Spinal teratomas that occur in adulthood are rare. The most accepted theory is that of a disembryogenic origin, but in this case we present the theory of misplaced germ cells.
We present a 54-year-old male with saddle hypoesthesia, 3/5 paraesthesia of the lower extremities, urinary incontinence, loss of anal sphincter tone, and patellar and achilles hyporeflexia, of 10 months’ evolution. The MRI showed a heterogeneous, cystic and solid intramedullary lesion with defined edges. Laminoplasty surgery of L2 to L4 and complete resection of the lesion was performed. The histopathological study found a mature teratoma.
The incidence of intraspinal teratomas is very low. There are two theories: the disembryogenic theory (most accepted) and the misplaced germ cell theory (our case). This pathogenesis of teratoma formation involves a niche of pluripotent cells from the primitive knot or a caudal cell mass that may precede the formation of a dysraphism. In this modified theory, teratoma growth sometimes causes disruption of the development field and dysraphism.
Los teratomas espinales, son raros que se presenten en edad adulta. La teoría más aceptada es disembriogénica, pero en este caso exponemos la teoría de células germinales del fuera de lugar.
Presentamos un masculino de 54 años, de 10 meses de evolución con hipoestesia en silla de montar, parestesia miembros pélvicos 3/5, incontinencia urinaria, tono de esfínter anal ausente, hiporeflexia patelar y aquilea. RMN lumbar muestra lesión tumoral heterogénea intramedular, quística y solida, bordes definidos. Se realiza cirugía de laminoplastia de L2-L4 y resección total de lesión tumoral, con resultado del estudio histopatológico de Teratoma maduro. La incidencia de teratomas intraespinales es muy baja. Existen 2 teorías: teoría disembiogénica (más aceptada) y teoría de células germinales fuera de lugar (nuestro caso). Esta patogénesis de formación de teratoma tiene nicho de células pluripotentes a partir de nodos de Hensen o masa celular caudal que puede preceder a la formación de disrafismo. Esta teoría modificada, del crecimiento de teratoma a veces causa interrupción del campo del desarrollo y disrafismo.
Spinal and, in particular, intramedullary teratomas rarely present during adulthood, especially without associated spinomedullary malformations.1 Various theories exist to explain the formation of spinal teratomas. The most accepted theory is that of disembryogenic origin, but in this case we discuss the pathogenesis of a spinal teratoma based on the misplaced germ cell theory.2 These tumours are slow-growing in nature. We present the following case.
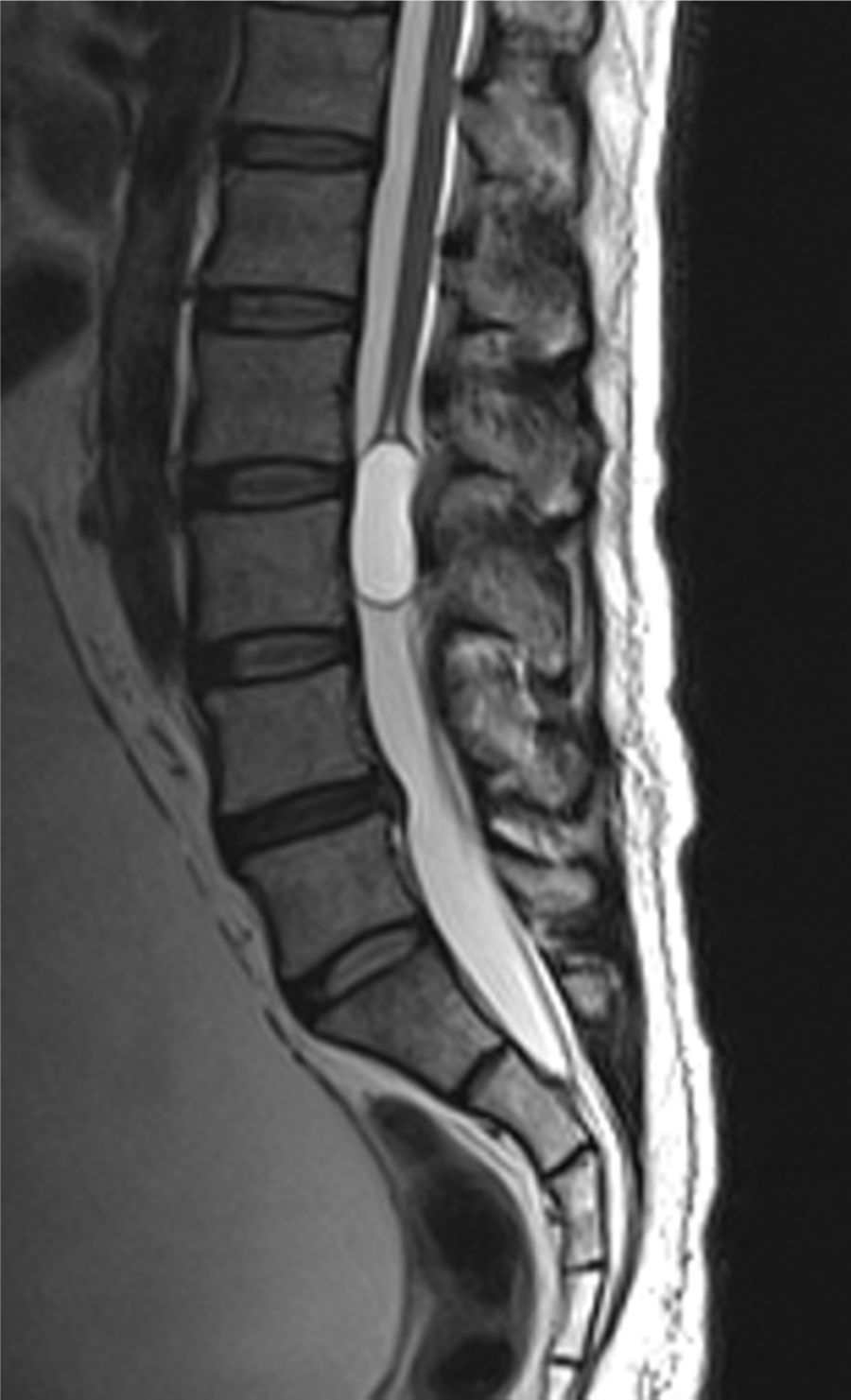
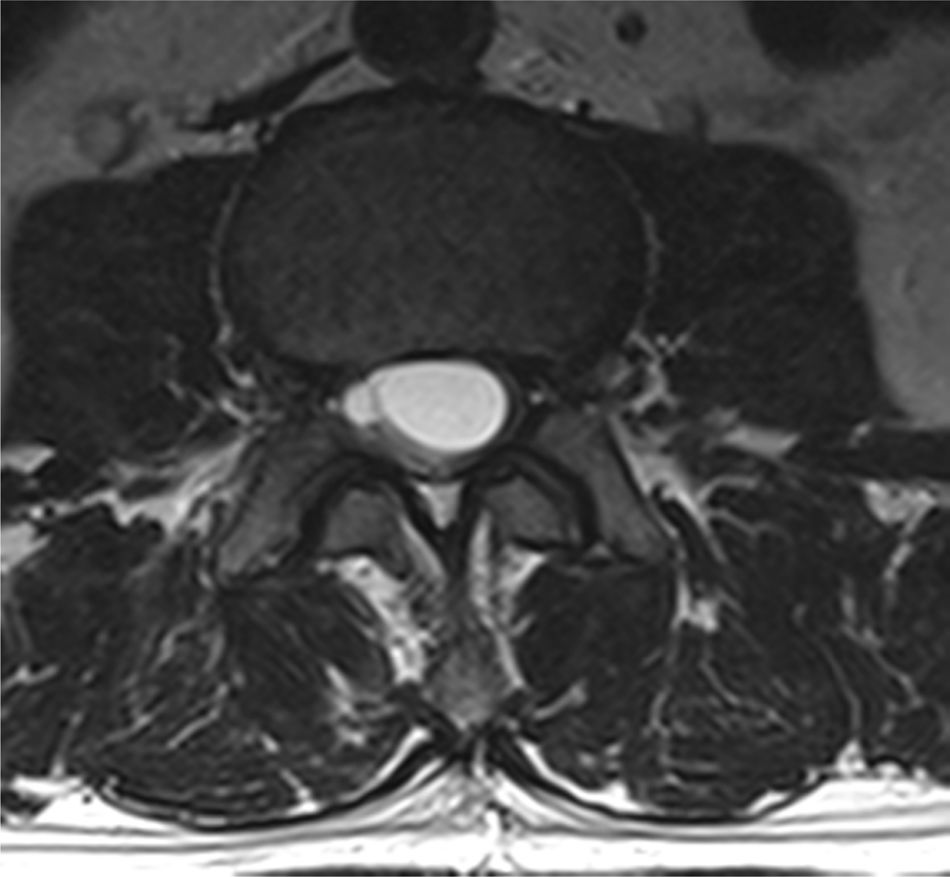
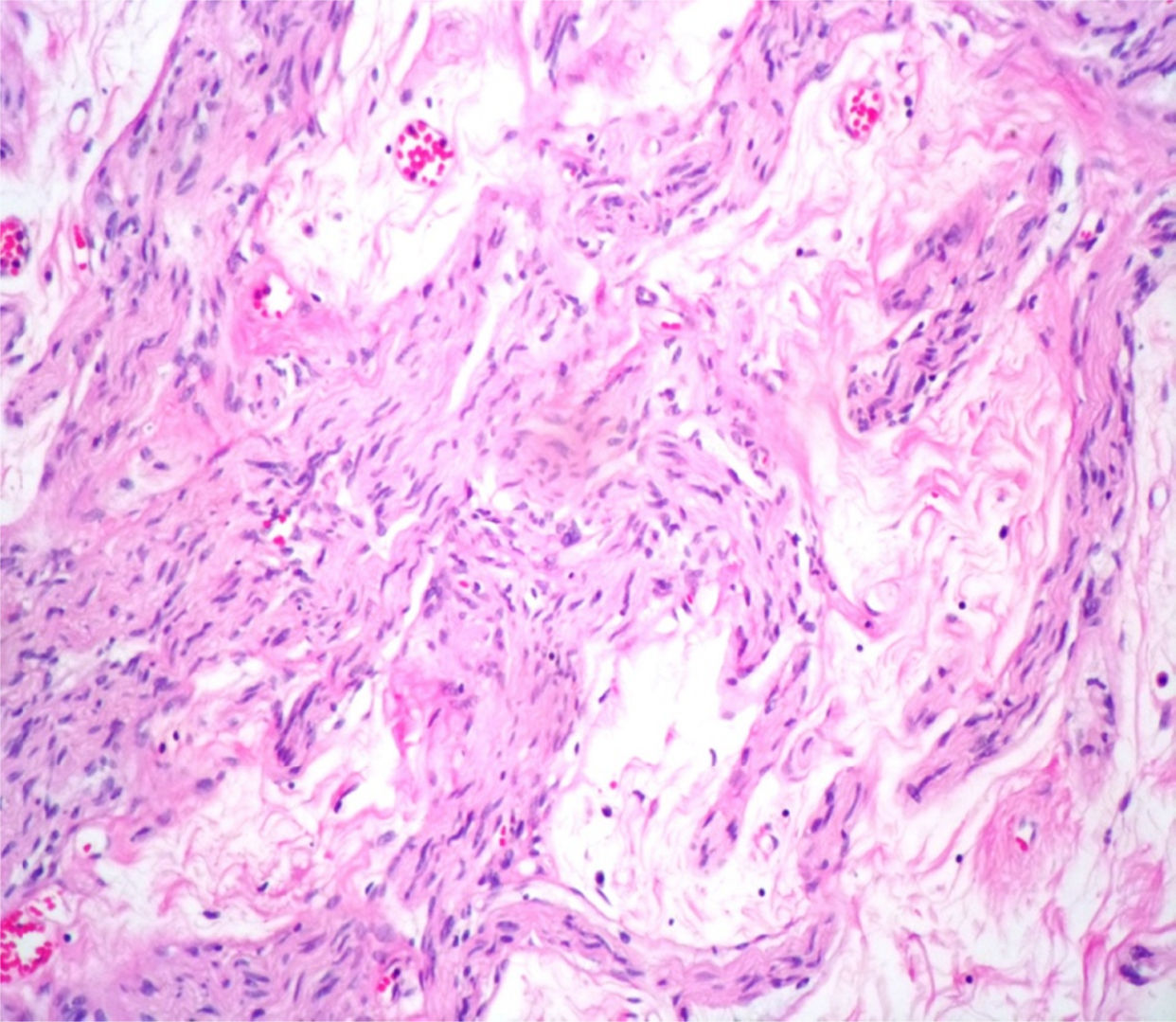
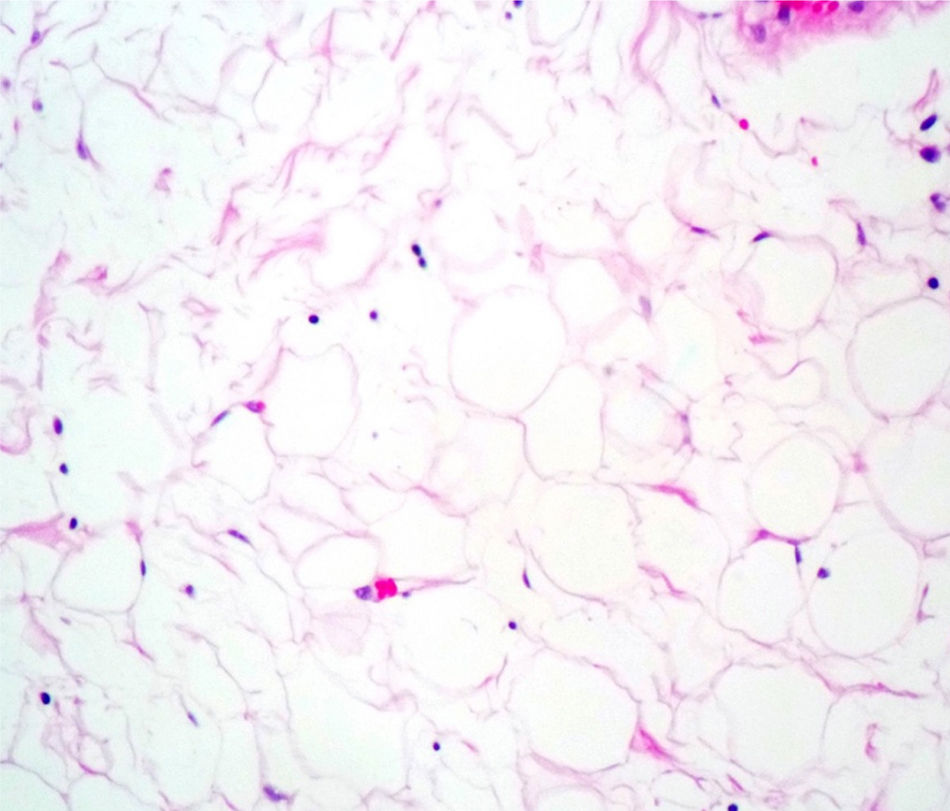
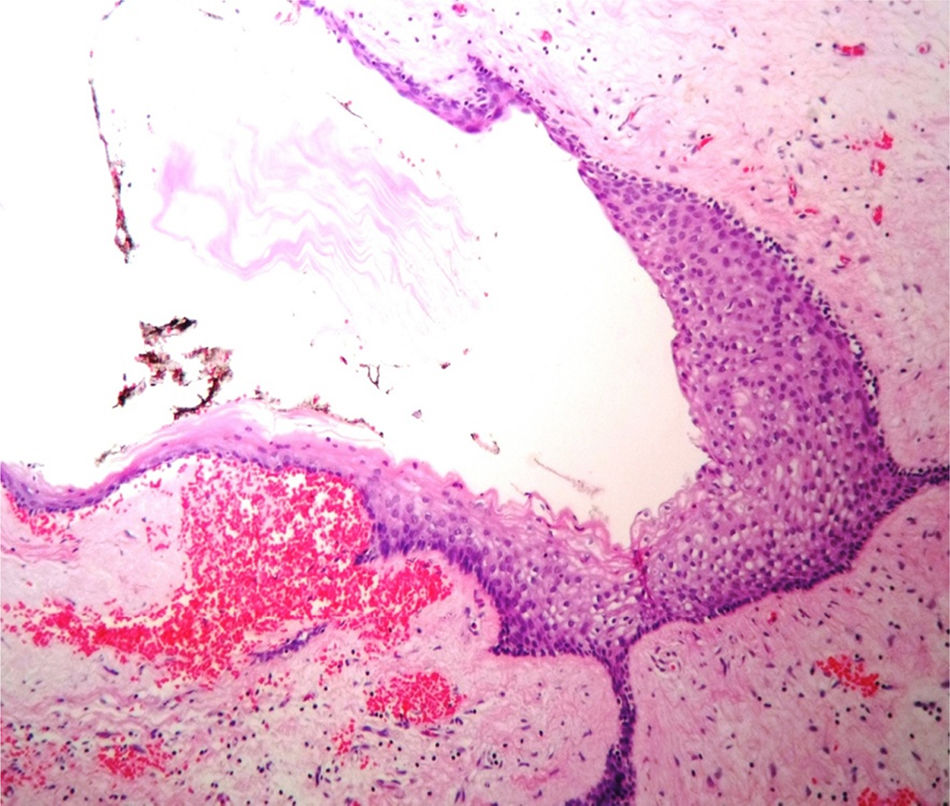
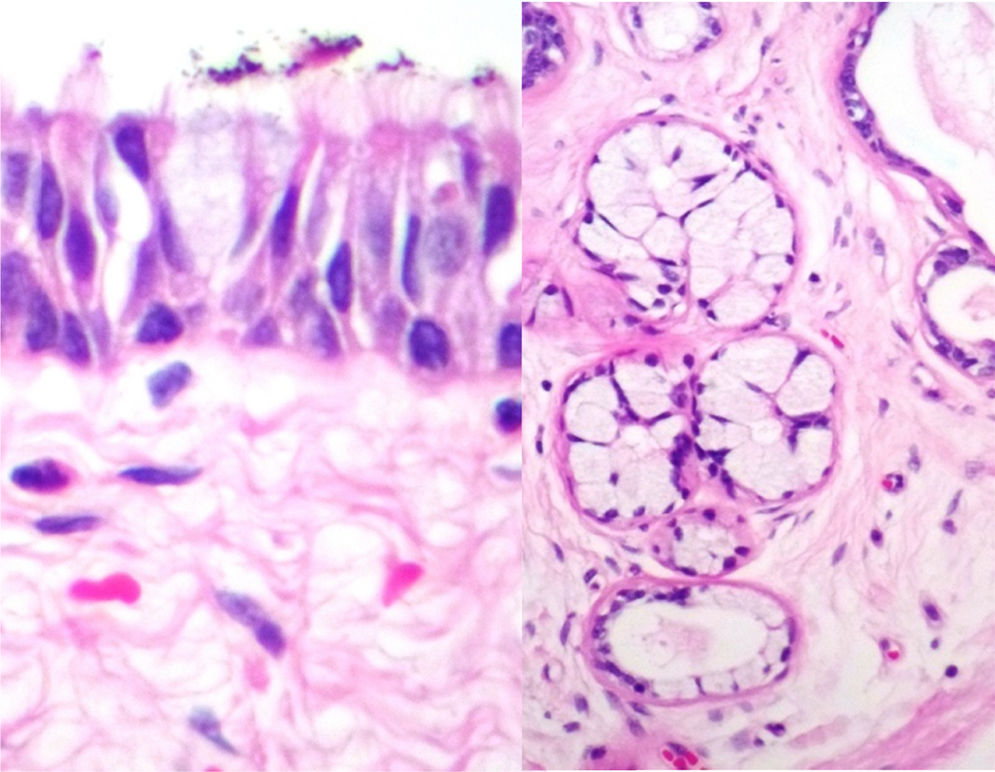
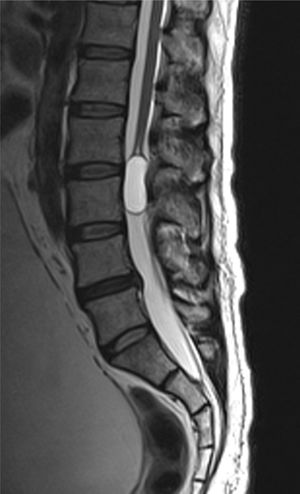
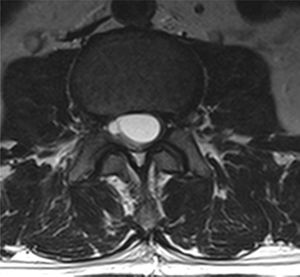
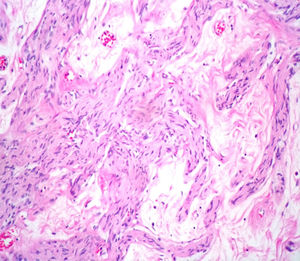
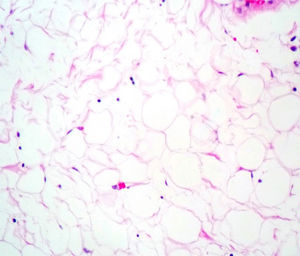
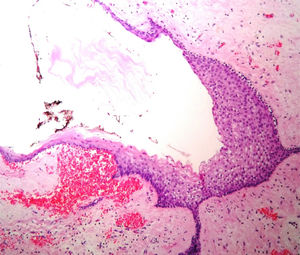
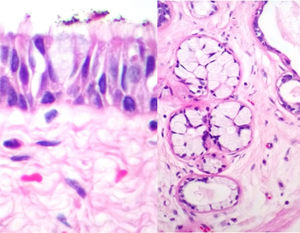
Case reportA male patient of 54 years of age was admitted to our hospital with 10 months’ evolution of lumbago with pain radiating to both lower extremities. In the last two months, his symptoms had gradually progressed with paraesthesia and saddle hypoesthesia, urinary incontinence and constipation. He reported no history of trauma or other spinal procedures. The neurological examination revealed cauda equina syndrome, urinary incontinence, loss of anal sphincter tone, paraesthesia of the lower extremities with a strength of 3/5 bilaterally and both proximally and distally, and bilateral patellar and achilles hyporeflexia. Magnetic Resonance Imaging (MRI) showed hyperintensity at the L2-L3 level in the T2 sagittal image (Fig. 1), while the axial image showed the same intraspinal, intradural and intramedullary feature to be heterogeneous, with the major component located centrally and a hypointense periphery (Fig. 2). It was decided to perform laminoplasty of L2 to L4, opening the dura mater, whereupon we observed that the major component was a central cystic lesion of friable, yellow-grey tissue. Consequently, the tumour was resected in its entirety. The histological examination revealed a mature teratoma with fully differentiated components (Figs. 3–6).
The incidence of intraspinal teratomas is very low, accounting for approximately 0.1–0.2% of all intraspinal tumours.1 In paediatric patients, 5–10% of spinal tumours are intraspinal teratomas.3 However, the incidence in adult patients is significantly lower than that observed in infants and children.4 Intraspinal teratomas have commonly been reported in relation to neural tube defects, including diastematomyelia and myelomeningocele, and linked to conus medullaris syndrome.5 The occurrence of intraspinal teratomas associated with congenital scoliosis is rare, and is more common in children and adolescents than adult patients.5
In children, tumoural lesions are generally found in the midline or at paraxial sites along the length of the neural tube,5 but they occur most often in the sacrococcygeal region.6
On the other hand, if we consider similar lesions such as dermoid cysts, in intra- and extramedullary locations, the absence of associated spina bifida with a vertebral dermoid cyst is extremely rare, with only seven cases reported in the literature to date.7
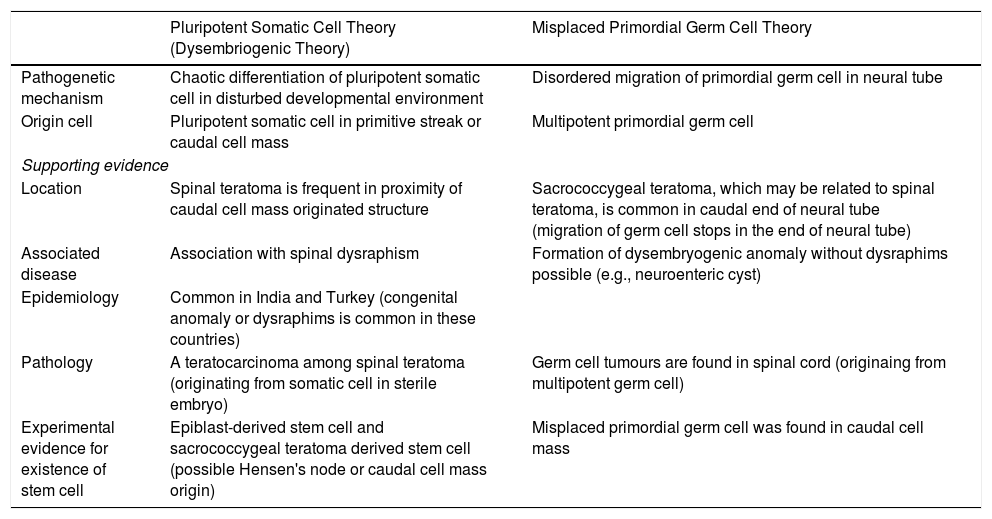
There are two theories regarding the origin of spinal teratomas8: the disembryogenic theory (the most accepted) and the misplaced germ cell theory (our case). This pathogenesis of teratoma formation involves a niche of pluripotent cells from the primitive knot or a caudal cell mass that may give rise to the formation of a dysraphism.8 In this modified theory, teratoma growth sometimes causes disruption of the development field and dysraphism.8 The modified dysmorphogenesis theory has no experimental evidence, but explains the cases of spinal teratoma without dysraphism.8 It may also be supported by the fact that there are around 22 cases of germ cell tumours found in the spinal cord.8 It also explains the typical midline location of germ cell tumours (intradural teratomas) and the rarity of extradural locations, as the cells must migrate farther from the midline.8Table 1 explains both of these theories to aid understanding.8
Comparison between pluripotent somatic cell theory and misplaced primordial germ cell theory.
| Pluripotent Somatic Cell Theory (Dysembriogenic Theory) | Misplaced Primordial Germ Cell Theory | |
|---|---|---|
| Pathogenetic mechanism | Chaotic differentiation of pluripotent somatic cell in disturbed developmental environment | Disordered migration of primordial germ cell in neural tube |
| Origin cell | Pluripotent somatic cell in primitive streak or caudal cell mass | Multipotent primordial germ cell |
| Supporting evidence | ||
| Location | Spinal teratoma is frequent in proximity of caudal cell mass originated structure | Sacrococcygeal teratoma, which may be related to spinal teratoma, is common in caudal end of neural tube (migration of germ cell stops in the end of neural tube) |
| Associated disease | Association with spinal dysraphism | Formation of dysembryogenic anomaly without dysraphims possible (e.g., neuroenteric cyst) |
| Epidemiology | Common in India and Turkey (congenital anomaly or dysraphims is common in these countries) | |
| Pathology | A teratocarcinoma among spinal teratoma (originating from somatic cell in sterile embryo) | Germ cell tumours are found in spinal cord (originaing from multipotent germ cell) |
| Experimental evidence for existence of stem cell | Epiblast-derived stem cell and sacrococcygeal teratoma derived stem cell (possible Hensen's node or caudal cell mass origin) | Misplaced primordial germ cell was found in caudal cell mass |
Spinal epidural teratoma: review of spinal teratoma with consideration on the pathogenesis: case report. Neurosurgery 67:E1818–E1825, 2010.
Spinal teratomas are heterogeneous in nature, and may have both solid and cystic parts.9 Spinal cysts (teratomas) can burst secondary to the trauma of surgery or can rupture spontaneously.10 The outcome when these teratomatous cysts burst is chemical meningitis or Mollaret's meningitis.10,11 This is described as aseptic meningitis, characterised by recurring episodes of severe headache, fever, vomiting, neck pain and pleocytosis in the CSF (cerebrospinal fluid).10,11 It is confirmed by the presence of large mononuclear cells known as Mollaret cells in the CSF.11 These cells characteristically disappear quickly as they undergo rapid lysis after 24h of illness.8,11,12 The necessary measures must be taken to prevent the rupture of cysts and thus avoid complications such as chemical meningitis, as was done in our case.
Moreover, in another case report, the dissemination of fat droplets was encountered due to the rupture of a cyst into the subarachnoid space.1 Typically, there is no communication between the cyst and the subarachnoid space.1 Scearce et al.10 reported that fat droplets reach the ventricular system via the perimedullary subarachnoid space through the backward flow of the foramina of Luschka and Magendie.10
The clinical manifestations of spinal tumours are non-specific, instead being dependent primarily on their anatomical location.13,14 Many patients with spinal cord tumours have clinical symptoms, including back pain, weakness in the lower extremities, altered sensation, altered gait, and sphincter dysfunction.10,12–14 Depending on the anatomical location, these may fit the descriptions of conus medullaris syndrome or cauda equina syndrome12; our patient presented the latter. Park et al.8 and their group found spinal teratomas predominantly between the lower thoracic vertebrae and the level of the conus medullaris, in this order of frequency.8
Images of the tumours obtained by Magnetic Resonance Imaging (MRI) guide the diagnosis of spinal teratoma.15 The morphological appearance on the MRI varies based on the location of the tumours. Intradural teratomas are often oval or lobulated heterogeneous masses, while extradural teratomas are more commonly found to have a “dumbbell” shape.5,6,15 Cases of extradural teratoma are frequently accompanied by malformation of the vertebral body, while intradural teratomas in adults are usually found under the dura mater, rarely invading the dura mater or vertebral body.3
Various tumour markers can be used for diagnosis, including β-human chorionic gonadotropin (β-hCG) in serum and α-fetoprotein (AFP).1,3 Despite the latter's utility, particularly for recurrence, this application is limited as it may have originated in non-secretory parts of the earlier lesion.
Definitive diagnosis requires a histopathological study. Teratomas are tumours that contain endodermic, mesodermic and ectodermic elements.5–7 They are composed of multiple tissues that are extraneous to the organ or site from which they originate. Due to the fact that they originate from pluripotent cells, teratomas can incorporate a wide range of tissues, including skin, muscle, bone, cartilage, intestinal mucosa, fat, teeth and hair.6,12
The treatment of choice continues to be surgical resection, total where possible, although teratomas with malignant histological characteristics presenting significant adherences that surround neural structures may require adjuvant radiotherapy and/or chemotherapy.3,5,6,10 In our case, total surgical resection of the tumour was achieved without damage to anatomical or neural structures which could cause temporary or permanent neural sequelae.
With regard to genetics, although there are no known genetic loci, growing evidence describes a possible association between chromosomal abnormalities and teratomas.6 This is the case for sacrococcygeal teratomas, which have been linked to distal 10q/17p trisomy or partial monosomy.6 Other studies report abnormalities including chromosome 1q26 trisomy mosaicism.6 Although it is rare, sacrococcygeal teratomas have been found with breakages in chromosome 6 with haploidy.6 Congenital sacrococcygeal teratomas express Ras, Fos and Jun oncogenes, as well as NM23 and p53 tumour suppressor genes, which imply that molecular pathways are involved in the formation and development of teratomas.6
ConclusionIntradural spinal teratomas are very rare, even more so in adults. The heterogeneous signal shown by MRI images can indicate the solid or cystic composition of the tumour lesion, which is extremely helpful for an early diagnosis of teratoma. The clinical picture is very non-specific, suggesting only compression of the spinal cord and/or medullary syndromes that could be caused by any other tumour lesion. Good outcomes are achieved with total surgical resection, as the clinical symptoms are caused primarily by compression of the spinal cord by the spinal teratoma. Extreme care is essential when resecting the tumour, in order to avoid breaking the cyst and disseminating its contents or post-surgical infections. Genetics is of little help, as there are no pathognomonic studies of this disease, and, as always with surgery, using histopathology to corroborate the result gives the most certain diagnosis.
Conflicts of interestThe authors have no personal, financial or institutional interest in any of the drugs, materials or devices described in this article.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.