Erectile dysfunction's physiopathology in uremia is complex and multifactorial, involving a combination of classical risk factors and specific uremia-related risk factors such as increased oxidative stress, endothelial dysfunction and inflammation. The aim of the study is to investigate the effect of chronic kidney disease (CKD) on vascular calcification and endothelial function of cavernosal bodies in apolipoprotein E deficient (apoE−/−) mice, a well known model of erectile dysfunction.
Materials and methodsEight-week-old male apoE−/− mice were randomly assigned to the following 3 groups: (i) subtotally nephrectomised (SNX apoE−/−, 12 mice), (ii) uninephrectomised (UNX apoE−/−, 11 mice) or (iii) sham operated (sham-op apoE−/−, 15 mice). At 16 weeks after surgery, aortas and penile erectile tissues were harvested for histological studies to assess atherosclerosis, vascular calcification, nitrotyrosine staining, total collagen content and macrophage staining.
ResultsAt sacrifice, SNX and UNX mice had significantly higher serum urea, total cholesterol, and triglyceride concentrations than sham-op controls. Atherosclerotic lesions in thoracic aorta were significantly larger in uremic apoE−/− mice than in controls. There were no atheromatous lesions in cavernosal bodies or penile artery observed in any group. However, SNX and UNX animals showed a significant increase in calcification score, collagen content and nitrotyrosine staining in cavernosal bodies when compared with controls. The degree of macrophage infiltration was comparable between the 3 groups.
ConclusionIn conclusion, even mild renal dysfunction, i.e., after uninephrectomy increases calcification score and aggravates endothelial function of cavernosal bodies in apoE−/− mice and this effect might be linked to increased oxidative stress in penile endothelium.
La fisiopatología de la disfunción eréctil en la uremia es compleja y multifactorial, e incluye una combinación de factores de riesgo clásicos y factores específicos asociados a la uremia, como el aumento del estrés oxidativo, la disfunción endotelial y la inflamación. El objetivo de este trabajo es examinar el efecto de la enfermedad renal crónica (ERC) sobre la calcificación vascular y la función endotelial de los cuerpos cavernosos en caso de deficiencia de apolipoproteína E en ratones (ratones ApoE−/−), un modelo bien conocido de disfunción eréctil.
Materiales y métodosLos ratones machos de 8 semanas de edad con «ApoE−/−mice» se distribuyen aleatoriamente en 3 grupos: 1) con heminefrectomía (SNX ApoE−/−), 12 ratones; 2) con nefrectomía única (UNX ApoE−/−), 11 ratones, y 3) operación de placebo (sham-op ApoE−/−), 15 ratones. Dieciséis semanas después de la cirugía, se retiraron los tejidos eréctiles de la aorta y el pene para realizar estudios histológicos con el fin de evaluar la aterosclerosis, la calcificación vascular, las sombras de nitrotirosina, el contenido de colágeno total y las sombras de macrófagos.
ResultadosDurante el sacrificio, los ratones con SNX y UNX reflejaron valores de urea sérica, colesterol total y concentración de triglicéridos significativamente más elevados, en comparación con los casos controlados con placebo. Las lesiones ateroscleróticas en la aorta torácica fueron mucho mayores en los ratones urémicos «ApoE−/−» en comparación con los controles. No hubo lesiones ateromatosas en los cuerpos cavernosos ni en la arteria del pene en ninguno de los grupos. Sin embargo, los animales con nefrectomía seminal y única mostraron un aumento significativo en la calcificación, presencia de colágeno y manchas de nitrotirosina en cuerpos cavernosos en comparación con los controles. El grado de infiltración de macrófagos fue comparable entre los 3 grupos.
ConclusiónSe ha concluido que incluso una disfunción renal menor, es decir, tras una nefrectomía única, aumenta la calcificación y exacerba la función endotelial de los cuerpos cavernosos en ratones «ApoE−/−», y este efecto puede estar asociado a un aumento del estrés oxidativo en el endotelio del pene.
Chronic kidney disease (CKD) in males causes sexual dysfunction with prevalence ranging between 71% and 98%.1 Erectyle dysfunction's (ED) in uremia is complex and multifactorial, involving a combination of classical risk factors (obesity, glucose intolerance, hypercholesterolemia, smoking and hypertension) and specific uremia-related risk factors (increased oxidative stress, endothelial dysfunction, inflammation). Endothelial dysfunction is associated with loss of nitric oxide (NO) bioavailability due to either reduced formation or accelerated degradation of NO. In turn, degradation of NO by increased reactive oxidant species including superoxide anion and oxidized lipoproteins might be a major mechanism underlying ED in these patients.2 In addition to the uremic milieu, peripheral neuropathy, autonomic insufficiency, peripheral vascular disease, and pharmacologic therapy all play an important role in the genesis of this problem.3,4
Endothelial dysfunction and accelerated atherosclerosis is one of the primary causes of morbidity and mortality in patients with CKD.5 ED and cardiovascular disease share the same risk factors. Accelerated atherosclerosis not only affects the coronary arteries but also may affect penile arteries in men, thus contributing to arteriogenic causes of ED in CKD patients. Thus atherosclerosis as a general health problem may be a link between these two entities.6
Since wild type rats or mice do not easily develop atheromatous lesions, we address these questions in the most common mouse model to study atherogenic mechanisms, namely the apolipoprotein E gene knock-out (apoE−/−) model mouse. ApoE−/− mice have delayed clearance of lipoproteins, and when placed on low-cholesterol, low-fat diets, their total serum cholesterol levels reach 11–13mM as a result of the accumulation of chylomicrons and cholesterol-rich VLDL remnants, as compared with 2–3mM in wild-type mice. Importantly, these genetically engineered mice develop not only fatty streaks but also widespread fibrous plaques at vascular sites that are typically affected in human atherosclerosis.7 Using this model, we have previously provided evidence that nephrectomy-induced uremia accelerated atherosclerosis and vascular calcification.8,9 Moreover, Xie et al. and others showed that this model provides a useful tool to analyze different cellular and molecular mechanisms underlying ED in the settings of generalized vascular disease and to test possible new therapeutic strategies.10,11 Therefore, the aim of the present study was to investigate the effect of CKD on vascular calcification and endothelial function of cavernosal bodies in apoE−/− mice.
MethodsAnimalsAll experiments were performed in male apoE−/− mice which were obtained from Charles River Laboratories (Lyon, France) and bred at our Medical Faculty animal facility. Animals were housed at a pathogen-free temperature controlled (25°C) facility with a strict 12-h light–dark cycle and they were given free access to water and a low-fat laboratory chow (Harlan Teklad Global Diet, 2014; Harlan, United Kingdom). The components of the diet as listed by the manufacturer were: 4% fat, 14.3% crude protein, 4.1% crude fiber, 0.6% calcium, and 0.9% phosphate. All experiments were in comply with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act. After sexual maturation at 4 weeks of age, the mice were separated by sex and housed in groups of up to 5. Only male mice were used for the present study.
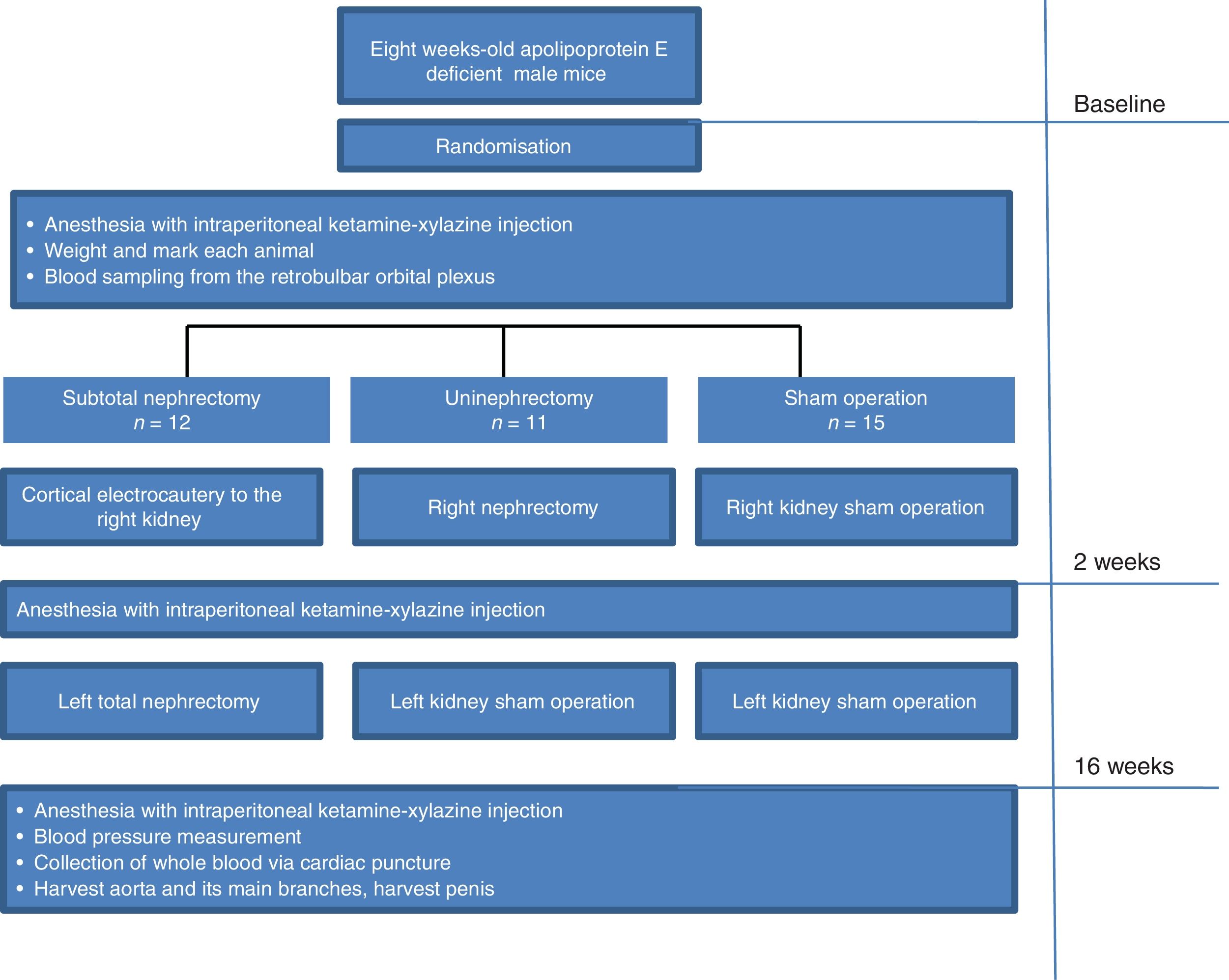
Study protocol and surgical proceduresFor creating CKD, 8 weeks old male apoE−/− mice were randomly assigned to the following 3 groups: (1) subtotally nephrectomised mice (SNX apoE−/−, 12 mice) that underwent cortical electrocautery to the right kidney and left total nephrectomy 2 weeks later, (2) uninephrectomized mice (UNX apoE−/−, 11 mice) and (3) left kidney sham operation mice (sham-op apoE−/−, 15 mice) that included decapsulation of both kidneys. The details about surgical procedures are previously reported by our group.9,12 All surgical procedures were performed under short-term anesthesia for a duration of 30–45min with one intraperitoneal injection composed of 75-μL Xylazine Rompun 2% (Bayer HealthCare, Leverkusen, Germany) in 25-mL vial, 300-μL Ketamine clorketam 1000 in 10-mL vial, and 1.53-mL 0.09% NaCl. The amount of the anesthetic fluid administered was 100–120μL/20-g body weight. Fig. 1 shows the detailed timeline explaining the study protocol.
After the completion of the study, the mice were anesthetized with intraperitoneal ketamine–xylazine injection (100mg/kg and 20mg/kg, respectively), and whole blood was collected via cardiac puncture. Thereafter, the aortic tree was perfused with 20-mL phosphate-buffered saline via 26G cannula inserted in the left ventricle, allowing unrestricted reflux from an incision in the right atrium. For aortic root sections, the ventricular edge and approximately 1mm of the aortic root were immediately dissected under a microscope and cryomounted in perpendicular position in an optimal cutting temperature–embedding medium
The aorta and its main branches were dissected from the left subclavian artery to the iliac bifurcation. All procedures were performed under dissecting microscope using a protocol previously described in detail.12
For histologic and immunocytochemical analyses of cavernosal bodies, the penis was harvested at time of sacrifice and cryomounted in perpendicular position in an optimal cutting temperature-embedding medium.
Biochemical analysisBlood samples were obtained from the retrobulbar orbital plexus at baseline and 16 weeks after surgery. Serum urea, total cholesterol and triglycerides concentrations were measured using a Hitachi 917 autoanalyzer (Roche, Meylan, France). Serum urea was used to evaluate the degree of CKD induced in the animals. Serum calcium and phosphorus concentrations were measured only at sacrifice using a Hitachi autoanalyzer, as mentioned.
Quantification of atherosclerotic lesionsAortic and penile lesion area was determined from 7-μm thick serial cryosections followed by oil red O staining and analysis of intimal area atherosclerosis. Evaluation of the atherosclerotic plaque area of the entire aorta opened longitudinally was made by the “en face” method using the same image analysis system as described previously.13 The acquisition of images and analysis of lesions were performed in a blinded fashion.
Quantification of calcification in aorta and cavernosal bodiesWe performed von Kossa staining in cryosections of the aortic root and cavernosal bodies to evaluate vascular calcium deposits. The precision and the accuracy of this method have been reported elsewhere with semiautomated measurement software.8 Data were expressed as the relative proportion of calcified area to total surface area, as reported previously.8
Quantification of monocyte–macrophage infiltration, collagen content, and nitrotyrosine expression in cavernosal bodiesMonocyte macrophages infiltration, collagen content and nitrotyrosine were measured as reported previously.14 Briefly, for nitrotyrosine analysis the sections were preincubated in peroxidase blocking solution (Dako Cytomation) before incubation with biotinylated nitrotyrosine monoclonal mouse antibody (Cayman Chemical, SpiBio, Massy, France). The sections were treated with peroxidase-labeled streptavidin (Dako) for 15min followed by reaction with diaminobenzidine/hydrogen peroxidase. For monocyte–macrophage infiltration, aortic sections were incubated with 10% normal goat serum at room temperature and incubated with a primary rat monoclonal antibody against mouse macrophages (clone MOMA-2; BioSource International, Camarillo, CA). The secondary antibody was a biotin–horseradish peroxidase-conjugated goat anti-rat IgG (Vector Laboratories, Biovalley, Marne la Vallée, France). Immunostainings were visualized after incubation with a peroxidase detection system (Vectastain ABC kit, Vector Laboratories) with 3-amino-9-ethyl carbazole (Sigma Aldrich) used as substrate. The lesion collagen content was determined by staining with Sirius red.
Blood pressure measurementsWe measured mean arterial blood pressure at the day of sacrifice using direct intra-arterial recording by a previously established method.13 Briefly, the mice were anesthetized with ketamine/xylazine (100mg/kg and 20g/kg, intraperitoneally), and a PE50 catheter, stretched to reduce the tip diameter, was inserted into the abdominal aorta. The catheter was filled with heparinized saline (20U/mL), and the distal end was attached to a BP transducer (Gould pressure processor). After a 5-min stabilization period, three BP tracings were obtained, and BP was averaged from the three measurements.
Statistical analysisData were analyzed by ANOVA, with 1 or 2 factors taken into account as appropriate, Scheffe's post hoc test and χ2 test. Results were expressed as mean±SEM. Differences between groups were considered significant at P<0.05.
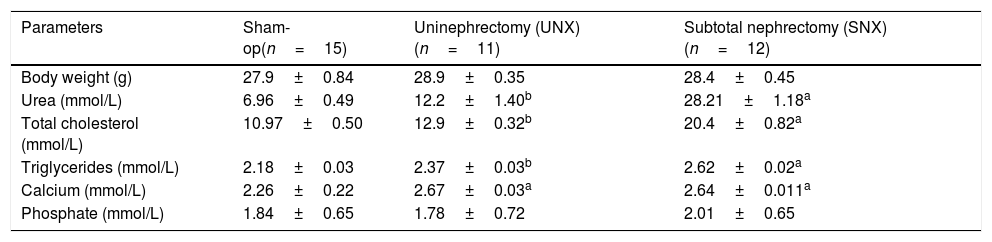
ResultsBody weight and serum biochemistryBody weight did not differ between the SNX, UNX and sham-op apoE−/− mice throughout the study (Table 1). Serum urea levels were measured to assess renal function. At 16 weeks after surgery, serum urea concentrations in SNX mice were 200% increased, as compared with those in the sham-op group (P<0.001, Table 1). Likewise, UNX mice showed significant increase in serum urea level compared to controls. Serum total cholesterol and triglyceride levels also were significantly higher in SNX and UNX mice, P<0.0001 and P<0.001, respectively (Table 1). SNX and UNX groups had higher serum calcium concentrations (P<.0001), whereas serum phosphate level did not differ between groups (Table 1). Mean arterial blood pressure did not differ between the groups (data not shown).
Effect of subtotal (SNX) vs unilateral nephrectomy (UNX) on body weight and serum concentrations of urea, total cholesterol, triglycerides, calcium and phosphate.
| Parameters | Sham-op(n=15) | Uninephrectomy (UNX)(n=11) | Subtotal nephrectomy (SNX)(n=12) |
|---|---|---|---|
| Body weight (g) | 27.9±0.84 | 28.9±0.35 | 28.4±0.45 |
| Urea (mmol/L) | 6.96±0.49 | 12.2±1.40b | 28.21±1.18a |
| Total cholesterol (mmol/L) | 10.97±0.50 | 12.9±0.32b | 20.4±0.82a |
| Triglycerides (mmol/L) | 2.18±0.03 | 2.37±0.03b | 2.62±0.02a |
| Calcium (mmol/L) | 2.26±0.22 | 2.67±0.03a | 2.64±0.011a |
| Phosphate (mmol/L) | 1.84±0.65 | 1.78±0.72 | 2.01±0.65 |
Values are means±SEM.
Determinations done 16 weeks after subtotal nephrectomy, left unilateral nephrectomy or sham-op.
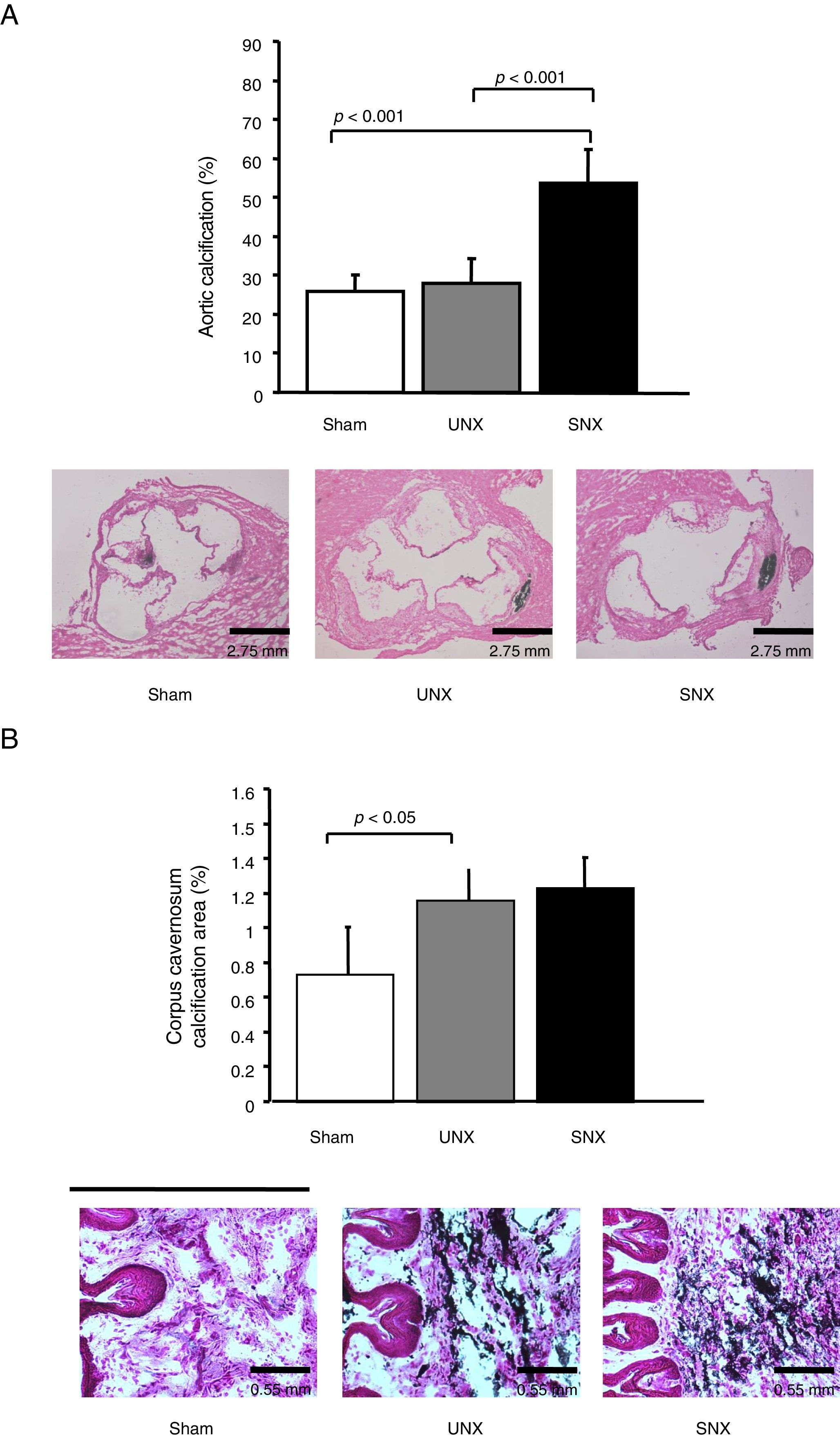
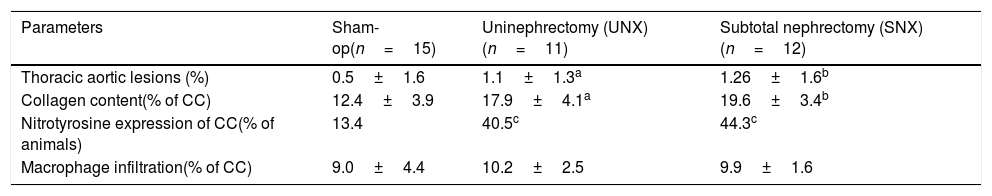
Atherosclerotic lesions in thoracic aorta were significantly larger in SNX and UNX mice than non-uremic controls (Table 2). There were no atheromatous lesions in cavernosal bodies or penile arteries observed in any group. However, SNX animals showed a significant increase in calcification score in both aorta and cavernosal bodies when compared with controls (Fig. 2A and B). In addition, calcification score in the cavernosal bodies was significantly higher in UNX mice, as compared with controls (Fig. 2B). No such difference was observed in the aorta between UNX and controls (Fig. 3A).
Cavernosal bodies and aortic tissue characteristics of apoE−/− mice at study end.
| Parameters | Sham-op(n=15) | Uninephrectomy (UNX)(n=11) | Subtotal nephrectomy (SNX)(n=12) |
|---|---|---|---|
| Thoracic aortic lesions (%) | 0.5±1.6 | 1.1±1.3a | 1.26±1.6b |
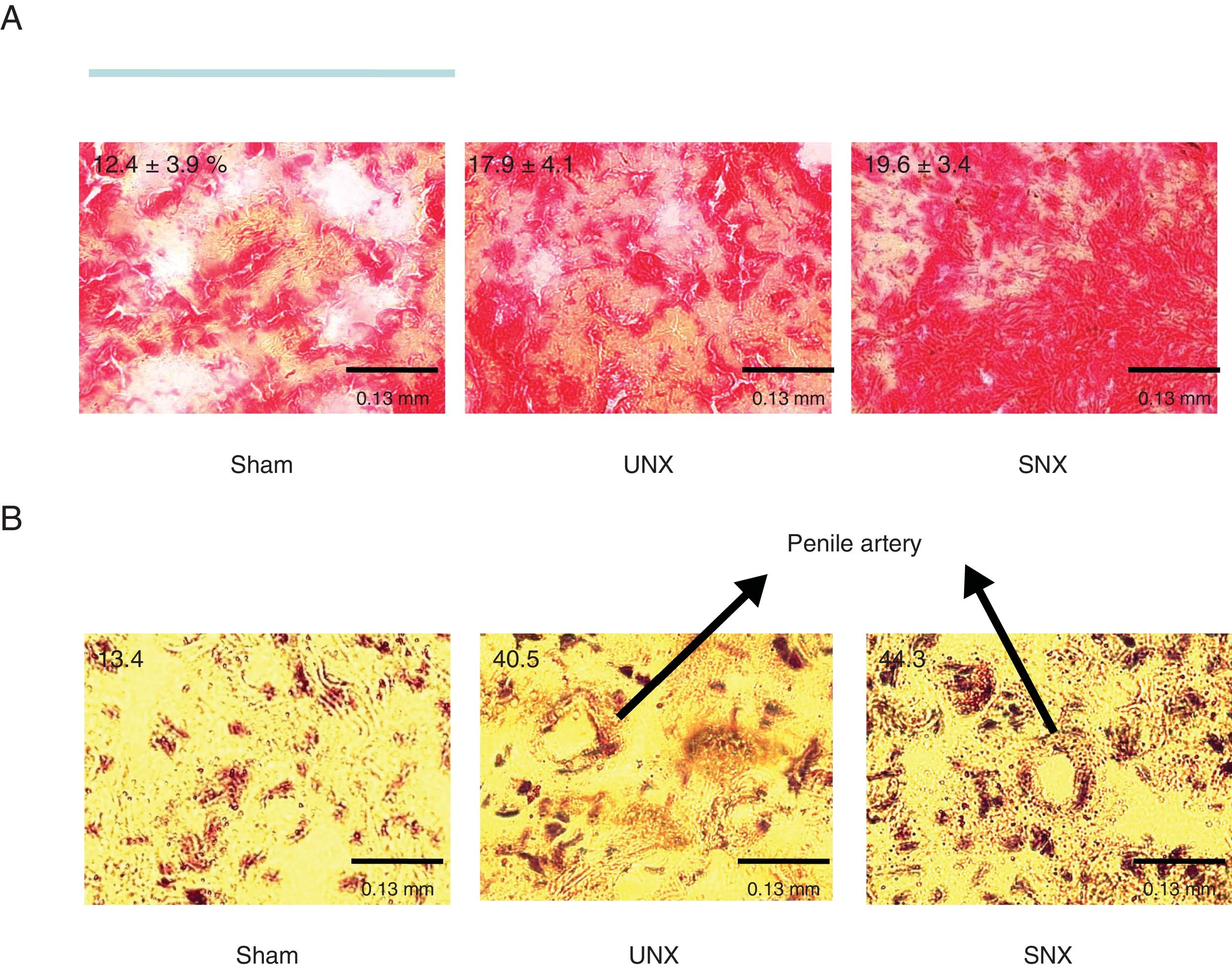
| Collagen content(% of CC) | 12.4±3.9 | 17.9±4.1a | 19.6±3.4b |
| Nitrotyrosine expression of CC(% of animals) | 13.4 | 40.5c | 44.3c |
| Macrophage infiltration(% of CC) | 9.0±4.4 | 10.2±2.5 | 9.9±1.6 |
CC, corpus cavernosum.
Values are means±SEM.
Effects of subtotal nephrectomy (SNX) vs unilateral nephrectomy (UNX) on vascular calcification in the aortic root and the cavernosal bodies in male apolipoprotein E deficient mice. (A) Upper panel: SNX animals showed a significant increase in vascular calcification in the aorta when compared with controls. Lower panel: Representative findings of calcification in the aortic root in the 3 study groups. Note the 3 aortic valves, cut in a perpendicular position to obtain a perfect image. Calcification is marked in black. Magnification 2.5×. (B) Upper panel: SNX and UNX mice showed a significant increase of calcification score in the cavernosal bodies when compared with controls. Control (n=15), UNX (n=11), and SNX (n=12). Values are means±standard error of the mean. Lower panel: Representative findings of calcification in the cavernosal bodies. Magnification 10×.
Representative findings of collagen content and nitrotyrosine staining in cavernosal bodies in the 3 study groups. (A) An increased Sirius red staining representing collagen content is observed in subtotal nephrectomy (SNX) and unilateral nephrectomy (UNX) mice. Numbers are showing the percentage of collagen content from whole surface area measured. (B) An increased brown staining showing nitrotyrosine staining. Numbers are showing the percentage of animals with positive nitrotyrosine expression. Arrows are pointing to a penile artery. Magnification 40×.
To investigate whether SNX or UNX, respectively, had an impact on cavernosal bodies morphology, detailed histological studies analyzing nitrotyrosine expression, and collagen content and macrophage infiltration, were performed. Collagen content was higher in cavernosal bodies of SNX and UNX mice than in those of controls (Table 2 and Fig. 3A). Furthermore, SNX and UNX mice showed increased nitrotyrosine expression in the cavernosal bodies compared with sham-operated controls (Table 2 and Fig. 3B). The percentage of lesion cross-section area occupied by macrophages, as revealed by MOMA-2 staining, was comparable between the 3 groups (Table 2).
DiscussionIn the present study we show that uremic state develops structural changes in the cavernosal bodies in apoE−/− mice. We found increased calcium deposits, collagen content and nitrotyrosine expression in cavernosal bodies of uremic apoE−/− mice despite the absence of atherosclerotic lesions. We did not find evidence of changes in local markers of inflammation (MOMA-2 staining). These findings are in line with our previous reports8,15 of increased vascular calcification in uremia and extend them by showing that even mild renal dysfunction, i.e., after uninephrectomy, increases calcification score and aggravates endothelial function of cavernosal bodies in uremic apoE−/− mice.
Vascular calcification is a prominent feature of CKD16 and it is predictive of increased cardiovascular morbidity and mortality.17 Coronary, pelvic and penile artery plaques in patients with end stage renal failure are characterized by increased medial thickness and marked intimal and medial calcification.16,18 In our previous studies, we have shown that uremic apoE−/− mice have increase in both medial and plaque calcification in the aorta.8 In the present study, we show that uremia increases calcification score of cavernosal bodies in these mice. Interestingly, calcification of erectile bodies in uremic animals occurs even before any visible atherosclerosic lesions in the penile artery indicating that it might represent an important sentinel of impending systemic vascular disease in this population. Moreover, our results show that calcification in cavernosal bodies in UNX mice precedes aortic calcification indicating that even in mild uremia as the one observed in UNX animals, penile endothelium might be more susceptible to vascular dysfunction and calcification compared to that of aorta. In line with this contention, it has been shown that the distal internal pudendal arteries in uremic rats are more susceptible to vascular dysfunction and calcification compared to carotid artery and thoracic aorta.19
One of the possible molecular mechanisms of increased vascular disease leading to severe ED in uremia is increased oxidative stress. There is a range of oxidative stress markers present in the plasma of CKD patients indicating that CKD is a pro-oxidant state.20 The results of the present study and previous studies by us and others15,21 in uremic mouse models are in agreement with this hypothesis. In the present study, uremic animals showed increased nitrotyrosine expression in cavernosal bodies as compared with sham-op mice. This finding supports the link between oxidative stress and ED in CKD and points to the possible importance of nitrosative-oxidative stress in the pathogenesis of vascular disease of uremic mice. Nitrotyrosine is an indirect marker of peroxynitrite generation that results from the reaction between nitric oxide and superoxide. Peroxynitrite sustains oxidative injury to the endothelium and reduces nitric oxide availability. Nitrotyrosine has been shown to be present in proteins in a variety of clinical conditions, including atherosclerotic lesions in human coronary arteries, post-ischemic myocardium, and placenta during preeclampsia.20,22
In our study, there was no evidence of increased macrophage infiltration related to a probable inflammation of the erectile tissue. It remains unclear to which extent inflammation contributes to the enhanced calcium deposition in cavernosal bodies observed in the CKD apoE−/− mice. It is interesting that a recent study in men addresses the importance of inflammation in the cavernosal bodies and highlights the difference in marker concentrations between venous blood from the arm and penile blood.23 However, the authors failed to show any difference in terms of clinical erectile score predictivity, suggesting that the widely accepted association between inflammation and ED is more complex than was generally thought.23 However, C-reactive protein serum concentration was observed to be inversely related to that of fetuin-A (a negative acute-phase protein and an extracellular calcium regulatory molecule), and lower fetuin-A concentrations in sera were found in men with low, mild severe ED compared to controls.24
One of the proposed mechanisms responsible for uremia-related ED is dylipidemia, mostly due to an increase in oxidative stress and impaired NO bioavailability within the erectile tissue of the penis. There is a close link between dyslipidemia and ED, with endothelial dysfunction as a common mechanism. High cholesterol is a well-identified risk factor in the vascular disease and surely contributed to the development of accelerated atherosclerosis and calcification in our experimental model.
Increased production and deposition of extracellular matrix proteins, including collagens, are a hallmark of fibrosis. In the present study even after unilateral nephrectomy, we have observed a marked increase of collagen deposition and nitrotyrosine expression compared with control group suggesting that structural changes in the penis begin in early stages of uremia. Overall, the morphology of cavernosal bodies in UNX mice was similar to those of SNX mice characterized by higher degree of calcification, increased deposits of collagen, nitrotyrosine expression and virtual absence of smooth muscle cell, and macrophage infiltration, linking uremic state to more aggressive morphology changes.
There are certain limitations to the interpretation of our present findings. No animal model mimics human vascular disease perfectly. In addition, hypercholesterolemia per se promotes oxidative stress by still unclear mechanisms, and the degree of hypercholesterolemia in apoE−/− mice far exceeds that seen in human subjects.
In conclusion, even mild renal dysfunction, i.e., after uninephrectomy increases calcification score of cavernosal bodies in apoE−/− mice and this effect might be linked to increased oxidative stress in penile endothelium. We propose that calcification of erectile bodies may represent an important sentinel of impending systemic vascular disease in uremic population. We anticipate that this mouse model will be useful to test treatment strategies aiming to target vascular disease in context of mild or severe uremia.
Ethical approvalAll applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestAll authors declared no conflict of interest.