Around 15% of productive couples in the world are infertile. Recent years, biochemical mechanisms leads to male infertility are started to research. Redox regulation and oxidative stress (OS) show importance in the pathogenesis of infertility in male. Malondialdehyde (MDA) and Glutathione (GSH) are biochemical indicatives of sperm damage by reactive oxygen species (ROS). In addition, sperm are coated with a thick glycocalyx rich in sialic acids. It is aimed to determine and evaluate the differences between normozoospermic and oligozoospermic individuals according to sialic acid, MDA and GSH concentrations and correlations between spermyogram and these parameters.
Material and methodsThis study was carried out on seminal plasma of individuals who admitted to Selcuk University Faculty of Medicine IVF Unit Andrology Laboratory. The groups were divided into two as normozoospermics (n=30, sperm concentration≥15million/mL), and oligozoospermics (n=30, sperm concentration<15million/mL). Spermyogram were evaluated regarding WHO (2010) Kruger criteria. GSH, MDA and sialic acid concentrations were analyzed in seminal plasma. Diagnostic performance of sialic acid has been determined with ROC curve analysis.
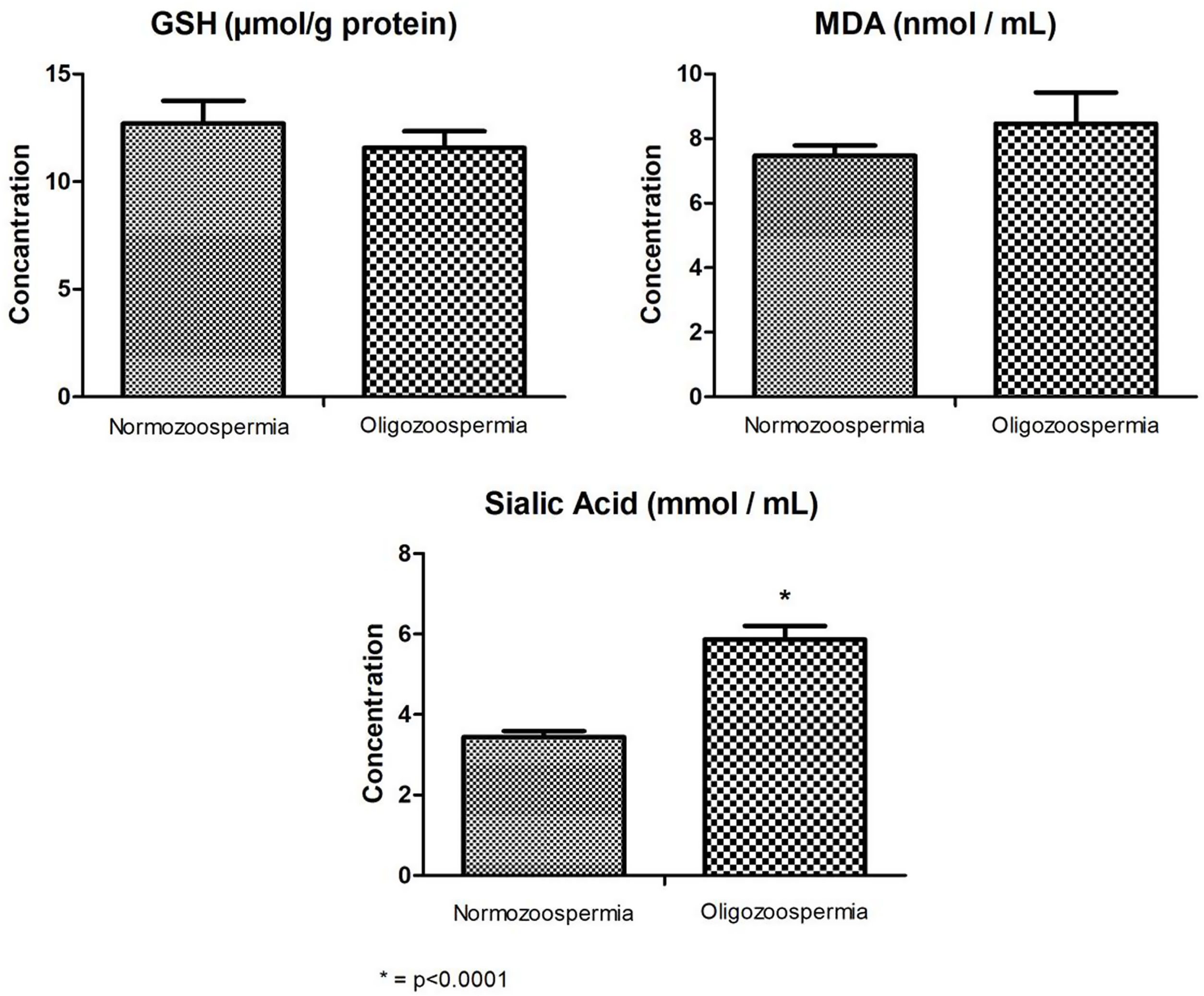
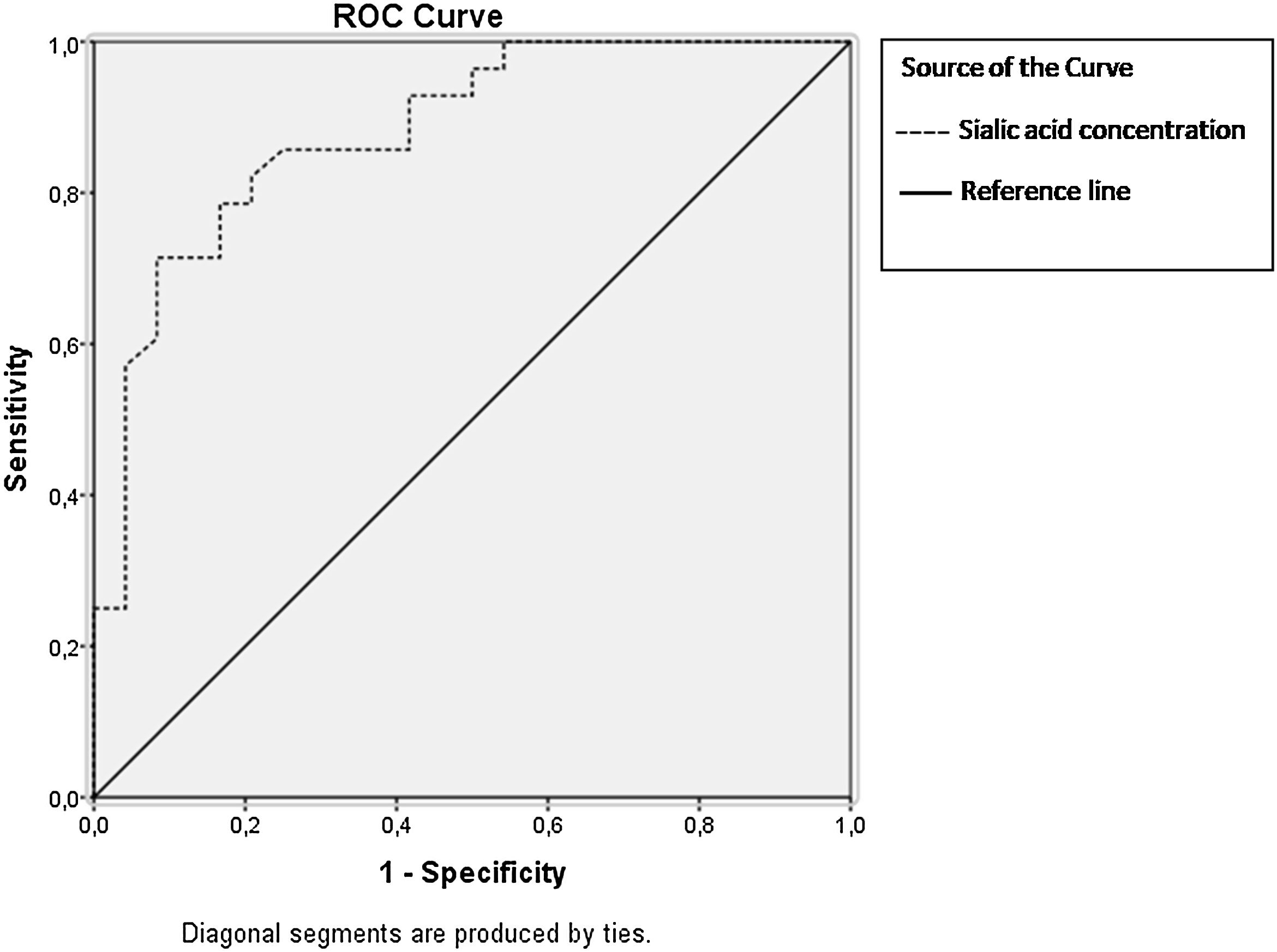
ResultsSialic acid levels were significantly lower in Normozoospermic than Oligozoospermic individuals (p<0.0001), MDA and GSH levels were not differ significantly in both groups (p>0.05). Sialic acid correlated significantly with most of the spermyogram findings. When diagnostic performance of sialic acid was evaluated, the cut off value of sialic acid found as 4.175nmol/mL by ROC curve.
ConclusionHigh seminal plasma sialic acid levels may be used as a biomarker and sialic acid is important determinant in oligozoospermia.
Infertility is defined as a couple of reproductive age does not develop pregnancy despite at least one year of regular sexual intercourse without using any contraceptive method.1 Today, approximately 15% of couples have infertility problems, and half of this problem is based on male fertility.2 The important role of oxidative stress (OS) in infertility pathogenesis in male has been demonstrated in many studies.3 Although basal level of reactive oxygen species (ROS) formation is required in order to perform male reproductive function, excessive increase of this formation causes negative effects on sperm motility and male fertility.4 Processing in sperm capacitation, hyperactivation, acrosomal reaction and sperm ovule fertilization depend on the low amount of ROS. High amounts of ROS neutralize antioxidants, which are protective factors in seminal plasma, and leads to OS.1 It has been reported that ROS occurs in sperm cells of 30–80% of infertilite male, and damages mitochondria and DNA.5 The reason why sperm cells are very sensitive to ROS is that; plasma membranes are rich in poly unsaturated fatty acids (PUFA) which is easily oxidized.4 It is known that there is a significant negative correlation between ROS production and semen quality.6 The final product of lipid peroxidation, which occurs in the formation of oxidative stress, MDA, denatures also the proteins and contributes to the formation of proinflammatory mediators such as cytokines.7 Biochemically, MDA is an indicator of the lipids which are damaged by ROS in spermatozoa. In dealing with overproduced ROS, the role of the antioxidant system in living organism is important, and GSH is included in these regulation.
GSH is an important anti-oxidantants that plays a role in the cell's response to oxidative stress. It is abundant in natural sperm cells and plasma.1 GSH is important in the modulation of intracellular ROS both in terms of protecting the cell against the harmful effects of oxidative stress and facilitating the intracellular signal transmission of ROS.8 GSH participates in many metabolic processes, such as biosynthesis of leukotriene and prostaglandins.9
The gangliosides that cover the outer surface of all mammalian cell membranes, including spermatozoa, are glycosphingolipids and contain Sias in their structure.10 Sialic acid are a subset of nine-carbonalpha-ketoaldonic acids that are involved in various biological functions.11 Sperm is covered with a thick (∼70nm) layer of glycocalyx rich in sialic acid.12 Even a slight change in the amount of glycocalyx has a major effect on sperm fertility.13 The maturity and integrity of the sperm glycocalyx, which plays an important role in sperm protection, is closely related to fertilization.14 Mammalian cells have a wide variety of sialic acid types.15 There are over 50 different forms of Sias in nature, but the two most common sialic acid found in mammals are N-acetylneuraminic acid (Neu5Ac) and Nglycolylneuraminic acid (Neu5Gc).16
The presence of sialic acid in human semen has a particular importance. Two important enzymes that play roles in sialic acid metabolism; Sialyltransferase and Sialidase.15 Sialyltransferase enzyme plays a role in the binding of sialic acid to carbohydrate groups.17 Sias plays an important role in cell–cell interaction and cell signaling. They are also thought to be important in the control of the immune system and in the formation of inhibitory self-signals.18 Sperm sialoma refers to the sum of sialic acid located on sperm. The sialoma of each mature sperm cell consists of millions sialic acid molecules and, it is obtained by during spermatogenesis, epididymal maturation and ejaculation; including seminal fluid components into the sperm membrane.19 These results demonstrated the presence of NeuNAc alpha (2,6) Gal/GalNAc glycoconjugate sequences on the plasma membrane of the motile human spermatozoon.20 In addition to, presence of two sialidases (neuraminidases Neu1 and Neu3) on mammalian sperm are shed during capacitation. Inhibiting sialidase activity interferes with sperm binding to the zona pellucida of the ovum. Loss of sialic acid from sperm may also unmask key receptors for sialylated ligands on the egg zona pellucida.19
The biological and molecular mechanisms involved in fertilization are not fully known and studies have contrast findings. Seen from this aspect, we aimed to identify the relationship between sialic acid, MDA, GSH levels and macroscopic, microscopic analysis of sperm is considered in the present study. Thus, it is tried to determine the role of this biochemical analyze in fertilization and whether they are related to various sperm anomalies. Also, we evaluated the diagnositic performance of sialic acid with statistical analyses, the Receiver Operating Characteristic curve (ROC) curve.
Materials and methodsThe present study was conducted with permision of Ethics Committee of Selcuk University with the number of 2019/259. All the volunteers signed and approved the “Informed Patient Consent Form”. Semen samples were taken from individuals who admitted to Selcuk University Faculty of Medicine IVF Unit Andrology Laboratory.
Collection and storage of sperm samples and evaluation of spermiogramAfter a ban on coitus lasting between 2 and 6 days, the sperm sample obtained by hospital masturbation method was collected in a special sterile plastic container. Samples were started to be evaluated for liquefaction after being kept in an incubator at 37°C for 20min. If liquefaction did not occur within 20min, an additional 15min was left in the incubator. Semen parameters such as sperm concentration, total number, total motility, progressive motility and normal morphology in semen samples were evaluated according to WHO (2010) criteria (semen volume 1.5mL; sperm concentration 15 million/mL; total sperm count 39 million; total sperm motility 40%; progressive sperm motility 32% A±B; and sperm morphology 4% Kruger criteria) and the results of those were recorded. The percentages of normal and abnormal spermatozoa forms were evaluated by scoring of at least 100 spermatozoa per preparation Sperm morphologic anomalies including head, neck-middle piece and tail abnormalities were evaluated.
The remaining semen samples, identified as bioawaste, were taken into falcon tubes and centrifuged at 1000rpm for 20min. After centrifugation, the supernatant portions of the samples defined as seminal plasma and were added to the eppendorf tubes. Eppendorf tubes were kept at −80°C until analysis. MDA, GSH and sialic acid analysis were determined in seminal plasma samples.
SubjectsIndividuals who were treated with any medication, chronic illness, azospermic, alcohol consumers, and smokers were excluded from the study. The study was conducted on 60 male individuals between the ages of 23–51 years old. Male (n=30) who are 31.17±5.02 (Mean±SD) and have sperm concentration≥15 million/mL has been defined as normozoospermia group. Individuals with Sperm concentration<15 million/mL and with age of 32.97±7.01 years old (Mean±SD) has been defined as oligozoospermia group.
Biochemical analysisMDA (nmol/mL), GSH (μmol/g protein) and sialic acid (mmol/mL) analysis were determined in seminal plasma by Mihara and Uchiyama method,21 Ellman method22 and Warren method,23 respectively. Colorimetric methods were used for all analyzing. MDA, GSH and sialic acid and protein levels were analyzed at Selcuk University Biochemistry and Physiology Research Laboratories. BMG LABTECH (Germany) device was used. Seminal protein levels were determined by BioRad colorimetric kit (cat no: 500-0002, BioRad Laboratory Inc., USA). The protein concentrations were indicated as g/mL. GSH values calculated as μg/g protein; corresponding value of the total protein concentrations (g/mL) divided to the each value of GSH (μmol/mL) in samples of seminal plasma. Therefore, GSH values determined in per g protein regarding the total protein values and expressed as GSH (μmol/g protein) in table and figure.
Statistical analysisStatistical analyses were performed using the SPSS program (version 21.0). The results were described mean±SE. Due to normal distribution of MDA and sialic acid levels, Independent-T test was applied to compare differences between two independent groups. Pearson correlations were used to assess the relationships between MDA, sialic acid and semen parameters. Due to not normally distribution of GSH levels, Mann–Whitney U test was used to compare. Spearman correlations were used to evaluate the relationships between GSH and semen parameters. Outcomes were statistically evaluated at 0.05 significant level (% 95 confidence levels). In addition, Receiver Operating Characteristic (ROC) curve were calculated to assess the performance of diagnostic value of sialic acid. The ROC curve is performed by plotting the true positive rate (sensitivity) against the false positive rate (1-specificity) at various threshold settings. It has been determined the optimal cut-off point for sialic acid regarding ROC curve. The areas under the curve (AUC) were calculated (95% confidence).
ResultsThe spermyogram findings of the groups are shown in the Table 1. The average age of normozoospermic group was calculated as 31.17±5.02 and the average age of group oligozoospermic was 32.97±7.01 years old. (Mean±SD) (p>0.05). The groups in present study were formed according to data of Table 1.
Spermyogram results in normozospermic and oligozospermic male (mean±SD).
| Semen paremeters | Normozoospermia (n=30) | Oligozoospermia (n=30) |
|---|---|---|
| Age (years old) | 31.17±5.02 | 32.97±7.01 |
| Volume (mL) | 3.69±1.25 | 4.7±1.29 |
| Concentration (million/mL) | 50.27±23.31 | 11.33±2.42 |
| Total number (million) | 188.64±96.89 | 51.03±14.48 |
| Total motility (%) | 68.91±8.63 | 67.33±9.25 |
| Progressive motility (%) | 55.41±9.82 | 50.33±11.96 |
| Non progressive motility (%) | 13.5±4.17 | 17±5.73 |
| Immotility (%) | 31.09±8.63 | 32.67±9.25 |
| TPMSC (million) | 108.62±62.19 | 25.83±9.93 |
| Normal morpholgy (%) | 2.27±0.70 | 1.33±0.52 |
| Head anomaly (%) | 88.96±1.96 | 91.17±1.84 |
| Amorphous head (%) | 76.05±4.95 | 80.50±4.59 |
| Large head (%) | 5.14±3.39 | 5.50±5.68 |
| Small head (%) | 2.64±1.94 | 1.50±1.52 |
| Long head (%) | 4.18±3.05 | 3.17±2.56 |
| Multiple head (%) | 0.96±1.46 | 0.50±0.84 |
| Neck or tail anomaly (%) | 8.86±1.46 | 7.50±1.52 |
| Neck-middle piece anomaly (%) | 12.86±2.93 | 17.83±3.19 |
| Tail anomaly (%) | 13.68±3.40 | 14.17±3.06 |
| Double tail (%) | 1.36±1.33 | 0.83±1.17 |
| Tail stump (%) | 0.27±0.55 | 0.5±0.84 |
| Dag defect (%) | 5.77±2.11 | 6.83±2.64 |
| Long tail (%) | 0.27±0.63 | 0.5±0.84 |
| Short tail (%) | 5.91±2.00 | 5.50±1.52 |
| Teratozoospermia index | 1.28±0.05 | 1.33±0.02 |
TPMSC: total progressive motil sperm count.
As stated in Fig. 1, Sialic acid levels (mean±SE) showed statistically significant lower levels in Normozoospermic (3.44±0.15mmol/mL) compared to Oligozoospermic individuals (5.86±0.34mmol/mL) (p<0.0001). In addition, MDA levels in Normozoospermic (7.47±0.32nmol/mL) were lower than Oligozoospermic (8.46±0.97nmol/mL) individuals, but it was not statistically significant (p>0.05). GSH levels (12.71±1.05μmol/g protein) in normozoospermic were measured as higher than Oligozoospermic (11.57±0.77μmoll/g protein), but it was not statistically significant (p>0.05).
As it shown in Table 2; Sialic acid concentration was correlated negatively with concentration (r=−0.551), total number (r=−0.551), total motility (r=−0.431), progressive motility (r=−0.517), Total Progressive Motil Sperm Count (TPMSC) (r=−0.567) and normal morphology (r=−0.665,). Positive correlations between sialic acid levels and immobility (r=0.431), head anomaly (r=0.463), neck-middle piece anomaly (r=0.628) and segmental mitochondrial aplasia (r=0.408) and teratozospermia index (r=0.528) were determined.
Correlations of the semen parameters with MDA, GSH and sialic acid levels (n=60).
| Semen parameters | MDA (nmol/mL) | Sialic acid (mmol/mL) | GSH (μmol/g protein) |
|---|---|---|---|
| Concentration (million/mL) | −0.031 | −0.551* | 0.291* |
| Total number (million) | −0.018 | −0.551* | 0.256 |
| Total motility (%) | −0.013 | −0.431* | −0.057 |
| Progressive motility (%) | −0.027 | −0.517* | 0.028 |
| Immotility (%) | 0.000 | 0.431* | 0.058 |
| TPMSC (million) | −.006 | −0.567* | 0.165 |
| Normal morphology (%) | −0.082 | −0.665* | −0.249 |
| Head anomaly (%) | 0.156 | 0.463* | 0.198 |
| Large head anomaly (%) | 0.401* | 0.048 | 0.082 |
| Multiple head anomaly (%) | 0.394* | −0.150 | −0.108 |
| Neck-middle piece anomaly (%) | 0.306 | 0.628* | 0.049 |
| Cytoplasmic droplet (%) | 0.377* | 0.239 | −0.022 |
| Segmental mitochondrial aplasia (%) | −0.049 | 0.408* | 0.258 |
| Teratozoospermia index | 0.090 | 0.528* | −0.029 |
MDA: malondialdehyde, GSH: glutathione, TPMSC: total progressive motil sperm count.
Positive correlations were found between MDA and large head anomaly (r=0.401) and multiple head anomaly (r=0.394) and cytoplasmic droplet (r=0.377) at p<0.05 significance level. A positive correlation was found between GSH and semen concentration at (r=0.291) p<0.05 level. Amorphous head, small head, long head, mitochondrial loss, dag defect, tail anomaly, double tail, tail stamp were not correlatted significantly with GSH, MDA and sialic acid levels (p>0.05).
In Fig. 2, ROC curves were plotted according to sensitivity and 1-specificity values for sialic acid concentration. It is valuable to note that at the highest susceptibility points, these are the least false positive Area Under Curve (AUC) values (lower band-upper band); sialic acid values reaches to the 0.881 thresholds. Furthermore, the cut-off value for sialic acid was determined as 4.175nmol/mL, which yielded an AUC of 0.881 thresholds (0.789–0.973). The findings show that the concentration of sialic acid is very important determinant in oligozoospermia based on biochemical aspect when it is evaluated with AUC value.
DiscussionSperm glycocalyx consists of more than 300 different glycoproteins and glycolipids. It is located on the main surface of male gametes to interact with the external environment.12 During spermiogenesis, the capacitation and acrosome reactions, glycoproteins and glycolipids on the sperm surface are re-arranged. Changes on the thickness of,13 and the maturity and integrity of sperm glycocalyx is closely related to sperm fertility.14 Decrease in sialic acid; causes a negative charge on the surface of the sperm, it can cause agglutination against the forward movement of the sperm.24 These acidic sugars are important in such as development, molecular recognition, immune regulation and carcinogenesis.25 Toit et al.26 found a relationship between sialic acid and sperm motility, pyruvate concentrations. They suggested sialidase can affect the concentration of sperm associated with sialic acid.26 In seminal plasma, positive correlation of pyruvate concentration with sialidase activity has been reported.26 Pyruvate can be used as a source of energy by the sperm cell in the presence of acetyl permease in seminal plasma.27 According to the results of Toit et al., 28 spermatozoal ATP concentrations showed a positive correlation with sperm-related sialic acid concentrations. They explained as follows; “Sialic acid's inability to perform its function in sperm metabolism by masking the receptors in the sperm membrane”. This will prevent ATP consumption, and therefore, this state will result in low energy efficiency and poor mobility in sperm. These results may be another reason to accept that sialic acid can play a key role in sperm motility.28 Levinsky et al.29 reported that sialic acid levels of spermatozoa were higher in oligozoospermic than normozoospermic individuals.28 The first findings by Singer et al.,30 which are indicated that increased sialic acid levels were observed with the immature form percentage.30 In patients with low morphologically normal sperm percentage, sialic acid concentrations in seminal plasma were significantly higher than controls. In the study with teratozoospermic individuals, they found that the concentrations of sialic acid found in seminal plasma and sperm were similar.31 This means that sialic acid increases equally on both sperm and seminal plasma.30 But contrast to these findings, Levinsky et al.,32 suggested that, the increased amounts of sialic acid in sperm means the lower concentrations in seminal plasma. Sialic acid levels are high in immature sperm. These immature forms are associated with the lack of maturation and, carry higher numbers of sialic residues.33 So, it can be postulated that the studies have opponency results, and there is still clearly not known mechanism between sialic acid and fertility.
In the present study, we analyzed the possible correlation between normozoospermia and Oligozoospermia individuals regarding the results of semen analysis and sialic acid. The results we found were compatible with the recent study results mentioned above and revealed the accuracy of our results. This study results show that seminal plasma sialic acid levels are lower in normozoospermia than oligozoospermia. In our study, no statistically significant difference was observed between the groups with sialic acid and large head, multiple head, cytoplasmic droplet anomalies. However there were statistically significant differences in concentration, total number, total motility, progressive motility, immotility, TPMSC, normal morphology, head anomaly, neck middle piece anomaly, segmental mitochondrial aplasia and Teratozoospermia index.
The differences between the other studies and our study and a new perspective of the present study are that, we determined relations between sialic acid and not only on total number and motility, and also all semen parameters. Furthermore, a ROC analysis was used to determine a cut-off value differentiating both normozoospermic and oligozoospermic to compare its diagnostic performance. Based on these findings, sialic acid was found to be significantly predictive in the differentiation of oligozoospermic male. In addition to Teratozoospermia index is an important result that means the number of morphological anomalies per sperm. Increases in sialic acid levels leads to increasing anomalies as it determined correlation coefficient r=0.528. While significant positive correlation was observed between sialic acid and mid-neck anomaly (r=0.628), negative correlations were found with normal sperm percentages (r=−0.665). We suggest that, higher seminal plasma sialic acid levels in oligozoospermia are important. To clarify the relations it is needed to analyze the enzymes which include in this process and which mechanism leads to stay high sialic acid levels and are more effective in seminal plasma.
High MDA and protein carbonyl (PC) levels indicate oxidative stress in abnormal semen, which means damages to lipids and proteins.34 Goswami et al. reported that proteins secreted from the prostate may increase the sialic acid content in the presence of oxidative stress.35 It has been reported that the presence of high sialic acid in abnormal semen may be due to oxidative stress.34
Oxidative damage can disrupt the role of spermatozoa in maintaining motility and eventually affect fertilization. Saraniya et al. also reported that MDA levels showed a significant and positive correlation with sialic acid levels.34 The increase of sialic acid in the abnormal semen and its correlation with MDA is strong evidence that this increase in sialic acid may be a protective response of the body to reduce the effect of ROS. All these results show that MDA and sialic acid have the potential to be used as abnormal semen biomarkers which support semen analysis.34 When we evaluated the relationship between MDA and semen parameters in our study, we could not find a relationship between MDA and total number and immotilite sperms, as similar to the results of Saraniya et al. According to our results in Table 2, the correlations of MDA with spermiogram analyze show that they may have a role in acrosome reactions.
Moreover, sperm capacitation which is the morphological and metabolic changes in spermatozoa required for fertilization. In the sperm capacitation process, the production of ROS that trigger and regulate many events, including protein phosphorylation, is a priority. Although the roles of biochemical mechanisms in this formation have not been fully elucidated, it is thought to be related to the subcellular parts of spermatozoons and changes in thiol groups of proteins localized to the plasma membrane during capacitation.9
Seen from this aspect, we analyzed the correlation between semen parameters and GSH. We determined a positive relationship between semen concentration (r=0.291) and GSH. Our study has the feature of being the first in this sense since we could not find a study that examined the relationship levels between GSH levels and any semen parameters.
The results showed that the group with infertile individuals with oligozoospermia had higher levels of sialic acid than normozoospermia, and the amount of sialic acid may be used as a biomarker to diagnose of infertility. In addition to semen analysis, it is thought that sialic acid analysis can be helpful in evaluation of fertile-infertile male. This importance will give new aspect on future studies on the definition of sialic acid relation with male reproductive. In addition, investigation of other structures and enzymes involved in oxidant–antioxidant balance will also contribute to the elucidation of mechanisms that cause DNA damage, lipid peroxidation, and protein oxidation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
AuthorshipStudy concept and design: EM.
Quality of data and algorithms: DD, ENK, FZE, NC.
Data analysis and interpretations: DD, ENK, NC, EM, FZE.
Data acquisition: DD.
Manuscript preparations: NC, EM, ENK.
Manuscript editing: NC, EM.
Manuscript review: EM, NC.
All authors read and approved the final manuscript.
Informed consentThis study was conducted with permision of Ethics Committee of Selcuk University with the number of 2019/259.
FundingNo funding was received.
Conflict of interestsThe authors declare that they have no competing interests.