Diclofenac sodium (DS) can have toxic effects on various tissues and organs, as well as causing foetal and new-born malformations. Thymoquinone (TQ), the basic bioactive compound of black seed oil, is an antioxidant and antineoplastic substance. The aim of our study was to explore the effects of DS and TQ exposure during gestation on offspring rat testicular histology.
Materials and methodsMother pregnant rats were divided into five groups: control, saline, DS, TQ and DS plus TQ (DS+TQ) four animals for each group. They were then treated as follows between day of 5 and 15 of gestation: the control group received no treatment. The saline group received physiological saline (1mg/kg/d) via the intraperitoneal (IP) route; the DS group received an intramuscular (IM) injection of DS (6.1mg/kg/d); the TQ group received TQ (5mg/kg/d) dissolved in drinking water; and the DS+TQ group received DS (6.1mg/kg/d) and TQ (5mg/kg/d) dissolved in water. After birth, the male rats were fed for four weeks, and at the end of this period offspring were sacrificed. Stereological methods, physical disector and Cavalieri principle were used for particle counting and volume estimation respectively.
ResultsThe results revealed a significant decrease in the total number of Sertoli and Leydig cells in 4-week-old rats in the DS group (p<0.05), and TQ not have provide protection against this adverse effect of DS.
ConclusionsIn this study, DS at a dose of 6.1mg/kg, equivalent to a dose of 1mg/kg in humans, decreased the number of Sertoli and Leydig cells, and TQ did not have a protective effect against the adverse effect of DS during the gestation period. These results show that new dose depend studies on TQ and DS interaction are requested to see protective effect of TQ.
El diclofenaco sódico (DS) puede provocar efectos tóxicos en diversos tejidos y órganos, además de producir malformaciones fetales y en recién nacidos. La timoquinona (TQ), el compuesto bioactivo básico del aceite de semilla negra, es una sustancia antioxidante y antineoplásica. El objetivo de este estudio fue estudiar los efectos de la exposición a DS y TQ durante la gestación sobre la histología testicular de crías de rata.
Materiales y métodosSe dividió a las ratas gestantes en 5 grupos: control, solución salina, DS, TQ y DS con TQ (DS+TQ). En cada grupo había 4 animales. A continuación recibieron el siguiente tratamiento entre los días 5 y 15 de gestación: el grupo de control no recibió tratamiento; el grupo de la solución salina recibió solución salina fisiológica (1mg/kg/día) por vía intraperitoneal; el grupo del DS recibió una inyección intramuscular de DS (6,1mg/kg/d); el grupo del TQ recibió TQ (5mg/kg/día) disuelto en agua potable, y el grupo DS+TQ recibió DS (6,1mg/kg/día) y TQ (5mg/kg/día) disueltos en agua. Después del nacimiento, a las ratas macho se las alimentó durante 4 semanas y al final de este período se sacrificaron las crías. Se utilizaron métodos estereológicos, el disector físico y el principio de Cavalieri para el recuento de partículas y la estimación de volumen, respectivamente.
ResultadosLos resultados revelaron una reducción importante del número total de células de Sertoli y Leydig en las ratas de 4 semanas en el grupo de DS (p<0,05). La TQ no ofreció protección frente a este efecto adverso del DS.
ConclusionesEn este estudio, el DS a una dosis de 6,1mg/kg, equivalente a una dosis de 1mg/kg en seres humanos, redujo el número de células de Sertoli y Leydig, y la TQ no produjo ningún efecto protector frente al efecto adverso del DS durante el período de gestación. Estos resultados muestran que se requieren nuevos estudios dependientes de la dosis en la interacción entre TQ y DS para comprobar el efecto protector de la TQ.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have anti-inflammatory, antipyretic and analgesic effects and are commonly used to alleviate pain, fever and inflammation.1 The therapeutic effects of NSAIDs are attributed to their ability to inhibit the activities of cyclooxygenase (COX) enzymes, which catalyze the synthesis of prostaglandins from arachidonic acid.2 Prostaglandins maintain organ function, produce thromboxane and protect the integrity of gastric mucosa.2 COX enzymes have two isoforms: COX-1 and COX-2. Although COX-1 is synthesized in all tissues, COX-2 is synthesized in the inflammatory response.2,6
As shown in previous research, NSAID administration during pregnancy increased the risks of miscarriage, congenital malformations and early closure of the ductus arteriosus. Reported adverse effects of NSAIDs administered to rats during the gestation period included nervous system, kidney, ovary, uterine, testicular and cardiovascular system damage in rat offspring.3–8
Diclofenac sodium (DS) is a NSAID used for pain, including dysmenorrhoea, and inflammatory conditions, such as rheumatoid arthritis and degenerative joint disease.9 Previous studies demonstrated that DS can be passed to the foetus via the placenta.10–13 Many studies revealed the effects of DS on the nervous system and various types of tissues, with researchers reporting that DS consumption in the prenatal period gave rise to pathological conditions, such as significant expansion of the periportal area, sinusoidal dilation, hepatocyte picnosis, bile duct proliferation and vacuolar degeneration in parenchymal cells in the postnatal period.14,15,11
Oxidative stress is the result of free radicals, such as reactive oxygen species (ROS), that form during cellular metabolism, and a deficiency of antioxidants, which detoxify these free radicals.16 NSAIDs induce ROS and apoptosis. Testicular tissue is particularly vulnerable to oxidative stress,17–20 and previous studies demonstrated that oxidative stress caused apoptosis and male infertility.16,21
Thymoquinone (TQ), the basic bioactive compound of black seed oil, has been used as an anti-inflammatory, antioxidant and antineoplastic substance for years. TQ is a volatile monoterpene quinone with bright yellow crystals.22,23 Previous research demonstrated that TQ protected many organs against damage caused by oxidative stress induced by free radicals, including hepatotoxicity induced by carbon tetrachloride,24 nephropathy induced by cisplatin25 and cardiotoxicity induced by doxorubicin.26
According to the literature, a DS dose of 6.1mg/kg/d in rats is equivalent to a dose of 1mg/kg/d in humans.27 There has been only one study on the effects of DS on testicular development,8 and there have been no studies on the possible protective effects of TQ. Therefore, it was aimed to investigate the adverse effects of prenatal exposure of DS on testicular development and the potential of TQ to provide protection against DS-induced adverse effects in the fourth week of the postnatal period, specifically in terms of the number of Sertoli cells, Leydig cells and spermatogonia, using unbiased stereological methods.
Materials and methodsAnimals and experimental proceduresThe animal experiments ethics committee of Van Yüzüncü Yil University, Turkey approved all the animal protocols described in this study. Twenty adult female albino Wistar rats weighing 200–300g were obtained from the experimental animals application centre of Van Yüzüncü Yil University. Five male rats were used for mating. The animals were fed standard food and had access to tap water ad libitum. They were maintained under a 12h light:12h dark cycle. Pregnancy was determined based on the observation of a vaginal plug (pregnancy d zero). The pregnant rats were housed in individual cages.
The pregnant rats were divided into five groups and treated as follows between day of 5 and 15 of gestation7,11,29: the control group received no treatment. The saline group received physiological saline (1mg/kg/d)13 via the intraperitoneal (IP); the DS group received an intramuscular (IM) injection of 6.1mg/kg/d of DS (Voltaren 75mg IM ampule, Novartis)28,30; the TQ group received 5mg/kg/d of TQ (Santa Cruz Biotechnology, sc-215986) dissolved in drinking water26,28,29; and the DS+TQ group received DS (6.1mg/kg/d) and TQ (5mg/kg/d) dissolved in water.28
The following formula was used to adapt the dose administered in the study to the human dose27:
Km: Calculated based on body surface area (BSA) for each species27
Km for human=37
Km for rat=6
According to formula: 1= Animal dose (for rat) (mg/kg)×637
Animal dose (rat) (mg/kg)=6.1mg/kg27
StereologySeven male offspring born from all maternal rats that of the same group were anesthetized with (ketamine–xylazine) at 4 weeks after birth.7,30,14,31 Then the thorax region was opened and fixed by intracardiac perfusion of neutral formalin. After perfusion, the right testis was removed by making an incision in the scrotal region, and the testis was then fixed in Bouin solution. After fixation, a fraction of 1/2 was made (first fraction). Fraction is to divide equal pieces the sample. The testis was embedded in paraffin after being passed through an alcohol and xylene series. Consecutive sections of 4μm thick were obtained using a microtome. The first section was selected randomly, and the other sections were systematically taken every 80th (second fraction). In this way, about 10–14 consecutive sections were obtained from each tissue block.
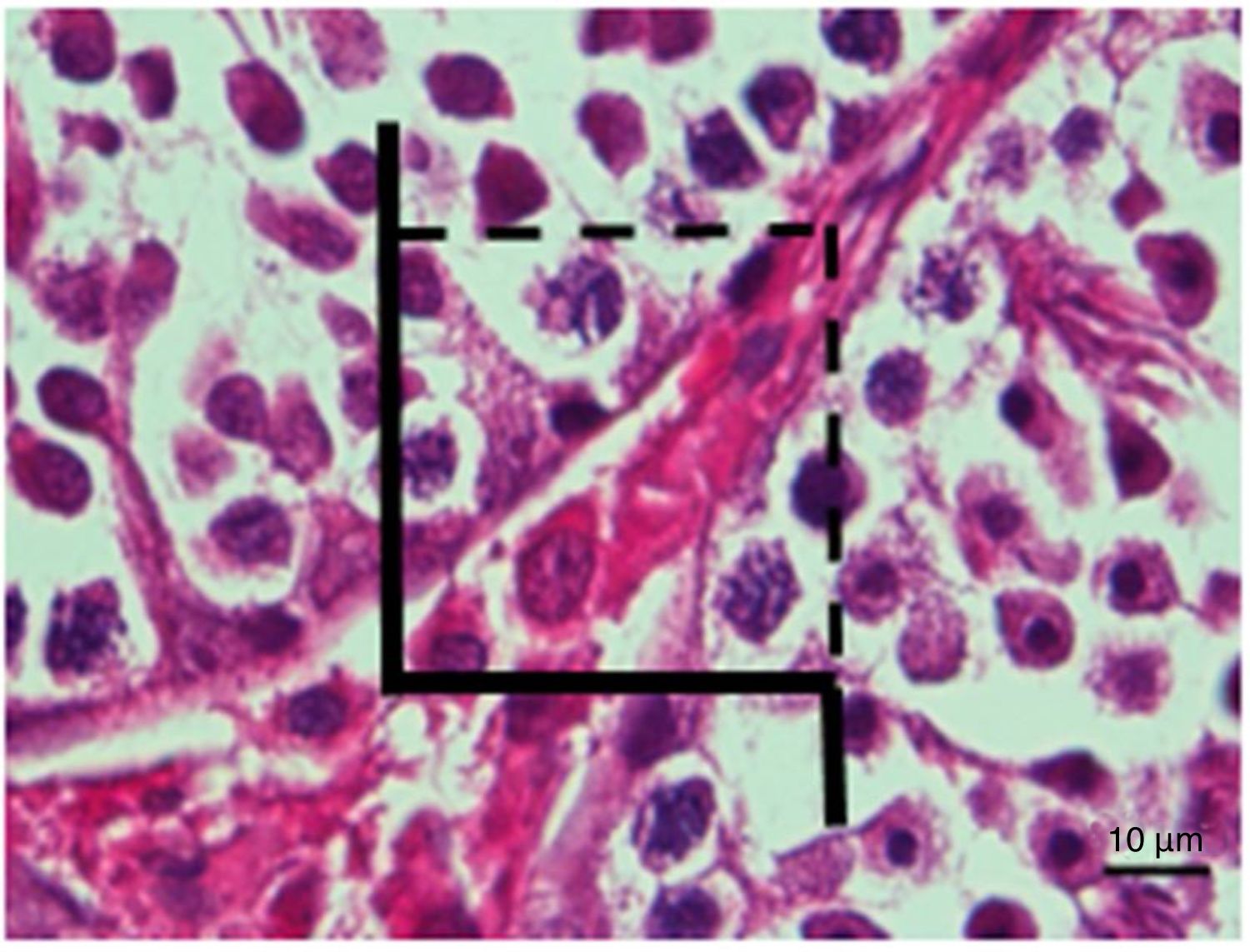
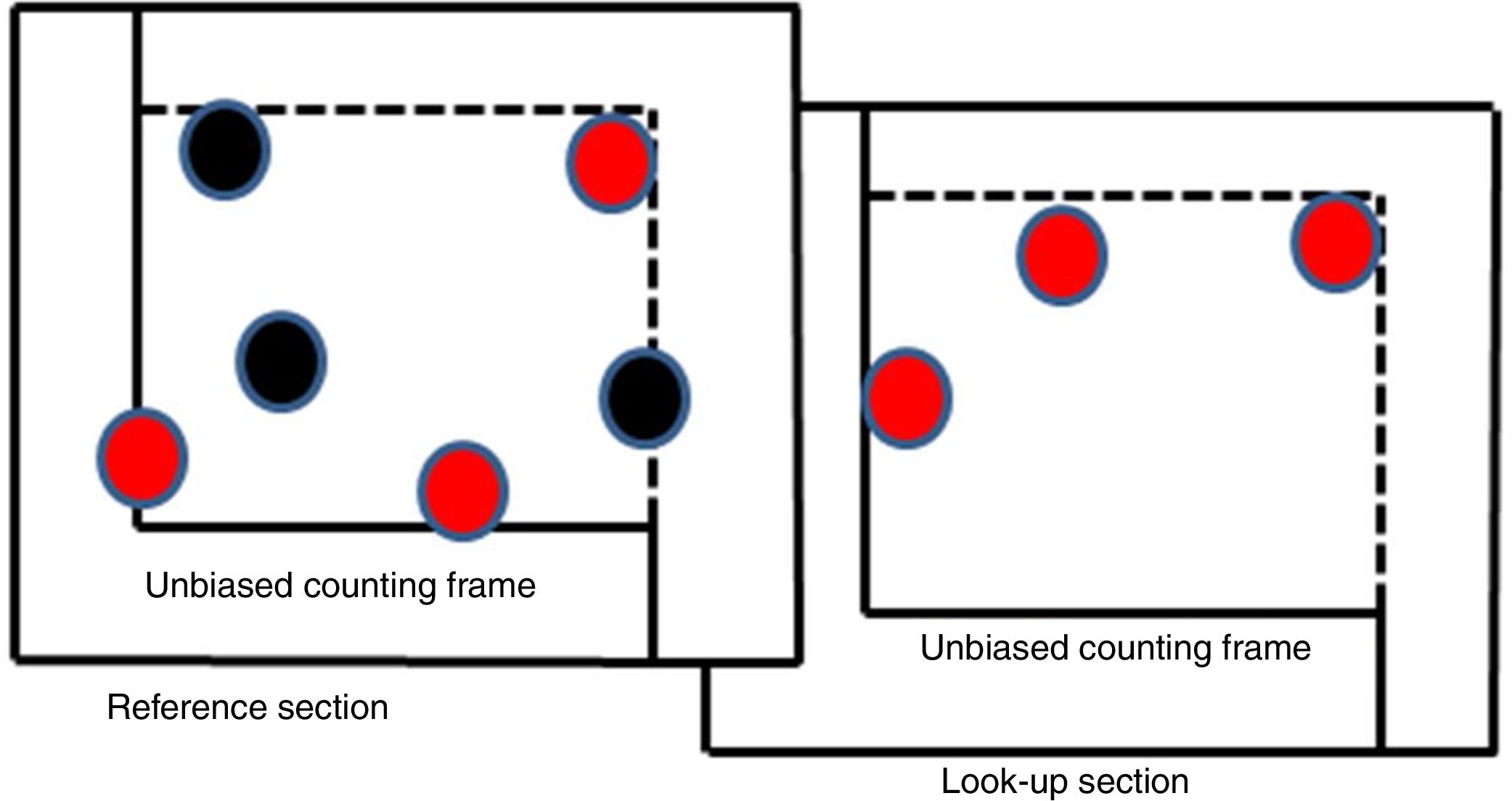
The tissue sections were stained with haematoxylin–eosin (HE) stain and then examined under a light microscope. Cavalieri's principle was applied for the total volume estimation of organ, and a dotted area measurement ruler was used in Cavalieri's principle. The disector counting technique was used to count the total number of cells using an unbiased counting frame (Figs. 1 and 2). When using the physical disector, the cells seen in the first section (reference section) but not in the second section (look-up section) were counted.
The following formulas were used for cell counting:
where N was the total cell number, Nv was the numerical density of the cells and Vref was the total volume of the tissue;where ∑Q− was the total number of disector particles (number of cells seen in the reference section but not in the look-up section) and ∑Vdis was the total disector volume;where ∑P was the total number of points corresponding to the tissue, a(p) was the area of a point, and t was the cross-section thickness.32The coefficient of error for each animal estimation was determined using a sampling adequacy test. For the determination of reliable number of animals for each group, the coefficient of variation was estimated. The Kruskal–Wallis test was applied for between-group comparisons.
Statistical analysisDescriptive statistics were applied for continuous measurements in our study, with median, mean, standard deviation, minimum and maximum. The Kruskal–Wallis H test was used to compare the measurements according to the groups, and the Bonferroni test was then used as a post hoc (multiple comparison) test to determine significant differences among groups. The statistical significance level was 5% in all the calculations. The SPSS (ver. 24) statistical package program was used for the calculations. Statistical significance was accepted at a p value of <0.05.
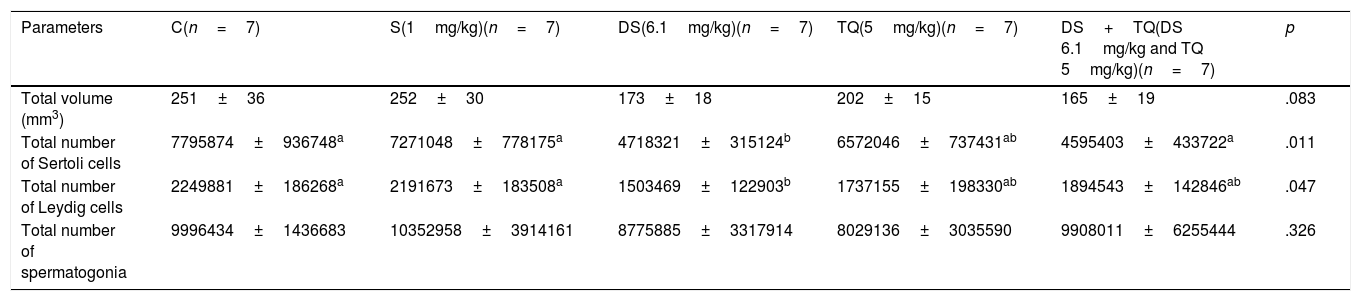
ResultsThere was no significant difference in the total testicular volume in any of the groups as compared with that in the control group (p>0.05) (Table 1). The total number of Sertoli cells significantly decreased in the DS group and DS+TQ group as compared with that in the control group (p<0.05) (Table 1).
Morphometrical values in the groups using standard error mean (SEM).
| Parameters | C(n=7) | S(1mg/kg)(n=7) | DS(6.1mg/kg)(n=7) | TQ(5mg/kg)(n=7) | DS+TQ(DS 6.1mg/kg and TQ 5mg/kg)(n=7) | p |
|---|---|---|---|---|---|---|
| Total volume (mm3) | 251±36 | 252±30 | 173±18 | 202±15 | 165±19 | .083 |
| Total number of Sertoli cells | 7795874±936748a | 7271048±778175a | 4718321±315124b | 6572046±737431ab | 4595403±433722a | .011 |
| Total number of Leydig cells | 2249881±186268a | 2191673±183508a | 1503469±122903b | 1737155±198330ab | 1894543±142846ab | .047 |
| Total number of spermatogonia | 9996434±1436683 | 10352958±3914161 | 8775885±3317914 | 8029136±3035590 | 9908011±6255444 | .326 |
Note: b shows a significant decrease compared to a (p<0.05). C: control, S: saline, DS: diclofenac sodium (6.1mg/kg), TQ: thymoquinone (5mg/kg), and DS+TQ (DS 6.1mg/kg and TQ 5mg/kg) (Kruskal–Wallis H test).
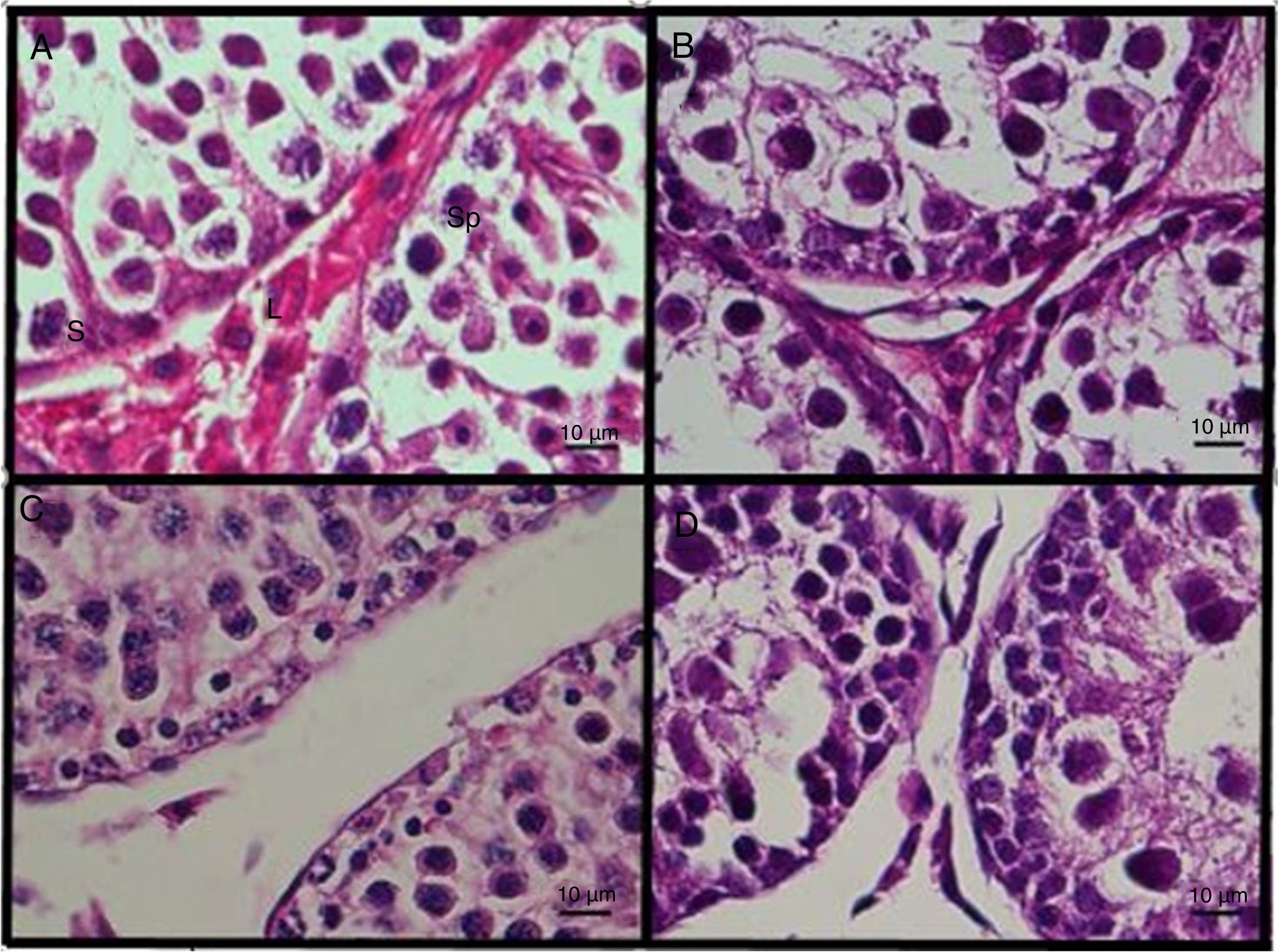
There was a significant decrease (p<0.05) in the total Leydig cell number in the DS group as compared with that in the control group (Table 1). Although apoptotic markers (e.g. pyknotic nuclei) of spermatogonia were observed in the section of DS group (Fig. 3C), there were no significant between-group differences in the total number of spermatogonia according to results of stereological analysis (p>0.05) (Table 1).
DiscussionMany drugs are oxidized to create free radicals. Therefore, ROS occurs often during drug use. Oxidative stress damages cell membranes, lipids, proteins and nucleic acids.33–35 Previous researches demonstrated that DS induced apoptosis in various cells and tissues by inducing the formation of ROS.36–38 Studies also reported that DS treatment during the prenatal period has resulted in significant differences in the total number of axons, decreases in myelin sheath thickness and morphological and histopathological changes in the optic nerve in offspring of rats.30 Furthermore, DS resulted in decreases in the number of neurons in the cornu ammonis of hippocampus,7 total number of Purkinje cells in the cerebellum11 and number of myelinated axons of sciatic nerves in offspring of rats.14
The adverse effects of some pharmacological agents on testicular and genital glands are well known. Sertoli cells play an important role in spermatogenesis, and Leydig cells are also the main source of androgen production. Toxins and chemical substances affect both types of cells. Deterioration in the functions of these cells can lead to sperm maturation and male reproductive system dysfunction, including a hormonal imbalance.39
In a previous histopathological study, the authors reported that DS caused a decrease in testicular weight and an increase in abnormal sperm cell counts.40 They not only observed a decrease in the number of Sertoli and Leydig cells, and but also tight junctions between the Sertoli cells were impaired that causes of releasing of spermatogenic cells from the seminiferous tubules. They also observed degenerative changes in seminiferous tubules and necrotic areas in its walls. The authors concluded that DS might decrease the testosterone level and disrupted spermatogenesis. The findings of the presented study are consonant with those found in the previous research, in which DS administration resulted in a significant decrease in the number of Sertoli and Leydig cells, as shown by stereological methods. Aslan et al.8 reported that DS at a dose of 3.6mg/kg/d did not cause a significant decrease in the total number of spermatogonia and Sertoli cells, whereas doses of 9mg/kg/d and 18mg/kg/d resulted in a significant reduction in these parameters. In the present study, a dose of 6.1mg/kg/d did not significantly reduce the number of spermatogonia cells, but it resulted with a significant decreasing of the number of Sertoli cells. Thus, higher doses of DS can affect spermatogonia.
Oxidative stress exposes the testis to free radicals. In response, antioxidant enzymes are released in the testis. This antioxidative defence mechanism counteracts the effect of the free radicals and provides protection against oxidative stress and many pathological conditions. However, it does not provide protection against extreme oxidative stress. In such cases, it needs external antioxidants.17
The role of TQ in inducing apoptosis by inhibiting the cell cycle via p53-dependent and independent mechanisms in cancer cells is well known. TQ also plays a role in the immune system by mediating the release of inflammatory mediators. Thus far, the chemotherapeutic effect of TQ has not been tested in the clinic. However, a large number of animal studies have provided promising results in terms of the anticancer effects of TQ. In one study, a combination of anticancer drugs clinically used in combination with TQ prevented chemotherapeutic side effects of robust cells outside the tumour, leading to improvements in therapeutic efficacy.41 A number of studies have investigated the mechanisms underlying the protective effects of TQ against oxidative stress-induced cell death and organ damage induced by free radicals.24,42–44 One study reported that TQ (10mg/kg, IP) administered to the testis provided protection against testicular injury (i.e. testicular torsion).45 Other studies reported that TQ (10mg/kg, IP) provided protection against the toxic effects of cisplatin and cadmium46,47 and against lead-induced testicular intoxication (5mg/kg, orally).48 In our study, in 4-week-old rats, TQ treatment did not cause significant differences in the total number of Sertoli cells, Leydig cells or spermatogonia or significant differences in the total testicular volume. We think that the discord in the findings of the present study as compared with those in the literature may be due to the manner, amount and duration of administration of TQ.
In conclusion, in the present study, when administered between day of 5 and 15 during gestation, a DS dose of 6.1mg/kg/d, which is equivalent to a dose of 1mg/kg/d in humans, decreased the number of Sertoli and Leydig cells. The DS treatment had testicular side effects, and TQ provided no protection against these side effects. A significant decrease in the number of Leydig cells may cause a reduction in the testosterone hormone level and lead to functional loss of Sertoli cells, alterations of the spermatogenesis process and postnatal male infertility. According to our findings, which were based on unbiased counting stereological methods, we advise caution in prenatal use of DS. It is recommended that immunohistochemical and biochemical studies should be conducted to determine the effects of DS and protective of TQ on the male reproductive system. Given the lack of a protective effect of TQ on testicular tissue in the present study, we suggest that different dose of the TQ should be used to provide some evidence on protective of TQ against the harmful effects of DS on testicular tissue during prenatal exposure.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interest.
FundingThis study was financially supported by Van Yuzuncu Yil University as a doctoral thesis project (project number: TDK-2016-5070).
We thank Van Yuzuncu Yil University for financial support.