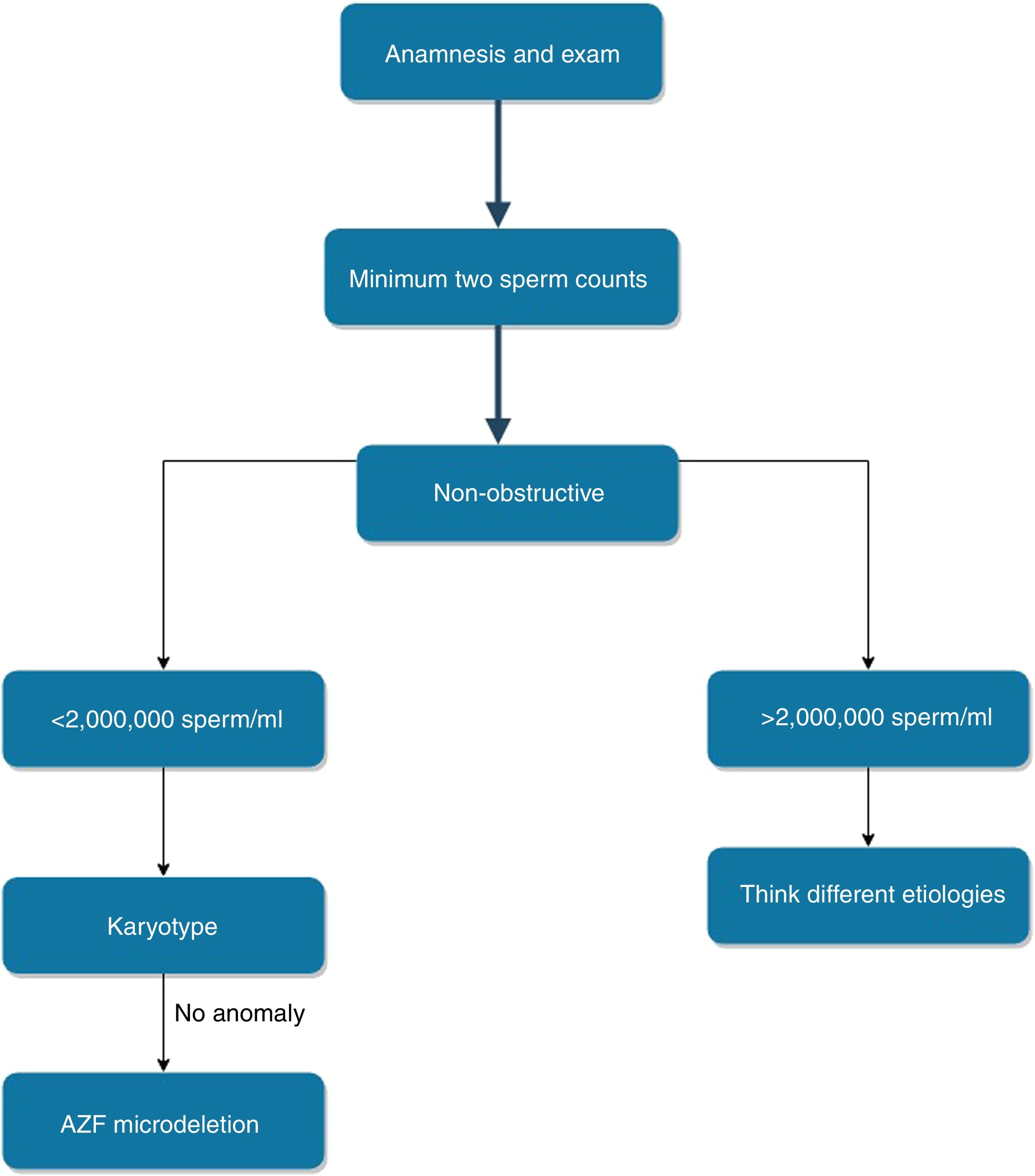
The first step in the investigation of male infertility should always be history, detailed physical examination and hormonal profiling. The test to be performed after this stage should be the spermiogram examined at least twice at different times.2 Semen analysis is the most valuable and collimator test for the study of genetic causes in male infertility. Less than 15 million spermatozoa per milliliter (ml) is defined as oligozoospermia, and severe oligozoospermia term is used for the cases where less than 5 million spermatozoa is counted per ml in semen. The term azoospermia is characterized by the absence of any sperm in semen analysis.8
The most widespread genetic reason for male infertility (especially azoospermia) is KS, which accounts for approximately 10–15% of azoospermia and severe oligozoospermia patients. Its prevalence is 1/500–1000 in the general population. Although tall stature, gynecomastia, increased follicle stimulating hormone (FSH) levels and small testicles are observed, these individuals are usually diagnosed in infertility clinics due to azoospermia.7 Y chromosome microdeletions are the most common molecular genetics reason and second most widespread cause of the genetic problem in male infertility.9,10 Y chromosome microdeletions occur in 1/4000 in the general population.11 AZF region deletions in the long arm of the Y chromosome (Yq11.23) have been associated with severe oligospermia and azoospermia and they are not found in men with normative spermiogram. The AZF region contains many critical genes for spermatogenesis, which are organized in three separate loci (AZFa, AZFb and AZFc). Each of these loci may be deleted individually or in combination. Different numerical and structural sperm anomalies are observed depending on the size and position of the deleted fragment.12,13 Many studies so far have demonstrated that AZF region deletions were observed in 5–10% of severe oligospermia cases and this rate increased to 10–18% in non-obstructive azoospermia group.2,14
In this study, we aimed to have a closer look to male infertility to address many essential questions which might have a clinical impact. The answers we were searching for were basically to whom, when, with which markers and, in what order. Complementary to finding answers to addressed questions we also aimed to offer new parameters. In addition to the laboratory and clinical part of the research, we wanted to touch on the cost aspect of the event.
Materials and methodsIn this retrospective cohort study, we aimed to investigate the genetic causes of infertility in more than 300 male individuals. The participants with following evidence and symptoms were not included in the study; patients with diagnosed malignancy, who had undergone urogenital surgery, individuals using medications that can change hormone values, cancer patients receiving chemo/radiotherapy and men with obstructive azoospermia. All patients were from Sanliurfa province in the east of Turkey. Research and ethics commission ratification is not needed to undertake a retrospective service assessment and this study was conducted in eligibility to the ‘Declaration of Helsinki 2013’. In total, 327 men with less than 15 million spermatozoa per ml in consecutive two spermiogram were investigated between January 2017 and December 2018, who was admitted to our hospital urology department due to infertility. Semen samples were collected after a minimum 3 maximum 5 days of sexual abstinence. All samples studied according to WHO guidelines. Patients were divided into two groups according to be the azoospermic (group A) and oligospermia (group O) in at least 2 semen analysis. In another respect, the patients were divided into five groups according to the number of spermatozoa in the ml detected in at least 2 semen analysis (group 1: azoospermia, group 2: 0–1 million spermatozoa in the ml, group 3: 1–2 million spermatozoa in the ml, group 4: 2–5 million spermatozoa in the ml, group 5: 5–15 million spermatozoa in the ml).
For chromosomal analysis, metaphases obtained from 72h of classical peripheral blood culture were examined by trypsin-Giemsa banding (GTG) and karyotype of patients was determined. A minimum of 20 metaphase fields with a minimum resolution of 550 bands per set was analyzed in each patient. For detection of AZF deletion(s), peripheral blood DNA was isolated using Magpurix Blood DNA Extraction kit 200 (Zinexts LSC, New Taipei City, Taiwan [R.O.C.]) according to the manufacturer's report. After the isolation of peripheral blood DNA, Y chromosome loci AZFa (sY84, sY86), AZFb (sY127, sY134) and AZFc (sY254, sY255) were amplified by multiplex polymerase chain reaction (PCR) of specific sequence tagged site (STS) markers using with BIO-RAD T100 Thermal Cycler (Bio-Rad Laboratories, CA, USA) specific primers using Devyser AZF kit (Devyser AB, Hagersten, Sweden). The SRY (sY14) and ZFX/ZFY were used as inner positive controls. Female sample, fertile male sample and sterile water were used as external control: negative, positive and blank controls, respectively. Results were scanned and analyzed with ABI Genetic Analyzers ABI 3500 and GeneMapper Software 5 (Life Technologies/Thermo Fisher Scientific, CA, USA) which support the detection of the set. If any deletion was not detected, the deletion of AZF region(s) was considered no complete deletion. If the deletion was detected in one marker, it was re-evaluated according to the European Academy of Andrology/European Molecular Genetics Quality Network (EAA/EMQN) guideline. If the deletion is detected in two consecutive markers, using with AZFa (sY82, sY83 for proximal region; sY1065, sY88 for distal region), AZFb (sY105, sY121 for proximal region; sY1192, sY153 for distal region), AZFc (sY160 for heterochromatin marker), it was evaluated that the deletion was complete or not complete with the Devyser AZF Extension kit (Devyser AB, Hagersten, Sweden). Patients with AZF deletion were not grouped as a/b/c because of possible problems in statistical analysis. Patients were divided into three groups according to chromosome analysis and AZF microdeletions results; XY with no AZF deletion group (N), XY with AZF deletion group (group D) and XXY group (group K), due to no other chromosomal anomaly is observed. Peripheral blood was taken from patients for FSH, LH and testosterone measurement before breakfast. All hormonal tests were examined with Immulite kits (Cruinn DL, Dublin, Ireland) according to the manufacturer's directives and the reference value range of our laboratory was used (FSH: 1.4–18.1IU/L; LH: 1.5–9.3IU/L; testosterone: 241–827ng/dl). Statistical data were analyzed using SPSS software version 25.0 (IBM SPSS, Chicago, IL, USA) program. Continuous variables are presented as mean±standard deviation and (min–max). The normality of the dispersion curves was evaluated using the Shapiro–Wilk and Kolmogorov–Smirnov test. Since the normal distribution was not observed after the evaluation, nonparametric tests were used (Kruskal–Wallis H test for multiple comparisons, Mann–Whitney U test for intragroup comparison). Categorical variables are submitted as percentages. The differences were examined using Fisher's exact test. For all tests p<0.05 was considered as significant.
ResultsThe study population comprised of 327 patients with oligospermia (group O) (n=107, 32.7%) and azoospermia (group A) (n=220, 66.3%) of whom 34 had KS (group K) (10.4%), 16 had AZF microdeletion with XY karyotype (group D) (4.9%), and 277 were had XY karyotype and no deletion at AZF region (group N) (84.7%). The mean age was 30.62±6.45 years group K, 30.44±6.67 years for group D, and 30.57±7.49 years for group N. There was no significant difference between the groups for age (p=0.96). When patients were grouped according to sperm count; in group 1–5, there were 220 (67.3%), 43 (13.1%), 8 (2.4%), 17 (5.2%) and 39 (11.9%) patients, respectively. There was no significant age difference in the patients in these five groups (30.6±7.4, 31.7±7.6, 31±8.1, 29.1±6.4 and 29.7±6.6, respectively) (p=0.88).
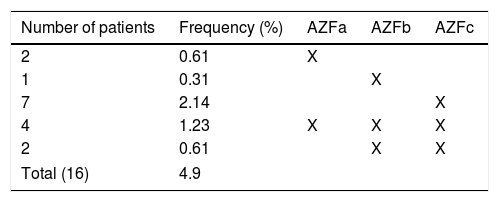
Individuals with no AZF microdeletion constituted 95.1% (n=311) of the study group. The overall frequency of AZF microdeletions was 4.9% (16/327). This frequency was 6.8% (15/220) in the azoospermic group and 0.94% (1/107) in the non-azoospermic group. No AZF microdeletions were detected in cases with sperm counts above 2million/ml. We identified the following microdeletions: AZFa: 2 patients (12.5%), AZFb: 1 patient (6.25%), AZFc: 7 patients (43.75%), AZFa+b+c: 4 patients (25%), and AZFb+c: 2 patients (12.5%) (Table 1).
There were no numerical and structural chromosome abnormalities noted in men with AZF microdeletions.
No structural anomaly was detected in any patient in the chromosomal analysis. No chromosomal abnormalities were detected in 293 (89.6%) of the 327 patients examined.
Numerical chromosomal anomalies were observed in 34 (10.4%) of 327 patients, and all of these anomalies were non-mosaic 47, XXY. All these 34 patients were azoospermic. Six of the 34 azoospermic KS patients underwent micro testicular sperm extraction (m-TESE), and spermatozoa were recovered from two of these individuals. The mean age of these two m-TESE positive patients was 22.5 years, and the mean age of the four m-TESE negative patients was 29.3 years. Only 5 of our patients with AZF deletion were preferred m-TESE. All of these patients had AZFc deletion. The successful result was obtained in 3 of these patients.
The mean FSH and LH levels in group K: 40.2±12.5IU/L (19–68IU/L), 22.1±9.8IU/L (6–48IU/L); it was decidedly lower in group D: 18.7±7.4IU/L (3–32IU/L), 8.8±3.3IU/L (4–16IU/L) and group N: 14.1±15.8IU/L (0–129IU/L), 6.9±7.1IU/L (0–58IU/L), respectively (p<0.001). When we look at the FSH and LH levels between the groups K and D, we saw statistically significant differences (p<0.001 and p<0.001, respectively). When we look at the FSH and LH levels between the groups K and N, we saw statistically significant differences (p<0.001 and p<0.001, respectively). When we look at the FSH and LH levels between the groups D and N, we saw statistically significant differences (p=0.006 and p=0.002, respectively). The mean testosterone level in group K: 226.9±145.7ng/dl (0.04–626ng/dl); it was significantly higher in group D: 350.7±201.1ng/dl (4–726ng/dl) and group N: 353.8±220.3ng/dl (0.6–1500ng/dl) (p=0.001). When we look at the testosterone levels between the groups K and D, we saw a statistically significant difference (p=0.029). When we look at the testosterone levels between the groups K and N, we saw a statistically significant difference (p<0.001). When we look at the testosterone level between the groups D and N, we saw no statistically significant differences (p=0.943).
Mean FSH levels in five groups (group 1–5) were: 21.4±18.8IU/L (0–129IU/L), 9.1±7.1IU/L (1–32IU/L), 8.6±9.1IU/L (1–24IU/L), 7.4±6.8IU/L (2–25IU/L) and 7.1±6.5IU/L (1–33IU/L), respectively. Mean FSH levels in the azoospermia group (group 1) were significantly increased compared to the other groups (p<0.001). There was no significant difference between the other groups in terms of mean FSH levels (p=0.73). Mean LH levels in five groups were: 10.4±9.8IU/L (0–58IU/L), 4.8±2.8IU/L (0–14IU/L), 6.5±3.7IU/L (2–12IU/L), 4.3±3IU/L (1–11IU/L) and 4.7±3.1IU/L (1–13IU/L), respectively. Mean LH levels in the azoospermia group (group 1) were significantly increased compared to the other groups (p<0.001). There was no significant difference between the other groups in terms of mean LH levels (p=0.26). Mean testosterone levels in five groups were: 317.6±204.1ng/dl (0.04–1500ng/dl), 406±233.4ng/dl (144–1493ng/dl), 390±242.7ng/dl (220–925ng/dl), 384.2±243.6ng/dl (164–1070ng/dl) and 366.6±231.8ng/dl (2.9–1133ng/dl), respectively. Mean testosterone levels in the azoospermia group (group 1) were significantly increased compared to the other groups (p=0.013). There was no significant difference between the other groups in terms of mean testosterone levels (p=0.37).
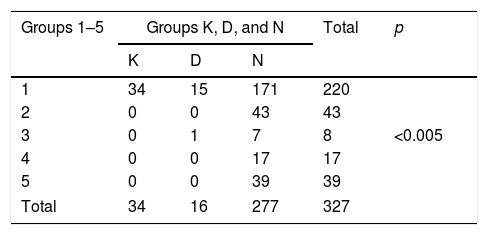
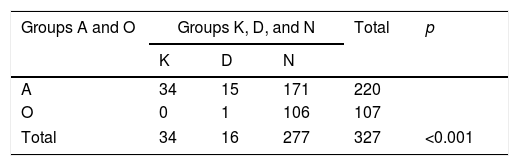
When we look at the anomalies detected according to the sperm count of patients; of the 220 azoospermic patients in group 1, 49 (22.3%; 15.5% for KS, 6.8% for AZF microdeletion) had anomalies. Of these 49 patients with anomalies, 34 (69.4%) were observed to have KS, and 15 (30.6%) have AZF microdeletion. In 107 patients with oligospermia, only 1 (0.94%) anomaly was observed and this was AZFc deletion. This abnormality was observed in a patient in group 3. This situation was statistically significant (p<0.001) (Table 2). This statistical significance continued in the same way when we grouped patients as azoospermic (group A) and oligospermic (group O) (p<0.001) (Table 3).
The anomalies observed when patients are grouped according to sperm count.
| Groups 1–5 | Groups K, D, and N | Total | p | ||
|---|---|---|---|---|---|
| K | D | N | |||
| 1 | 34 | 15 | 171 | 220 | |
| 2 | 0 | 0 | 43 | 43 | |
| 3 | 0 | 1 | 7 | 8 | <0.005 |
| 4 | 0 | 0 | 17 | 17 | |
| 5 | 0 | 0 | 39 | 39 | |
| Total | 34 | 16 | 277 | 327 | |
Group K: XXY patients’ group.
Group D: XY patients with AZF deletion group.
Group N: XY patients with no AZF deletion group.
Group 1: Patients with azoospermia.
Group 2: Patients with 0–1 million spermatozoa in the ml.
Group 3: Patients with 1–2 million spermatozoa in the ml.
Group 4: Patients with 2–5 million spermatozoa in the ml.
Group 5: Patients with 5–15 million spermatozoa in the ml.
The anomalies observed when patients are grouped according to azoospermia and oligospermia.
| Groups A and O | Groups K, D, and N | Total | p | ||
|---|---|---|---|---|---|
| K | D | N | |||
| A | 34 | 15 | 171 | 220 | |
| O | 0 | 1 | 106 | 107 | |
| Total | 34 | 16 | 277 | 327 | <0.001 |
Group K: XXY patients’ group.
Group D: XY patients with AZF deletion group.
Group N: XY patients with no AZF deletion group.
Group A: Patients with azoospermia.
Group O: Patients with oligospermia.
In the existing study, we examined AZF deletion and chromosomal abnormalities frequencies in 327 men with less than 15 million spermatozoa per ml in consecutive two spermiogram, and its association with reproductive hormone parameters and sperm counts.
KS is the most common genetic condition of male hypogonadism, but the diagnosis is usually made after infertility. Almost all individuals with KS are azoospermic. In a study on this subject, 118 subjects with KS were examined and 114 patients showed azoospermia. The remaining four patients had severe oligospermia or cryptozoospermia. All four patients were under the age of 24 years. All of our patients are azoospermic. The average age of our KS patients is around 30 and we believe that this situation explains observed azoospermia in all our patients. In another respect, in a study conducted in individuals with KS, mean age of TESE-positive patients was 17.3 years and mean age of TESE-negative patients was 26.6.7 This condition was also observed in our patients (22.5 vs 29.3 years). These two findings are probably related to progressive testicular failure. The importance of early diagnosis and directing to early TESE procedure is evident in patients with KS.
Among all the patients we evaluated in our study, the rate of KS was 10.4% and in the azoospermia group, this rate increased to 15.5%. The rate of KS detection in infertile patients is 7–13% in the literature, and our study is consistent with the data.15 The incidence of chromosomal abnormality in the normal population is 0.6%, while in azoospermia this rate is 15%.2 The rate we found in our study was consistent with the literature. Interestingly, in our study, all of the chromosomal anomalies found were 47, XXY. In a study including 118 patients with KS, only 1 (0.8%) patient had AZF deletion which was AZFc.7 Simoni et al.16 identifying only one AZF deletion in 208 patients with KS. In a similar study, 77 patients with KS were examined and no AZF deletion was observed.17 In the study conducted in China, no AZF deletion was observed in 80 individuals with KS.18 In different ethnic groups, the AZF deletion rate did not exceed 1% in any study in which more than 20 KS subjects were studied.7 This situation was also confirmed by our study. Therefore, we do not consider AZF deletion screening as appropriate for individuals with KS diagnosis. Again, with the same conclusion, we do not find it appropriate to request simultaneous AZF deletion screening and chromosome analysis.
Since AZF region contains many genes for successful spermatogenesis, it is a major factor for male fertility. Among all the patients we evaluated in this study, the rate of AZF deletion was 4.9% and in the azoospermia group, this rate increased to 6.8%. The rate of AZF detection in infertile patients is 1.3–10.7% in the literature, and our study is consistent with the data.7,13,19 The main reasons for such a wide percentile spectrum are: ethnicity, patient selection and the used method. In this study, AZFc deletion was the most frequent (43.8%), followed by AZFa+b+c (25%), AZFb+c (12.5%), AZFa (12.5%) and AZFb (6.25%) deletions. The most common AZF deletion in our study was AZFc which is in a harmony with most of the published studies.13,15,19–21 The main reason for this condition is the plentiful non-allelic homologous recombination (NAHR) regions in the AZFc region. The presence of these NAHR areas makes the AZF region dynamic in terms of losses and gains.22 The second most frequent AZF deletion is regionally variable.20,23–25 According to another study conducted in a close geographical location to us, the second most common AZF deletion was observed as AZFa+b+c.13 AZFa+b and AZFa+c are the least observed AZF deletions in studies conducted so far.21 In accordance with the literature, we have not encountered with these deletions in our study. There is no definite information on why AZFc is the most common AZF deletion, but repetitive sequences in this region may be the cause. Latest published literature shows that patients with azoospermia and oligospermia are potential m-TESE candidates. However, in people with AZFa, AZFa+b+c, AZFb and AZFb+c deletion, the chances of obtaining sperm are very close to zero. Those with other AZF deletions are more likely to obtain sperm with m-TESE.2,22,26 The m-TESE procedure was performed in patients with only AZFc deletion in our patient group. Our success rate in this patient group was 60%. This ratio seems to be consistent with 50–60% literature but it should be kept in mind that the number of our patients is low.26 We could not detect any other AZF deletions in our study.
On the other hand, the threshold to be used to scan AZF deletions is the biggest question in AZF deletions today. In our study, just one anomaly, AZFc, was observed in the 107 oligospermic patients. This patient had a sperm count of 1–2 million spermatozoa in the ml of semen. Of the 220 patients with azoospermia, 49 (22.3%; 15.5% for KS, 6.8% for AZF microdeletion) anomalies were detected. EAA recommends that all men with a sperm count of <5,000,000/ml should be examined for AZF deletions.11 The majority of individuals with AZF microdeletion are azoospermic, and only in very rare individuals have a sperm count of >2,000,000/ml as in our study. Lo Giacco et al.27 reviewed 12 separate studies in different ethnic groups (n=7870 individuals) and identified a total of 196 patient with AZF microdeletion. They found that 3 men had a sperm count of between 2 and 5million/ml. Of the 16 AZF deletions we detected in our study, 15 (93.75%) were observed in azoospermic individuals. A remaining patient had a sperm count of <2,000,000/ml. If we set the threshold value of our study to 5 or 2 million, instead of 15 million, we would not have a loss of sensitivity. However, we would have achieved a significant increase in specificity. At the same time, a significant amount of expenditure is being reduced (AZF deletion costs $250 in the UK, $70 in Turkey). Although some publications state that the threshold value should be lower than 0.5million/ml, it is clear that the sensitivity ratio will decrease in such a case. But, the results of our study and many recent studies clearly show that the threshold value of the EAA is controversial. In light of our study data and literature, it is our opinion that the threshold value should be set at 2million/ml (Fig. 1). We estimate that studies conducted in different ethnic groups and larger patient groups will support this opinion.
FSH, LH and testosterone are important parameters for male infertility. These parameters should always be considered to illuminate different etiologies such as central hypogonadism.20 In addition, the question often asked is whether these parameters can be used to elucidate the different etiologies of male sterility. Based on our data, there was a significant difference between the group K, group D and group N in terms of FSH and LH levels. This condition may be related to the inadequate response of Sertoli and Leydig cells. When we look at testosterone levels; no significant difference between group N and group D was found. However, a significant difference between group K with N and D groups was detected. While some published studies are aligned well with our, according to some other papers no statistical difference in terms of these hormone values between the groups was ovserved.20,28 This may be due to ethnic differences, the group of studied patients and the followed method. When we analyzed the hormone values as the difference between the groups (group 1–5), our observations can be summarized as: there were statistically significant differences in FSH, LH and testosterone levels between group 1 and other groups but no difference was observed between the other groups in terms of hormone values when excluding group 1. This result is similar to the results of another study conducted in our region.19 It is well known that FSH, LH or testosterone levels cannot be used as a prognostic marker in these patients. Because the values in all groups have a very wide distribution and this range is observed as intertwined. Again, due to the observation of the same distribution width and intertwined this situation also applies when the patients are evaluated as K, N and D groups.
The missing part of our study are not evaluating testicular volume in any patient group and not comparing AZF subgroup deletions with each other. The reason for this is that the testicular volume measurement is subjective and AZF subgroup deletion numbers are not statistically significant. On the other hand, we did not use a normospermic control group in our study because of the lack of AZF deletion in normospermic individuals in the large series data up to date and our aim was to focus on the difference between the infertility groups that we formed.2,7 It has been shown in many studies to date that identification of genetic conditions can help diagnose male infertility. On the other hand, it should be remembered that the possible effects of genetic problems on men's general health may affect male infertility.29–31 Next-generation sequencing and epigenetic analysis may open new horizons for male infertility detection, but confirmation studies are one of the most important obstacles, but it should be kept in mind that these studies have a serious economic burden, and this amount will increase with advances in genetics.32
ConclusionIn conclusion, an extensive data of patient group such as sperm count, hormone values, chromosomal anomaly results, AZF deletion types and frequency, was collected by this study. We integrated current literature findings with our data. Answers for the questions of to whom, which threshold, when and in what way were provided by this research. We believe that the data we present here would bring a new perspective to male infertility. We would like to note that these data should be supported by further studies covering different ethnic groups and more patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestNone.