To investigate the effects of combined tadalafil and testosterone usage on oxidative stress, DNA damage and MMPs in testosterone deficiency.
MethodsFifty rats were randomly divided into 5 groups (group-1: sham group-placebo, group-2: bilateral orchiectomy (ORX), group-3: bilateral ORX+tadalafil, group-4: bilateral ORX+testosterone, group-5: bilateral ORX+tadalafil+testosterone). Group-3 received tadalafil (5mg/kg/day, oral). Group-4 was administered testosterone undecanoate (100mg/kg i.m., single dose). Group-5 was administered a combination of tadalafil and testosterone undecanoate. All groups were compared with regard to serum nicotinamide adenine dinucleotide phosphate oxidase-4 (NOX-4), total thiol, matrix metalloproteinase-2 (MMP-2), MMP-3 and MMP-9, tissue inhibitor of metalloproteinases-1 (TIMP-1) and TIMP-2 and 8-hydroxy-2-deoxy guanosine (8-OHdG) levels.
ResultsTotal thiol levels of group-2 were significantly lower than the other groups and thiol levels were higher in group-1 and group-5 than in the other groups. NOX4, MMP2 and 9 levels in group-2 were higher than in the other groups. MMP-9 levels in group-5 were lower than in groups 3 and 4 (p=.001). The level of 8-OHdG in groups 2 and 3 was higher than in the other groups (p=.001). In correlation analysis, 8-OHdG, MMP2, and 9 levels were negatively correlated with total thiol, whereas NOX4 and 8-OHdG levels were positively correlated with MMPs values.
ConclusionsThe combination of testosterone with PDE-5 inhibitor suppresses MMP-9 levels and increases total thiol levels better than testosterone alone and tadalafil alone. Therefore, testosterone can be considered for use with PDE-5 inhibitor from the initial stage in case of testosterone deficiency.
Investigar los efectos del uso combinado de tadalafil y testosterona en cuanto a estrés oxidativo, daño del ADN y metaloproteinasas de la matriz (MMPs) en la deficiencia de testosterona.
MétodosSe dividió aleatoriamente a cincuenta ratas en cinco grupos (grupo-1: grupo de simulación-placebo, grupo-2: orquiectomía bilateral (ORX), grupo-3: ORX bilateral+tadalafil, grupo-4: ORX bilateral+testosterona, grupo-5: ORX bilateral+tadalafil+testosterona). El grupo 3 recibió tadalafil (5mg/kg/day, oral). El Grupo 4 recibió undecanoato de testosterona (100mg/kg i.m, dosis única). El Grupo 5 recibió una combinación de tadalafil y undecanoato de testosterona. Se comparó a todos los grupos con respecto a los niveles séricos de nicotinamida adenina dinucleótido fosfato oxidasa-4 (NOX-4), tiol total, metaloproteinasa de la matriz 2 (MMP-2), MMP-3 y MMP-9, inhibidor tisular de metaloproteinasas-1 (TIMP-1) y TIMP-2, y 8-hidroxi-2-deoxi guanosina (8-OHdG).
ResultadosLos niveles totales de tiol del grupo 2 fueron significativamente menores que en el resto de grupos, y los niveles de tiol fueron mayores del grupo 1 y el grupo 5 con respecto a los demás grupos. Los niveles de NOX4, MMP2 y 9 en el grupo 2 fueron mayores que los del resto de grupos. Los niveles de MMP-9 del grupo 5 fueron menores que los de los grupos 3 y 4 (p=0,001). El nivel de 8-OHdG de los grupos 2 y 3 fue mayor que los del resto de grupos (p=0,001). En el análisis de correlación, los niveles de 8-OHdG, MMP2, y 9 guardaron una correlación negativa con tiol total, mientras que los niveles de NOX4 y 8-OHdG se correlacionaron positivamente con los valores de MMPs.
ConclusionesLa combinación de testosteronay el inhibidor de PDE-5 suprime los niveles de MMP-9 e incrementa los niveles totales de tiol, de mejor manera que testosterona y tadalafilen solitario. Por tanto, puede considerarse el uso de testosterona con el inhibidor de PDE-5 en las etapas iniciales de deficiencia de testosterona.
Testosterone has critical importance for gender differentiation, penis and testis formation, muscle development, regulation of lipid and glucose metabolism and cognitive functions. In addition to deterioration in reproductive function, erectile function, libido, bone mineral density and mood, testosterone deficiency is accepted as the one of the risk factors for cardiovascular diseases, metabolic diseases and obesity.1,2
Testosterone deficiency has been associated with increased oxidative stress, changed matrix metalloproteinase (MMP) enzyme activity, penile tissue fibrosis and apoptosis.3–5 Oxidative stress results from an imbalance between increased reactive oxygen species (ROS) and the antioxidant system as a result of an increase in endogenous synthesis in situations involving external factors or pathogens. Excess ROS production attacks proteins, lipids, thiols and nucleic acids and disrupts their structures.6 Nicotinamide adenine dinucleotide phosphate oxidase (NOXs) is a family of enzymes that catalyze the oxidation of nicotine amide adenine dinucleotide phosphate (NADPH). There are 7 members of this enzyme family (NOX 1–5 and DUOX 1,2), and these enzymes are thought to be responsible for endogenous ROS production. The increase of especially NOX4 among these enzymes causes excessive production of endogenous ROS.7 Thiol-containing organic molecules are an important part of the antioxidant system. A decrease in total thiol concentration is an important indicator of increased oxidative stress.8 As a result of DNA being attacked by ROS, strand breaks and base modifications occur. 8-hydroxy-2-deoxy guanosine (8-OHdG), one of these base modification products, is used as an important indicator of oxidative DNA damage.9
Matrix metalloproteinases-2 (MMP-2) and MMP-9 are key enzymes in the extracellular matrix and play a role in collagen degradation. It was shown that tissue inhibitor of metalloproteinases-1 (TIMP-1) increased and MMP-2 levels decreased in patients with erectile dysfunction (ED) and that sildenafil, a phosphodiesterase-5 (PDE-5) inhibitor, inhibits MMP-2.10 Administration of high levels of testosterone decreases MMP-1 expression but does not have an effect at low concentration.11 Phosphodiesterase-5 inhibitors reduce oxidative damage by increasing antioxidant enzyme activities.12 It was shown that sildenafil decreased NADPH oxidase activity and that NADPH oxidase mediated superoxide increased PDE5 expression.13,14 However, the effect of combined use of testosterone and PDE5 inhibitors is not known.
We investigated the effects of testosterone, tadalafil and combined testosterone and tadalafil usage on oxidative stress markers, DNA damage and MMPs in orchiectomy-induced testosterone insufficiency. In this context, the aim was to gain an idea about whether the PDE5 inhibitor combination with testosterone should be administered at the beginning of hypogonadism.
Materials and methodsAnimals and experimental protocolThis study was approved by the Institutional Animal Experimental Ethics Committee (approval number: 2018/12). This study was carried out according to the rules stated in the revised Helsinki declaration. The study included a total of 50 adult male Wistar Albino rats with a mean weight of 250–350g. The animals were housed under a 12-h light/dark cycle at 22±1°C with 50–60% humidity. The rats were randomly divided into 5 groups;
Group-1 (n=10): sham group (placebo surgery)
Group-2 (n=10): bilateral orchiectomy (ORX)
Group-3 (n=10): bilateral ORX+tadalafil
Group-4 (n=10): bilateral ORX+testosterone
Group-5 (n=10): bilateral ORX tadalafil+testosterone.
The surgical procedures were performed under anesthesia with 20mg/kg ketamine (Ketazol®, Richter Pharma, Wels, Austria). The animals in the sham group (Group-1) underwent scrotal midline incision to release the testicles and then were closed. The animals in groups 2–5 underwent bilateral orchiectomy as well as scrotal midline incision. Nothing other than orchiectomy was applied to group-2. One month after orchiectomy, group-3 received 5mg/kg/day tadalafil (Longis®, Santa Pharma, Istanbul, Turkey) orally once a day for one month. Group-4 underwent testosterone replacement after 30 days of orchiectomy with a single intramuscular (i.m.) dose of 100mg/kg testosterone undecanoate (Nebido, Bayer Schering Pharma, Berlin, Germany). Group-5 underwent both testosterone undecanoate and tadalafil administration. During the study, all rats were fed with standard pellet feed.
SamplesAt the end of the two-month process, intracardiac blood samples were taken from the rats following anesthesia and all animals were sacrificed. A portion of the blood samples were taken into biochemistry tubes that did not contain anti-coagulants. Tubes were centrifuged at 3000×g for 10min to obtain serum samples. The other blood samples were transferred to hemogram tubes containing ethylene diamine tetra acetic acid (EDTA) and stored at −80°C.
Biochemical analysisThe levels of NOX4, MMP-2, MMP-3, MMP-9, TIMP-1, TIMP-2 and total thiol were measured with commercially available enzyme-linked immunosorbent test kits (ELISA, YL biont, Shanghai YL Biotech Co., Ltd). Oxidative DNA damage detection was performed in two steps. The leukocyte DNA was isolated from whole blood samples with a commercially available DNA isolation kit (Invitrogen, CA, USA). Isolated DNA samples were incubated with formic acid at 150°C for 1h and hydrolyzed. In the second stage, hydrolyzed DNA samples were diluted with 1/1 volume of acetonitrile, and the quantitative analysis of serum 8-OHdG was performed with the high-performance liquid chromatography-electrochemical detection method. The level of 8-OHdG was expressed as 8-OHdG molecules per 106dG.
Statistical analysisData were analyzed using Statistical Package for Social Sciences (SPSS) Version 22 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean and standard deviation. The normal distribution of the data was determined by Kolmogorov–Smirnov test. Since the data showed normal distribution, differences between the groups were determined by Kruskal–Wallis analysis. The Tukey's honestly significant difference test was performed to determine which group values caused the differences. The means with a p value of 0.05 or less were considered significant compared to each other. A p value of<0.05 was considered significant.
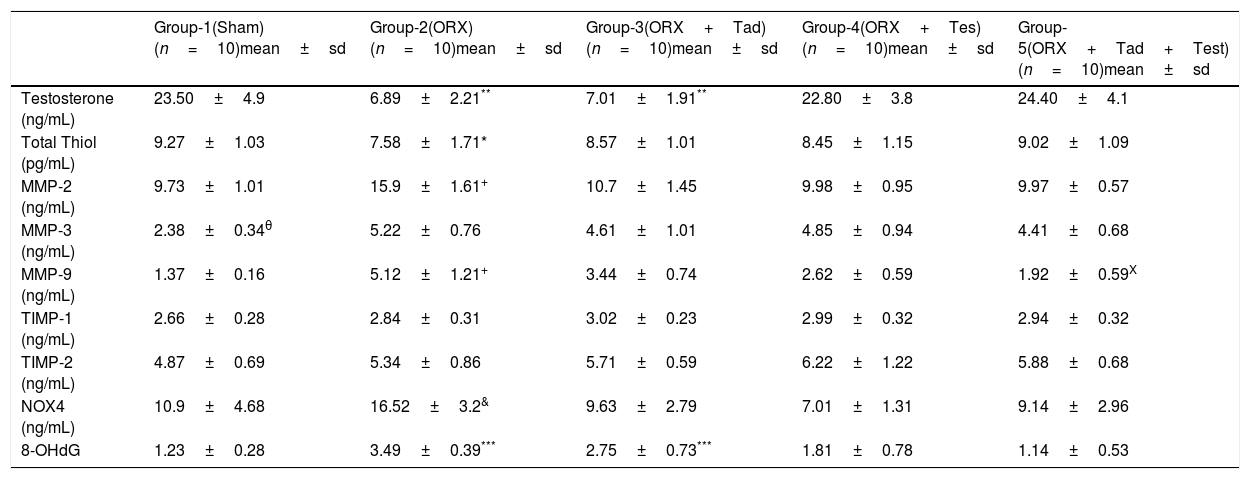
ResultsTable 1 presents the comparisons of the biochemical parameters. Serum testosterone levels were significantly lower in group-2 and group-3 compared to the other groups (p=0.001). On the other hand, there was no significant difference between testosterone levels in groups 1, 4, and 5.
Comparison of the groups with regard to testosterone, oxidative stress markers, MMP and total thiol levels.
| Group-1(Sham)(n=10)mean±sd | Group-2(ORX)(n=10)mean±sd | Group-3(ORX+Tad)(n=10)mean±sd | Group-4(ORX+Tes)(n=10)mean±sd | Group-5(ORX+Tad+Test)(n=10)mean±sd | |
|---|---|---|---|---|---|
| Testosterone (ng/mL) | 23.50±4.9 | 6.89±2.21** | 7.01±1.91** | 22.80±3.8 | 24.40±4.1 |
| Total Thiol (pg/mL) | 9.27±1.03 | 7.58±1.71* | 8.57±1.01 | 8.45±1.15 | 9.02±1.09 |
| MMP-2 (ng/mL) | 9.73±1.01 | 15.9±1.61+ | 10.7±1.45 | 9.98±0.95 | 9.97±0.57 |
| MMP-3 (ng/mL) | 2.38±0.34θ | 5.22±0.76 | 4.61±1.01 | 4.85±0.94 | 4.41±0.68 |
| MMP-9 (ng/mL) | 1.37±0.16 | 5.12±1.21+ | 3.44±0.74 | 2.62±0.59 | 1.92±0.59X |
| TIMP-1 (ng/mL) | 2.66±0.28 | 2.84±0.31 | 3.02±0.23 | 2.99±0.32 | 2.94±0.32 |
| TIMP-2 (ng/mL) | 4.87±0.69 | 5.34±0.86 | 5.71±0.59 | 6.22±1.22 | 5.88±0.68 |
| NOX4 (ng/mL) | 10.9±4.68 | 16.52±3.2& | 9.63±2.79 | 7.01±1.31 | 9.14±2.96 |
| 8-OHdG | 1.23±0.28 | 3.49±0.39*** | 2.75±0.73*** | 1.81±0.78 | 1.14±0.53 |
p values were determined according to Kruskal–Wallis test.
Total thiol levels of group-2 were significantly lower than the other groups (p=0.03) and thiol levels were higher in group-1 and group-5 than the other groups (p=0.045).
MMP-2 and MMP-9 levels in group-2 were significantly higher than in the other groups (p=0.001) and MMP-9 levels in group-5 were lower than in groups 3 and 4 (p=0.001). MMP-3 levels were lower in group-1 compared to the other groups (p=0.001). However, group 2 had the highest numerical value.
TIMP-1 and TIMP-2 levels were similar in all groups.
The level of NOX4 was higher in group-2 than in the other groups (p=0.003). There was no significant difference between the other groups.
The levels of 8-OHdG in groups 2 and 3 were higher than in the other groups (p=0.001). However, there was no significant difference between groups 2 and 3.
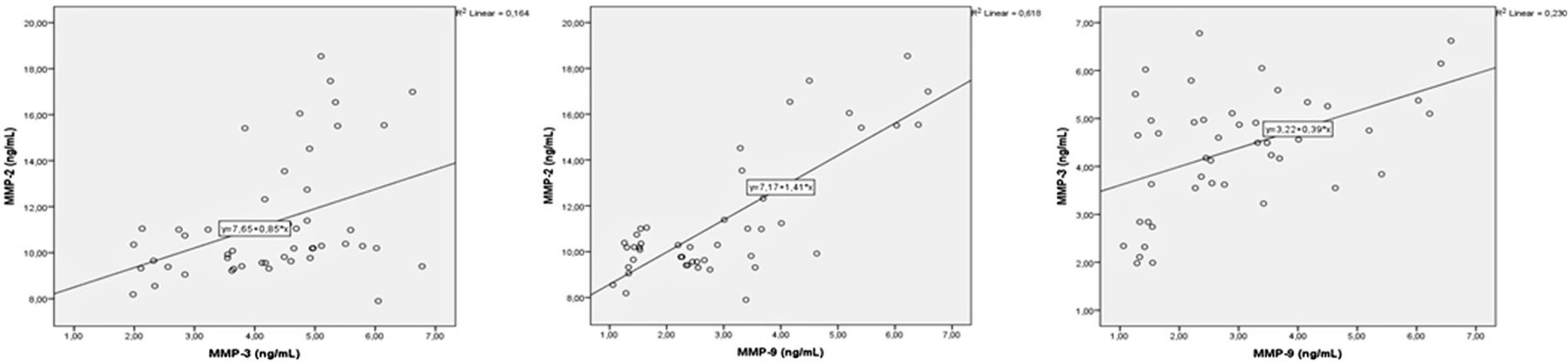
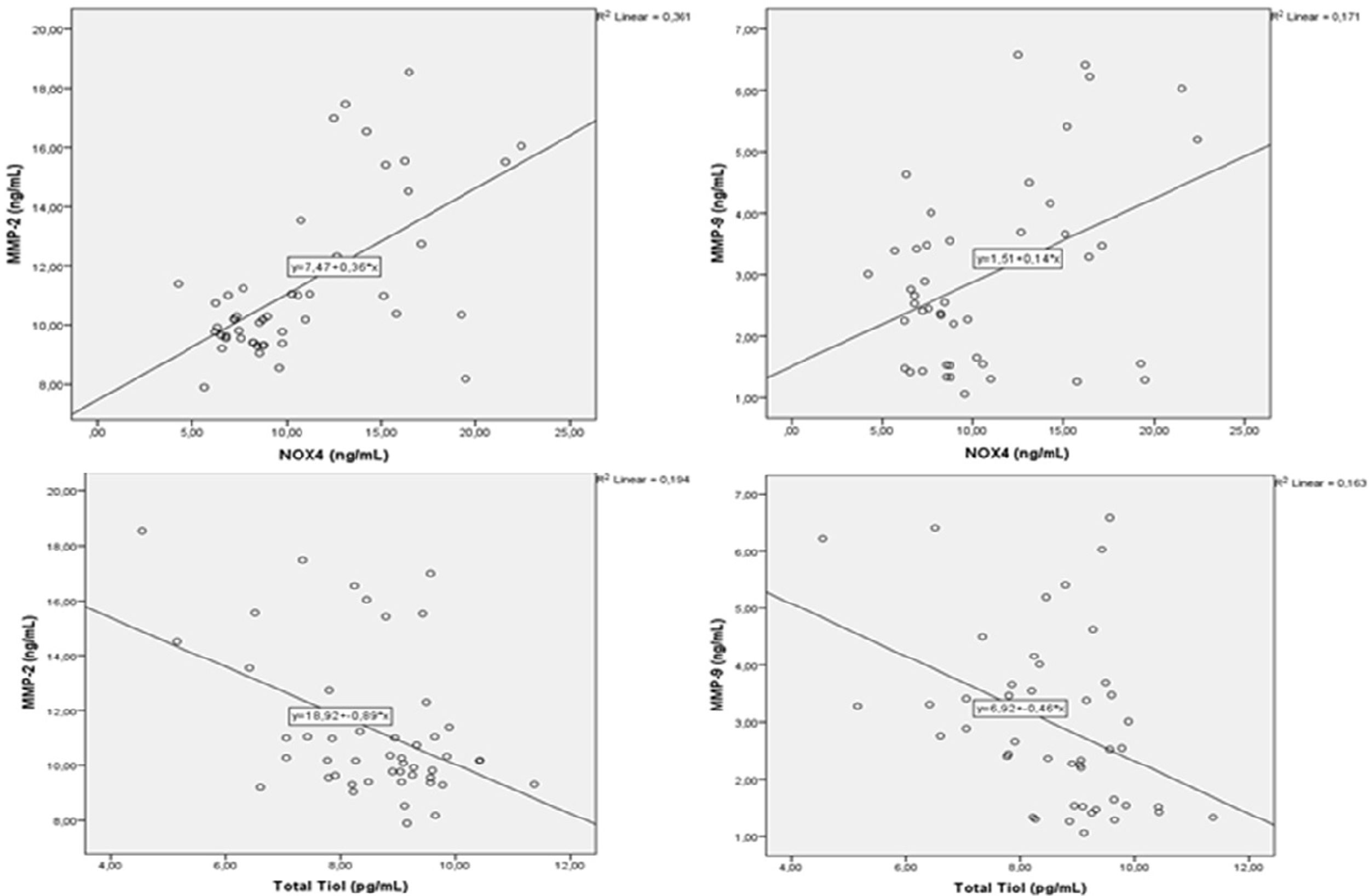
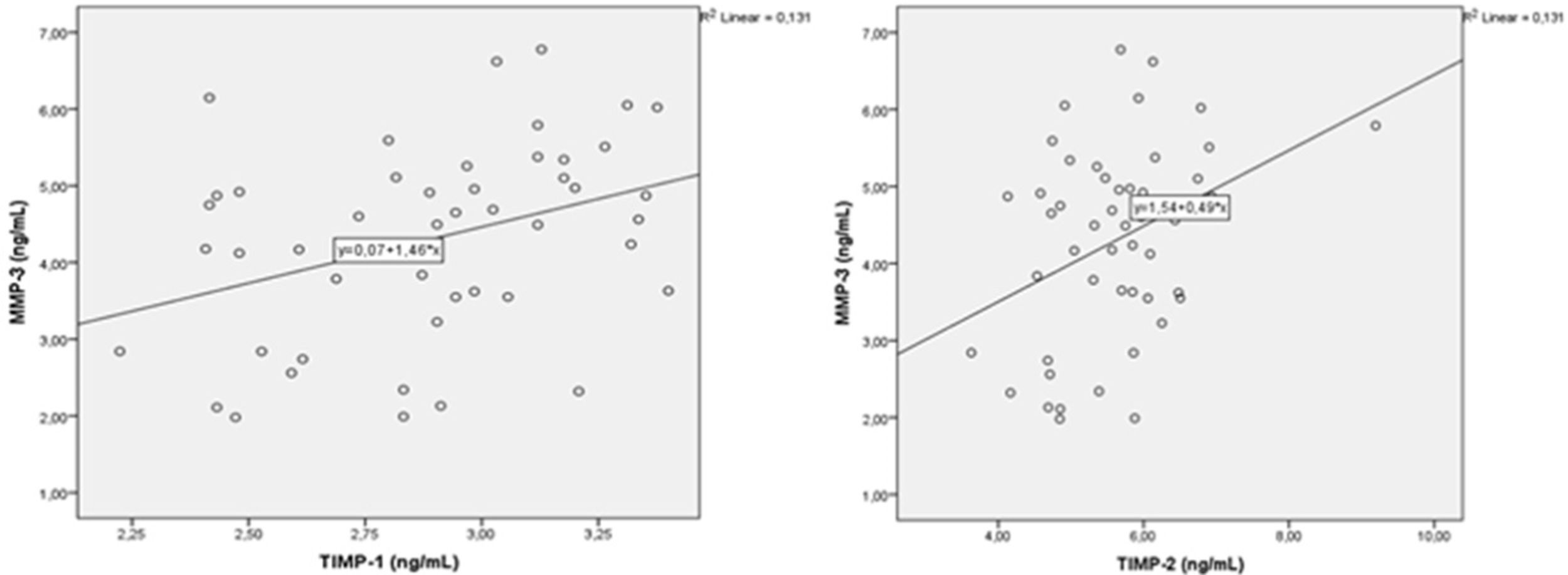
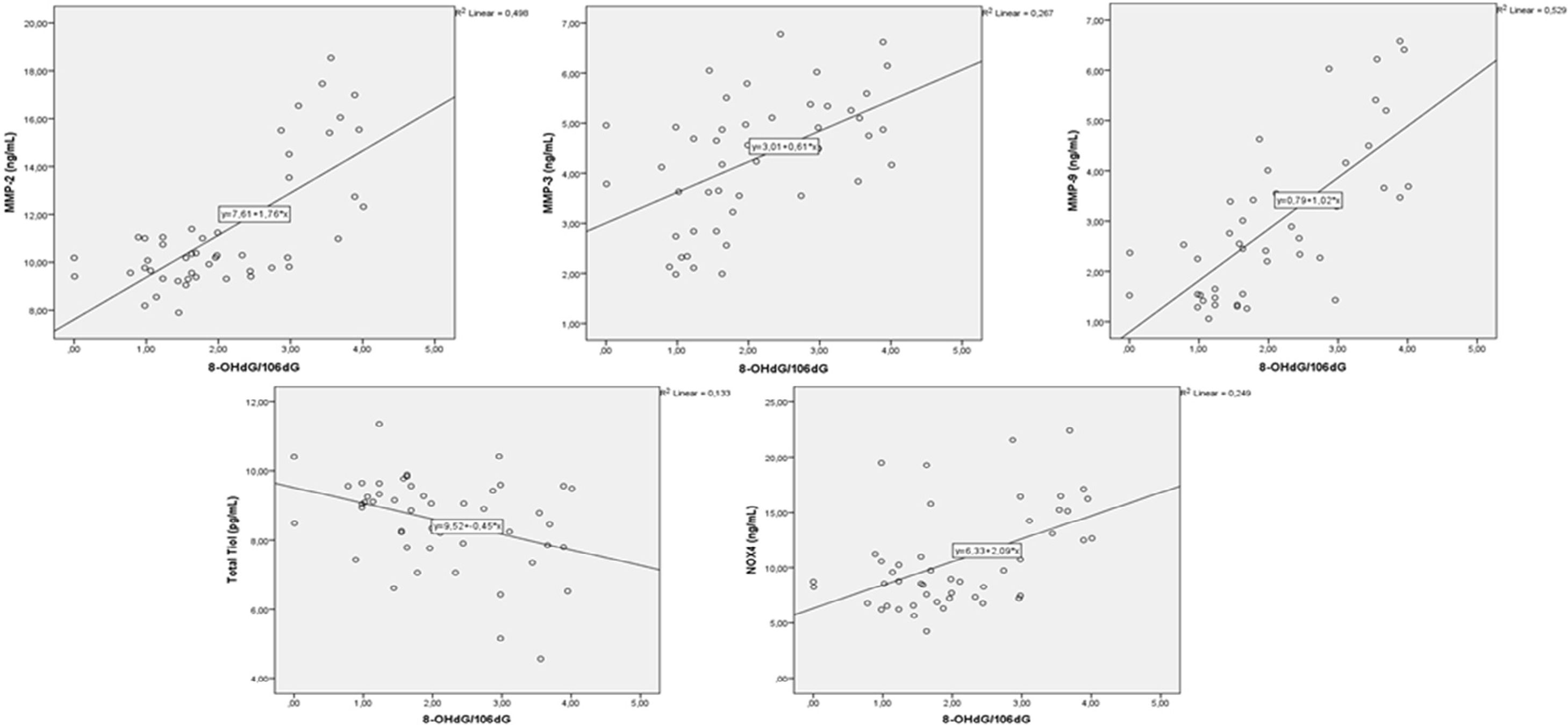
A correlation analysis was performed between MMPs and oxidative stress parameters. There were positive correlations between MMP-2 with MMP-3 and MM-9, and between MMP-3 and 9 (p=0.004, r=0.405, p=0.001, r=0.786, p=0.001, r=0.480, respectively) (Fig. 1). There was a negative correlation between MMP-2 and total thiol (r=−0.440, p=0.002). MMP-9 showed negative correlation with total thiol (p=0.005, r=−0.404) and showed positive correlation with 8-OHdG and NOX4 (p=0.001, r=0.727, p=0.004, r=0.413, respectively) (Fig. 2). MMP-3 showed positive correlation with TIMP-1 and TIMP-2 (p=0.012, r=362) (Fig. 3). MMP-3 was positively correlated with 8-OHdG (p=0.001 r=0.517). There was a negative correlation (p=0.011, r=0.365) between antioxidant total thiol and 8-OHdG values and a positive correlation between NOX4 and 8-OHdG (p=0.001, r=0.499) (Fig. 4).
DiscussionThis study demonstrated that serum testosterone levels in orchiectomized rats were low compared to normal rats and rats with testosterone replacement therapy. Also, it was determined that NOX4 and 8-OHdG levels were significantly high and antioxidant total thiol levels were significantly low due to testosterone deficiency. All these findings indicate that testosterone deficiency is an important cause of oxidative stress increase. In addition, we found a significant change in MMP 2, 3 and 9 levels. This variation, especially in the MMP 2 and 9 levels, was positive according to the NOX4 and 8-OHdG levels, while it was negative compared to total thiol levels. This suggests that increased oxidative stress due to testosterone deficiency leads to a significant change in MMP levels. But did increased MMP levels induce NOX4 and 8-OHdG levels? Or did increased NOX4 and 8-OHdG levels induce MMPs levels? More detailed studies are needed to clarify this situation.
The relationship between testosterone and oxidative stress is still controversial. It was reported that testosterone was shown to be a pro-oxidant and to have antioxidant effects.15,16 Meydan et al. showed that testosterone deficiency in orchiectomized male rats caused increased oxidative damage and decreased antioxidants.17 Li Zhang et al. showed a significant decrease in antioxidant (superoxide dismutase; SOD and glutathione peroxidase; GSH-Px) enzyme levels and a significant increase in malondialdehyde (MDA) levels, due to testosterone deficiency in orchiectomized rats. They also observed a significant improvement in the activities of antioxidant enzymes (SOD, GSH-Px) after testosterone replacement therapy.18 In addition, Mancini et al. showed that testosterone deficiency leads to a significant decrease in Coenzyme Q10 levels, an antioxidant, and a significant increase in Coenzyme Q10 level occurred with testosterone supplementation.19 Pintana et al. showed that testosterone replacement therapy improves metabolic parameters and cognitive functions by decreasing oxidative stress in orchiectomized rats.20 Klapcinska et al. reported that testosterone replacement restores reduced SOD and GSH-Px activities in castrated rats.21 We evaluated the response to testosterone replacement therapy in orchiectomized rats. Also, in orchiectomized rats with testosterone replacement therapy, total thiol levels were high according to the ORX group, and NOX4 and 8-OHdG levels were low. In addition, in the group with tadalafil as well as testosterone therapy, these values were changed in a similar and but more pronounced way compared to the ORX+testosterone group. This result indicates that administration of tadalafil together with testosterone may significantly reduce oxidative stress and increase antioxidant capacity in orchiectomized rats. It also shows that this practice may lead to negative correlation between total thiol levels and 8-OHdG values, and an increase in total thiol levels can lead to decreased oxidative DNA damage.
Testosterone deficiency has been associated with an increased risk of vascular disease. In relation to this situation, MMPs play a role in the remodeling of the veins. With in vitro studies, androgen hormones were shown to affect MMP enzyme activities.22,23 Mountain et al. showed that dihydrotestosterone (DHT) alters the activation of MMP-2. It was shown that MMP2 activity increased especially with low DHT concentration, while MMP-2 activation decreased with high DHT concentrations.24 In another study, Liao et al. reported that androgens increase MMP-2 activity but they have no effect on MMP-9.25 We found that there was a significant increase in serum levels of MMP-2, MMP-3 and MMP-9 with deficiency of orchiectomy-induced testosterone, in line with the above studies. In addition, MMP2 and 9 levels showed a negative correlation with total thiol values, while NOX4 and 8-OHdG values were positively correlated. This indicates that MMPs may increase with the increase in oxidative stress.
PDE-5 inhibitors cause vasodilation by relaxation of vascular smooth muscle cells. Thanks to these effects, they are used in the treatment of erectile dysfunction and pulmonary hypertension. In addition, PDE5 inhibition has been shown to perform vascular remodeling through MMPs.26 Also, it was shown that PDE5 inhibitors decreased oxidative stress by decreasing oxidant enzyme levels and increasing antioxidant enzyme levels.14 Sun et al. showed that MMP-2 levels significantly increased in endothelin-induced pulmonary artery smooth muscle cells and MMP-2 production was significantly suppressed by PDE-5 inhibitors.27 The results of our study are relatively supportive of these findings. In experimental studies, testosterone insufficiency was shown to cause a significant decrease in NO synthase activity, which is the target of PDE5 inhibitors.28,29 In addition, testosterone replacement therapy was reported to regulate the cavernosal phosphodiesterase-5 (PDE-5) gene and protein expression with hypogonadotropic hypogonadism.30
ConclusionsTestosterone deficiency causes an increase in oxidative stress markers and MMP levels and a decrease in total thiol levels. Oxidative stress and MMPs levels synergistically increase, but total thiol correlates negatively with oxidative stress and MMPS levels. The combination of testosterone with PDE-5 inhibitor suppresses MMP-9 levels and increases total thiol levels better than testosterone alone and tadalafil alone. Therefore, testosterone can be considered for use with PDE-5 inhibitor from the initial stage in case of testosterone deficiency. The routine use of PDE-5 inhibitor with testosterone in hypogonadism will become clear after clinical trials.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was supported by “Presidency of Individual Research Projects”, Van Yuzuncu Yıl University (TSA-2019-7321) and with opportunities from the Department of Biochemistry, Faculty of Medicine, Van Yuzuncu Yıl University.