To analyze existence of an association between methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism with male infertility.
Materials and methodsA case–control study was conducted from June 2017 to August 2018 in which 88 infertile and 40 fertile were recruited. Polymerase chain reaction (PCR) – restriction fragment length polymorphism (RFLP) assay was carried out to study the allelic frequency of C677T polymorphism. The differences in allelic and genotypic frequencies of C677T locus between fertile and infertile groups were evaluated by the Pearson chisquare test. A logistic regression model was used to calculate Odds ratios and 95% confidence intervals, p value<0.05 was considered significant. The Hardy–Weinberg equilibrium was tested using HWE software.
ResultsIn infertile subjects, frequency distribution of CC allele was (60.2%), the CT allele was (30.7%) the TT allele was (9.1%) and in the fertile controls the frequency was CC allele (75%), CT allele (20%) and TT allele (5%) respectively. Analysis revealed MTHFR 677 CC genotype associated significantly with male infertility (p<.046, OR=2.385; 95% CI=1.014–5.608); Frequency of CT (30.7%) and TT (9.1) genotypes were higher in infertile men as compared to CT (20%) TT (5%) in fertile controls but statistically these were not significantly different (p=0.097; OR=0.455; CI=0.179–1.153 and p=0.431; OR=0.526; CI=0.107–2.599 respectively). Significant association of age and BMI with MTHFR genotypes and infertility was observed.
ConclusionOur results showed that MTHFR C677T polymorphism is not a risk factor for male infertility in our Pakistani population.
Observar el efecto del polimorfismo C677T en metilenetetrahidrofolato reductasa (MTHFR) en la infertilidad masculina.
Materiales y métodosSe realizó un estudio de casos y controles desde junio de 2017 hasta agosto de 2018 en el que se reclutaron 88 infértiles y 40 fértiles. Se llevó a cabo el ensayo reacción en cadena de la polimerasa (PCR) - polimorfismo de longitud de fragmento de restricción (RFLP) para estudiar la frecuencia alélica del polimorfismo C677T. La prueba de chi-cuadrado de Pearson se utilizó para estimar las diferencias en las frecuencias alélicas y genotípicas del locus C677T entre fértiles e infértiles. Los cocientes de probabilidad se obtuvieron mediante el análisis de regresión logística con intervalos de confianza del 95%, siendo significativo un valor de p<0,05. Se aplicó el equilibrio Hardy-Weinberg (HWE).
ResultadosEn sujetos infértiles, la distribución de frecuencia del alelo CC fue del 60,2%, la del alelo CT, del 30,7%, la del alelo TT, del 9,1%, y en los controles fértiles la frecuencia fue alelo CC fue del 75%, la del alelo CT, del 20%, y la del alelo TT, del 5%, respectivamente. El análisis reveló el genotipo CC MTHFR 677 asociado significativamente con infertilidad en los hombres (p<0,046, OR=2,385; IC95%: 1,014-5,608). La frecuencia de los genotipos CT (30,7%) y TT (9,1) fue mayor en hombres infértiles en comparación con CT (20%) y TT (5%) en controles fértiles, pero estadísticamente estos no fueron significativamente diferentes (p=0,097, OR=0,455; IC95%: 0,179-1,153, y p=0,431, OR=0,526; IC95%: 0,107-2,599, respectivamente). Se observó asociación significativa de edad e IMC con genotipos MTHFR e infertilidad.
ConclusiónNuestros resultados mostraron que el polimorfismo MTHFR C677T no está asociado con la infertilidad por factor masculino en nuestra población pakistaní.
DNA methylation is a crucial epigenetic feature of DNA that depends on a methyl donor. S-adenosyl-l-methionine (SAM) is the primary methyl group donor for most biological methylation reactions.1 The enzyme Methylene tetrahydrofolate reductase (MTHFR) reduces the 5–10-methylenetetrahydrofolate in to 5-methyltetrahydrofolate (biologically active form) which in turn donates a methyl group to converts homocysteine into methionine, the methionine itself donates a methyl group for the synthesis of S-adenosylmethionine which act as a methyl donor for methylation of DNA and proteins.2 The DNA methylation is essential for several biological processes including spermatogenesis. Folate is very important for the maintenance of genome integrity due to its role in DNA synthesis, repair and methylation. Studies have shown that the folate metabolic pathway plays an important role in spermatogenesis. It is widely shared that mutations in the genes encoding key enzymes involved in folate metabolism are potential risk factors for male infertility.3 A single nucleotide polymorphism (SNP) C667T in the coding region of human MTHFR gene results in the production of thermolabile protein Methylene tetrahydrofolate reductase enzyme (MTHFR) by replacement of amino acid alanine by valine (p. Ala222-Val) with almost 30% less enzymatic activity in heterozygotes (CT) individuals and almost 70% in mutant individuals as homozygotes (TT).4 The C667T polymorphism in the gene MTHFR leads to hypomethylation that involve the entire genome or specific CpG sites variations in the DNA methylation pattern which are common among the healthy people in association to age, sex or tissue specific, however inter individual variations that are influenced by factors including genetic as well as environmental have also been reported.5 The homozygous C677T mutation in the MTHFR gene has been studied to be associated with certain cardiovascular disorders, neural tube defects, and cancers.1 Studies have revealed a higher activity of MTHFR in adult mice testis as compared to other vital organs it gives an idea that the enzyme MTHFR plays a crucial role in the process of spermatogenesis.6 Since the enzyme MTHFR plays an important role in the folate and homocysteine metabolism and in humans, the folic acid supplements have reported to increase total sperm count. Therefore, C677T mutation in MTHFR gene have been reported to be associated with men infertility.7
We therefore, hypothesized that C667T mutation may be the reason for infertility in our Pakistani male individuals.
Materials and methodsSubjectsThis was a case control study conducted from June 2017 to August 2018. Sample size was calculated by Open Source Epidemiologic Statistics for Public Health. The prevalence (70%) of MTHFR gene mutation was taken from the literature.4 Sample size for unmatched case control study was calculated with 95% confidence interval and a power of 80% by Fleiss method with continuity correction, which recommended 72 cases and 36 controls. We included a total of 128 subjects to minimize the risk of drop outs, therefore included 88 infertile patients (cases) aged 22–67 years from Islamabad Clinic Serving Infertile Couples, Pakistan. Forty healthy fertile individuals with age ranging from 26 to 67 year and who have fathered at least one child have been selected as controls. All the patients as well as controls were examined by specialists. The BMI was noted as 18.95–37.0kg/m2 for infertile men (non-normozoospermic) and 18.30–30.31kg/m2 for healthy fertile controls (normozoospermic).
The selection criterion: patients having any anatomical defects, cryptorchidism, orchitis, chromosomal abnormalities, any previous surgery, and radiotherapy or had any infertility treatment previously were excluded from the study. The semen samples from each infertile patient were collected and analyzed for azoospermia, oligoasthenozoospermia, asthenozoospermia, oligoasthenoteratozoospermia. Semen analysis from normal subjects was considered as normozoospermia to serve as control.
The present study was approved by the Ethical Review Committee (ERC) of Islamabad Clinic Serving Infertile Couples, Pakistan. The ERC approved informed consent was distributed among all the study participants. Five ml venous blood was collected from each participating individual and sent to Multidisciplinary Lab (MDL) Aga Khan University (AKU) for further analysis.
Molecular analysisDNA extractionGenomic DNA was purified from blood, by using the Wizard Genomic DNA Purification Kit (Promega, USA). The protocol was followed according to the vendor's instructions. The eluded DNA samples were dissolved in 250μl TE buffer; the purity of DNA was checked by optical density (OD) at the ratio of 260/280 and stored at −40°C for further use.
Polymerase chain reaction (PCR)To analyze the C677T polymorphism in the coding region of MTHFR gene following sets of primers 5′-CATCCCTATTGGCAGGTTAC-3′ (forward) and 5′-GACGGTGCGGTGAGAGTG-3′ (reverse) (7) were used in this study. The PCR was carried out according to the method adapted by (7) with some modifications. Briefly, in a volume of 10μl master mix 1μg genomic DNA, 5μl Ruby taq master mix (Affymetrix, Inc. Cleveland, Ohio, USA), 1μl forward and 1μl reverse primers, 2μl DNAase/RNAase free water (World Wide Scientific Karachi, Pakistan) were used. Thermal cycling conditions were set as, pre heat at 94°C for 10min followed by denaturation at 94°C for 20s annealing at 60°C for 45s and extension at 72°C for 30s for 35 cycles with a final extension at 72°C for 10min. Gel electrophoresis was performed with 2% agarose gel containing 2% ethidium bromide. The gel harboring PCR product was analyzed on GelDoc, and a 265bp DNA fragment was yielded.
Restriction fragment length polymorphism (RFLP) for analyzing single nucleotide polymorphism C677T in the MTHFR geneThe quantity and quality of DNA in the amplified PCR product was checked by analyzing the amplified DNA at 260nm and 280nm via the Nano-drop Spectrophotometer (Thermo Scientific, Germany). The PCR products were digested with restriction enzyme HinfI (New England Biolabs Inc. IPSWICH, MA, USA). The RFLP protocol for 10μl restriction reaction mix containing 1.5μl amplified PCR product (1μg DNA), 0.2μl restriction enzyme, 1μl NEB buffer, 7.3μl RNase/DNAse free water. Reaction mixture was incubated in a water bath at 37°C for 3h; the digested fragments were subsequently identified by gel electrophoresis on 3% agarose gel with Gel Doc 1000 system (Bio-Rad, Hercules, CA, USA).
Statistical analysisCalculation of the allele and genotype frequencies of the patients and controls were done by counting the results on agarose gel. The differences in allelic and genotypic frequencies of C677T locus between fertile and infertile groups were evaluated by the Pearson chi-square test. A logistic regression model adjusting the effects of age, body mass index (BMI) was used to calculate Odds ratios and 95% confidence intervals, The p value<0.05 was considered as statistically significant. All the statistical analysis was carried out with SPSS 16 statistical software (SPSS Inc., Chicago, Illinois, USA). The Hardy–Weinberg equilibrium was tested using HWE software.
ResultsA total of 128 male individuals including 88 infertile (non-normozoospermic) patients identified as Azoospermia, Oligozoospermia, Asthenozoospermia etc. with mean age 35.94±0.873 years and mean BMI 27.0891±0.319kg/m2. While, 40 normal controls (normozoospermic) with mean age 39.92±1.380 years and mean BMI 24.1572±0.412kg/m2.
The distribution of C677T polymorphism in the MTHFR gene is summarized in Table 1.
Genotype frequencies of MTHFR c 677C>T polymorphism among infertile (Non-Normozoospermic) and fertile (Normozoospermic) men and their association with male infertility.
| Infertile (non-normozoospermic) Men n=88 | Fertile (normozoospermic) (controls) Men n=40 | p value | Odd ratio | 95% CILower–upper | |
|---|---|---|---|---|---|
| Sperm parameters (Mean±SD) | |||||
| Sperm count (mill/ml) | 33.97±17.22 | 89.47±49.36 | <0.001 | 1.075 | 1.047–1.105 |
| Sperm motility (%) | 35.02±14.23 | 67.93±14.25 | <0.001 | 1.141 | 1.096–1.188 |
| Sperm morphology (%) | 2.68±1.54 | 4.12±1.56 | <0.001 | 1.772 | 1.359–2.311 |
| MTHFR 677C>T genotype (N, %) | |||||
| CC | 53 (60.2) | 30 (75) | .046a | 2.385 | 1.014–5.608 |
| CT | 27 (30.7) | 8 (20) | .097a | 0.455 | 0.179–1.153 |
| TT | 8 (9.1) | 2 (5) | .431a | 0.526 | 0.107–2.599 |
| CT+TT | 35 (39.7) | 10 (25) | 0.42a | 0.844 | 0.148–4.801 |
Note: p value assessed using Mann–Whitney U test for sperm parameters and Fisher's exact test (two-tailed) for genotype comparison between infertile with fertile (control) groups. OR: Odds ratio; CI: 95% confidence interval.
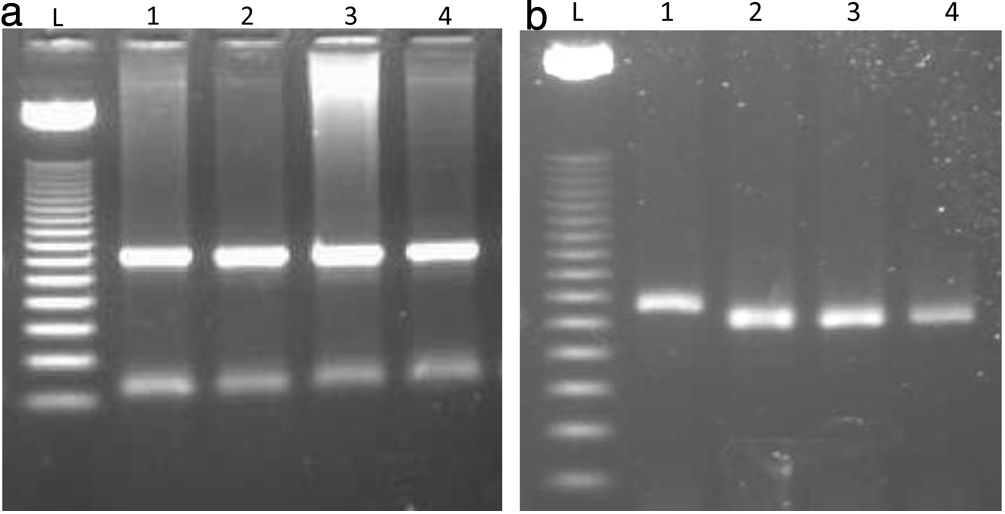
To identify the C677T polymorphism in the coding region of MTHFR gene among infertile and fertile groups, all the subjects were analyzed by means of PCR-RFLP method. The PCR was performed with 265bp product that was analyzed on 2% agarose gel (Fig. 1a). RFLP was performed by digesting the yielded PCR product with restriction enzyme Hinfl (New England Biolabs Inc. IPSWICH, MA, USA). The genotypes were observed on the basis of differences in the length of DNA fragments, the wild type homozygous (CC) allele showed one band of 265bp fragment, the heterozygous (CT) allele showed three bands of 265, 171 and 94bp fragments and the rare or mutant homozygous (TT) allele showed two bands of 171 and 94bp fragments because there is a cut site for Hinfl on T allele (Fig. 1b).
(a) Polymerase chain reaction (PCR) analysis of MTHFR 677C>T gene polymorphism. Lanes L ladder 50bp. 1–4 showing amplified product of 265bp. (b) MTHFR 677C>T polymorphism was analyzed by PCR-RFLP. The 265-bp PCR product was digested with HinfI. Lane L – 50bp DNA marker. Lane 1 showing the C allele with 265bp band as it was not cut by the enzyme Hinfl. Lane 2, 3 and 4 are the T allele showing 171bp fragments. In this gel image 94bp is not visible because it is a very small fragment and it appeared very light on gel which we were not able to capture on chemidoc imaging system (Bio-Rad).
The frequency distribution of CC allele was (60.2%), the CT allele was (30.7%) the TT allele was (9.1%) in our infertile subjects and the in the fertile controls the frequency was CC allele (75%), CT allele (20%) and TT allele (5%) respectively (Table 1).
The statistical analysis of our results revealed an association of 677 CC allele with fertility (p<0.046, OR=2.385 and CI 1.014–5.608) (Table 2). The allele frequency of heterozygous (CT) alleles was higher in infertile patients as compared to controls but difference was not statistically significant (Table 2). The homozygous mutant T allele and the T allele in heterozygotes (CT) were significantly more in infertile patients than the controls (Table 2). The strength of the association between genotypes and male infertility was determined by odds ratios. The MTHFR 677 C>T polymorphism showed a very weak association with infertility in our male population. Males with CT (p=.097, OR 0.455 and CI 0.179–1.153) and TT genotype (p=0.431, OR 0.526 and CI 0.107–2.599) exhibited somewhat higher risk of infertility disorder than males CC genotypes (Table 2).
A significant association was found between physical factors such as age and BMI and C677T genotype polymorphism with infertility status of men. The BMI showed high significant association with all the alleles of MTHFR C677T genotype (p<0.001). This indicates that age and BMI are the major risk factors for the MTHFR C677T genotype polymorphism contributing infertility in our male population.
DiscussionMale infertility is a multifactorial disease; several environmental and genetic factors play an important role in the disruption of the process of spermatogenesis which is controlled by firmly organized events of gene expression. MTHFR plays an important role in folate and homocysteine metabolism, the levels of tHcy could affect synthesis and methylation of DNA molecules. DNA methylation and DNA synthesis play important role in the spermatogenesis.8 Several studies have proved experimentally that certain enzymes of folate pathway like MTHFR are crucial for spermatogenesis. Any gene mutation or polymorphism of the MTHFR together with folate deficiency can alter nucleotide synthesis and may cause infertility.9
The association between 677C>T polymorphism in the coding region of MTHFR gene and risk of male infertility was first reported by10 in Germany. Later, many studies have reported the association between MTHFR 677C>T polymorphism and the risk of male infertility but with inconsistent results.
We found a significant association between the MTHFR C677C allele and male infertility (p<0.046, OR 2.385, 95% CI 1.014–5.608) though, the frequencies of heterozygous CT allele (30.7%) and homozygous mutant TT (9.1%) allele were higher in infertile (non-normozoospermic) group as compared to fertile group (normozoospermic) CT allele (20%) and TT allele (5%) yet the results were not statistically significant (p=0.097 and p=0.431 respectively) (Table 2). Therefore, no significant association has been found in the heterozygous (CT) alleles, (OR 0.455, 95% CI.179–1.153) and TT allele (OR 0.526, 95% CI 0.107–2.599) with infertility, this gives an idea that C677T polymorphism is not a risk factor for infertility in our Pakistani men. A meta-analysis of twenty-six studies by Gong M et al. reported significant association of MTHFR 677C>T polymorphism with increased risk of male infertility.9 Furthermore, a similar tendency was observed in both Asian and Caucasian ethnicity with increased risks of infertility due to MTHFR 677C>T in an inclusive analysis of all infertile subgroups, except oligospermia subgroup.8 Another meta-analysis supports MTHFR C677T, A1298C, and MTRR A66G polymorphisms as the risk factors with increased chances of male infertility in Asians.11 Similarly another meta-analysis suggests the role of MTHFR C677T polymorphism in male infertility.12 This mutation was also discovered to be a risk factor for male infertility in azoospermic and oligoasthenoteratozoospermia (OAT) patients in a trial sequential analysis by Kang et al.13 Nevertheless prevalence of the MTHFR C677T polymorphism in fertile and sub fertile men from Netherlands concluded that the frequency of the MTHFR genotypes in fertile and sub fertile men was not statistically different.14
Studies showing positive association of C677T polymorphism with male infertility among the Asian populations as, Irfan et al. reported a positive association between MTHFR C677T polymorphism and infertility in a total of 437 idiopathic infertile men and 218 normospermic fertile men from Rawalpindi, Pakistan.15 Similarly, a positive association between C677T polymorphism and male infertility in 160 infertile patients selected from different cities of Pakistan.16 A positive association of T allele with infertility further suggested that homozygous MTHFR C677 mutation could have played a physiologically protective role in Indian populations.17 An association of C677T polymorphism with male infertility stated that the C677T polymorphism in MTHFR gene could be a genetic risk factor for male infertility in North Indian population.18 Our results are contradictory to these findings which could be due to a small sample size that might not have drawn sufficient conclusion or due to lack of stratification on the basis of specific cause of male infertility, age, type of occupation, food habit, lifestyle, type of environmental exposure and ethnicity.
Different studies among Asian populations have shown a positive association between T allele and male infertility.17,19 But there are some other reports showed no association of this polymorphism with male infertility. Vani et al. studied 206 infertile men and 230 controls from Hyderabad, India but was unable to report any significant association between MTHFR C677T polymorphism and the growing risk of male infertility.20 Another research from New Delhi India showed no significant differences in the frequency of MTHFR CT (p=0.44) between oligozoospermic infertile men and controls.21 No significant association between C677T and A1298C polymorphisms in the MTHFR gene with male infertility from Iran was observed.22
There was no significant association of the MTHFR, MTR, MTRR gene polymorphisms with non-obstructive male infertility in a Polish population.23 No evidence of an association between reduced sperm counts and polymorphisms in enzymes involved in folate metabolism was observed in the French population.24
Among the three (C677T, A1298C and G1793A) MTHFR polymorphisms C677T variant was associated with an increased risk of idiopathic male infertility in Iran.25 The frequency of the rare TT genotype in MTHFR C677T polymorphism in infertile German patients was increased by 9.3% as compared with the control group, but the frequency of CT heterozygotes was decreased by 8%.10
We observed significant association of age and BMI with C677T genotype polymorphism and infertility status of men. Studies have shown that obesity is an independent risk factor for alteration in sperm count, motility and morphology leading to male infertility.26 Multiple interrelated mechanisms like oxidative stress, inflammation and apoptosis in the testis explain the detrimental effect of obesity on male fertility.27,28 Infertility related with age is associated with sperm DNA fragmentation, age-related endocrine changes like reduction in testosterone as well as increased susceptibility to reactive oxygen species.29 However, age and BMI were adjusted via regression analysis method in our study.
These contradictory reports from different populations of the world give an impression that association of susceptibility of the MTHFR C677T polymorphism with male infertility may be due to some specific environmental factors like folate intake in diet.10 Furthermore, the effect of MTHFR polymorphisms depends on gene versus environmental factors mainly nutrient and gene versus ethnical or racial communications.30 It is known that folate deficiency occur frequently, and the related hyper homo cysteinaemia is considered as a risk factor for various diseases, including acute myocardial infarction (AMI), coronary artery disease (CAD) and infertility.19 Pakistanis belong to the South Asian population (part of under developing world) with the highest known rate of CAD; where folic acid deficiency also appears to be highly prevalent in our population.31
Our present study confirms our previous study31 reporting lack of association of MTHFR 677C>T mutation with clinical diagnosis of acute MI (AMI) in a Pakistani population. Results of our two studies indicate that MTHFR C677T polymorphisms do not contribute to an inherited genetic susceptibility of certain disease pattern including AMI and infertility in our population. Our study is limited by a very small sample size and the fact that its calculation was based on the prevalence of CC allele, but clinically significant change of the base-line frequency was not considered due to budget constraints.
ConclusionMTHFR gene plays a major role in regulation of spermatogenesis; C667T allele variants can disturb the process ensuing male infertility. The results of the present study showed that MTHFR C677T polymorphism is not significantly associated with increased risk of male infertility. However, a well-planned and meticulously organized epidemiological study with large sample size will be needed to confirm our findings in the future.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the ethical standards of the committee of responsible human experimentation and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was supported by Dean's research grant AKU. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestAuthors have declared no potential conflict of interest relevant to this article.
The authors would like to express their humble gratitude to the office of Dean Faculty of Health Sciences and Dr. Perwaiz Iqbal the Chair Department of Biological & Biomedical Sciences Aga khan University, Karachi Pakistan for their support to carry out this research project. The authors appreciate and thank Miss Farzana Abubakar Yousuf for help in statistical analysis, Mrs. Asra for help in optimization of lab procedures.