There is currently little evidence available about the metabolic behaviour in adult patients with Turner syndrome (TS). Metabolic complications are common in adult TS patients, increasing morbidity and impairing quality of life. Body composition is altered in TS, secondary to the short stature. Metabolic damage in patients with TS is an important medical issue due to complications observed in adulthood.
AimStudy some of the aspects involved in the origin of the metabolic damage.
MethodsWe conducted an observational, cross-sectional, comparative, descriptive study in 20 adult patients with TS and 20 control patients matched by age, waist circumference, waist circumference/height ratio (W/Hr) as sensitive parameters for metabolic risk. Anthropometric, body composition, resting energy expenditure data and blood samples for blood chemistry, lipid and thyroid profile were considered. Multivariate analysis of variance and the Student's T-test were used to analyse the data all the patients’ data were corrected according to the predicted specific formula for adult TS.
ResultsStatistically significant differences in energy expenditure (REE) modifications and in free fat mass per weight percentage were observed.
ConclusionDifferences in anthropometric values and REE in TS could be implicated in the metabolic damage, and are attributable to the syndrome and not to the body composition.
En la actualidad existe poca evidencia disponible sobre el comportamiento metabólico en pacientes adultos con síndrome de Turner (ST). Las complicaciones metabólicas son comunes en pacientes adultos con ST, incrementando la morbilidad y deteriorando la calidad de vida. La composición corporal es alterada en ST, secundario a la baja estatura. El daño metabólico en pacientes con ST es un importante problema médico debido a las complicaciones observadas en la edad adulta.
ObjetivoEstudiar algunos de los aspectos que intervienen en el origen del daño metabólico.
MétodosSe realizó un estudio observacional, transversal, comparativo y descriptivo en 20 pacientes adultos con ST y 20 pacientes control emparejados por edad, circunferencia de cintura y relación de la circunferencia de cintura/altura (W/Hr) como parámetros sensibles para el riesgo metabólico. Datos de antropometría, composición corporal y el gasto de energía en descanso, así como muestras para la química sanguínea y los perfiles de lípidos y tiroideo fueron considerados. Se utilizó el análisis multivariado de varianza y la prueba T-Student para analizar los datos de todos los pacientes. Los valores fueron corregidos de acuerdo con la fórmula específica prevista para el adulto con ST.
ResultadosSe observaron diferencias estadísticamente significativas en el gasto de energía en reposo (REE) y en la masa libre de grasa por porcentaje de peso.
ConclusiónLas diferencias en los valores antropométricos y REE en ST podrían estar implicados en el daño metabólico y son atribuibles al síndrome y no a la composición corporal.
Turner syndrome (TS) is one of the most common sexual chromosomal abnormalities, affecting 1/2500 live female birth, and approximately 1.5 million women around the world have TS.1 It is associated with threefold increased mortality and a decrease in life expectancy by 13 years.2 TS is characterised by total or partial monosomy of the second sex chromosome, short stature, skeletal abnormalities and gonadal dysgenesis observed with a lack of oestrogens throughout life. The most common systemic complications are heart and renal defects,3 endocrine autoimmune diseases, including mainly hypothyroidism,4 osteoporosis,5 and metabolic syndrome in adulthood.6 When the syndrome is diagnosed in childhood there is close medical supervision; however, in adulthood close surveillance by a multidisciplinary group decreases, despite a high rate of complications. Metabolic complications are common in adult TS patients, increasing morbidity and impairing quality of life.7 Women with TS in adulthood are at high risk for hyperlipidaemia, ischaemic heart disease, atherosclerosis, hypertension, and insulin resistance.8,9 Type 2 diabetes mellitus (DM) is 2–4 times as common in women with TS compared with the general population and occurs at younger ages.2,10 Patients with TS are more likely to be overweight and obese. Body composition is altered in TS, secondary to the short stature; Turner females are primarily growth-retarded along the longitudinal axis. Approximately 3–4 SDS compared with a reference population reduces height, sitting height, and arm-span. The hands and feet are reduced in size to a lesser extent, while head circumference, biacromial diameter, and bi-iliac diameter are comparable to those of healthy women.8–11 Fat mass (FM) and body mass index (BMI) are higher in adult Turner patients compared with age-matched controls, and lean body mass (LBM) is low.11 In addition, distinct differences in regional body composition are present in young TS girls (9–15 years) in comparison with age and BMI-matched controls, and in adults excess visceral fat and hepatic adipose tissue have been documented.12 The metabolic damage in patients with TS is an important medical issue due to complications observed in adulthood. There are not many studies in adult TS females. The aim of the present manuscript is study some of the aspects involved in the origin of the metabolic damage, a detailed analysis of body composition and resting energy expenditure (REE) was conducted in a group of adult patients with TS. This study will help in early intervention for TS patients, give some tools for the nutritional management in order to decrease the metabolic damage and the long-term multidisciplinary monitoring, which should contribute to reduced morbidity,13 improved life expectancy, and higher quality of life.
Material and methodsAn observational, transversal, comparative, descriptive study was performed. Twenty women with Turner syndrome (TS) confirmed by cytogenetics were included. All the patients were from the adult Turner Syndrome Clinic of the Hospital General de Mexico. The study was accurate to consider the anthropometric differences observed in the TS, and in order to have a homogeneous group, we included 20 women without TS as a control group, the patients and the controls were matched by age, waist circumference, waist circumference/height ratio (W/Hr) considered as sensitive parameters for metabolic risk, also we considered the body mass index (BMI), however we known that secondary, to the anthropometric differences between de TS and the controls this is not the best parameter. In fasting conditions, anthropometric measurements, bioelectrical impedance data, indirect calorimetry data and blood samples were obtained. Lipid profile and thyroid function were measured in all subjects. The Research and Bioethics Committee of the Institution approved the protocol.
AnthropometryThe anthropometric measurements used were the average of 3 different measures taken by the same person. A fixed stadiometer with 0.1-cm sensitivity was used to obtain the height. Patients stood without shoes, heels together, and the head on a line horizontal to the Frankfort plane. Weight was measured without shoes and with light clothing using a scale with a sensitivity of 0.05kg. Body size was classified according to the relationship between the circumference of the waist, measured with anthropometric fibreglass tape, and the height. BMI (Quetelet index) was assessed according to World Health Organization criteria.
Analysis of body compositionBioelectrical impedance analysis is a non-invasive, simple and safe method to estimate fat-free mass, total body water and fat mass. The resistance and reactance were measured at a single frequency (50kHz) in four channels (BIA Quantum IV RJL Systems, Inc., Michigan. USA). The data were processed with the software BC RJL formula NHANES-III (National Health and Nutrition Examination Survey).14 The results obtained were corrected according to the predicted formula specific for adult Turner syndrome: FAT-FREE MASS by BIA (g)=−11.068+0.307×S2/R (cm2/Ω)+0.286×weight (kg)+0.14×stature (cm).15
Indirect calorimetric analysisResting energy expenditure in both groups was measured by indirect calorimetry using a ReeVue1800 calorimeter (KORR Medical Technologies. Utah. USA) after 12h of fasting when the patient was awake, relaxed, and sitting comfortably and without previous physical activity. The test was performed over 10min in each subject.
Statistical analysisA statistical comparison between groups was performed by analysis of variance (MANOVA), using total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, TSH, percentage of body fat and REE as dependent variables; age and BMI were used as covariates. Homogeneity between groups was analysed using the Student's T-test test for independent samples.
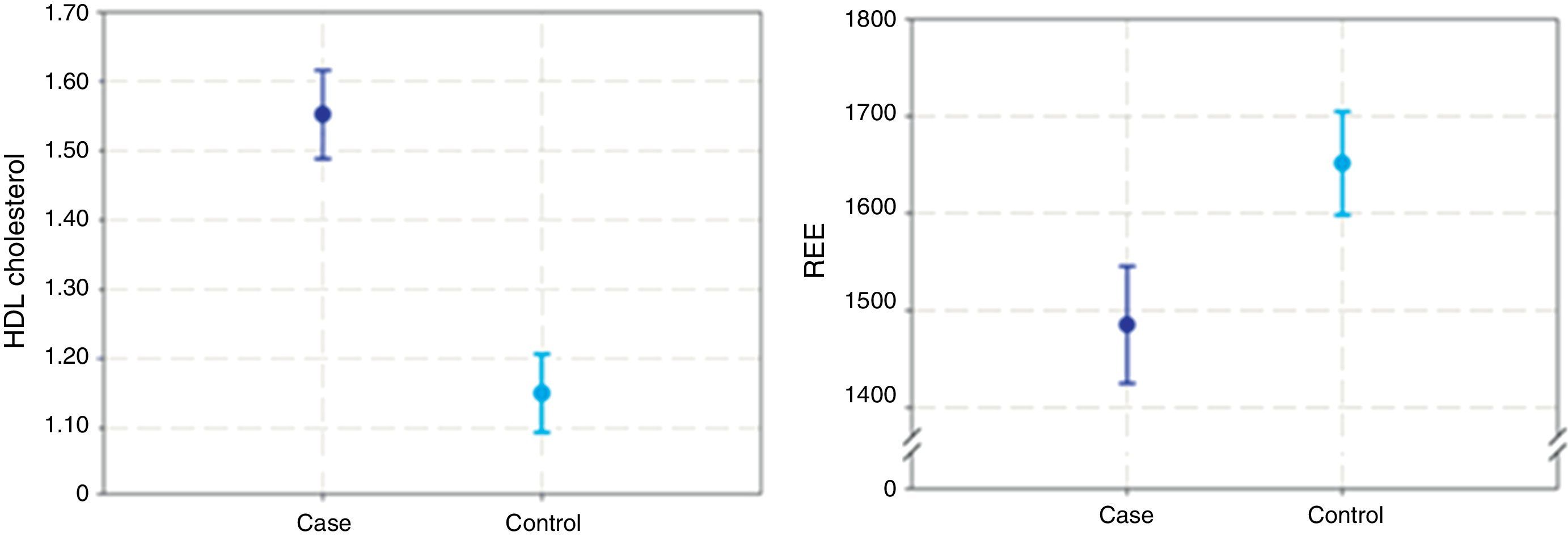
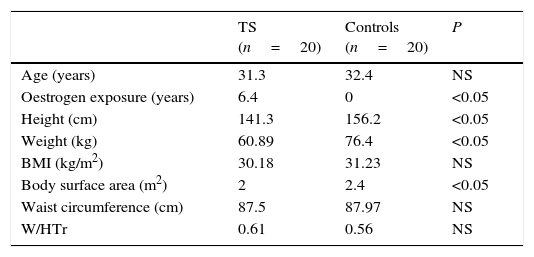
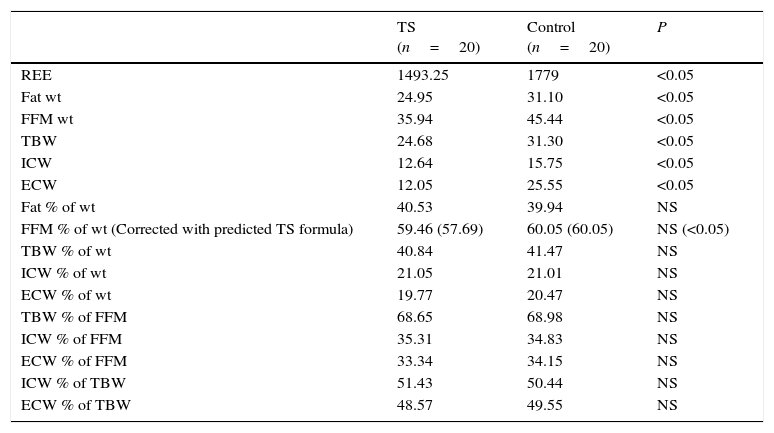
ResultsTwenty consecutive adult women with Turner syndrome, were included in the study. The karyotype was 45,X in 17 patients, 46,X,i(Xq) in two, and 45,X/46,XY in one. All the patients, including the one with the XY mosaic genotype, had all the clinical features of Turner syndrome. The complications observed in our group were three cases of hypothyroidism (under treatment), one case of autoimmune liver disease, and one case of carbohydrate intolerance. All of them were under medical supervision. The TS group had been undergoing oestrogen treatment for an average of 15 years. In 13 out of the 20 cases poor compliance was observed. It is important to note that none of the patients received growth hormone treatment. No other drugs were being taken at the time of the study. Twenty control women without Turner syndrome who were matched for age, waist circumference, waist circumference/height ratio and BMI to the TS group were included. T-student showed no statically significant differences in the anthropometric parameters and age were observed within groups indicating that the statistical analysis performed in the study groups was accurate to evaluate the differences between them (Table 1). The waist circumference/height ratio is a good parameter to evaluate metabolic risk and is used in most of the clinic during the approach of overweight and obese subjects. Values above 0.5 are sensitive parameters to differentiate those patients with high metabolic risk, an average W/Hr of 0.6 was observed in the total sample including TS and non-TS cases, indicating high metabolic risk. This results shows that the metabolic risk observed in our study group is independent of the syndrome and is more dependent of the body weight.16,17 Average BMI in TS patients were 30.1±7.5kg/m2; the values for the control group were 31.2±4.70kg/m2 (Table 1). Independent the anthropometric characteristic in the TS, the BMI in both groups were outside the normal. The patients with TS were overweight or obese; these observations were confirmed by body composition analysis (Table 2). Total body fat, FFM wt, and FFM % were higher in the TS group. These results confirm previous observations that TS patients have differences in body composition from normal females. Here we confirmed this observation in adult TS patients, nevertheless, it is important to mention that de FFM % of wt were corrected according to a specific equation to predict body composition in TS, the corrected value showed difference between the groups being higher in the patients. Both groups had a high risk for metabolic disease. Trying to explore if basal metabolism and modifications in the resting energy expenditure (REE) could participate in specific way in TS, increasing metabolic risk, a calorimetric analysis was performed. REE revealed statically significant lower resting energy expenditure in the TS group compared to the control group (p≤0.05) (Fig. 1B). No statistically significant differences were observed in triglycerides, LDL or cholesterol between groups, although HDL cholesterol was significantly higher in the TS group (p=0.01) (Fig. 1A). However, we considered important to mention that 8 patients and 12 controls had elevated triglycerides. Suggesting that high triglycerides have an important role in the metabolic risk in our population, more than the cholesterol does.
Clinical and anthropometric measures in TS patients and controls.
| TS (n=20) | Controls (n=20) | P | |
|---|---|---|---|
| Age (years) | 31.3 | 32.4 | NS |
| Oestrogen exposure (years) | 6.4 | 0 | <0.05 |
| Height (cm) | 141.3 | 156.2 | <0.05 |
| Weight (kg) | 60.89 | 76.4 | <0.05 |
| BMI (kg/m2) | 30.18 | 31.23 | NS |
| Body surface area (m2) | 2 | 2.4 | <0.05 |
| Waist circumference (cm) | 87.5 | 87.97 | NS |
| W/HTr | 0.61 | 0.56 | NS |
W/HTr=waist circumference/height ratio.
NS=non-significant.
Analysis of the body composition.
| TS (n=20) | Control (n=20) | P | |
|---|---|---|---|
| REE | 1493.25 | 1779 | <0.05 |
| Fat wt | 24.95 | 31.10 | <0.05 |
| FFM wt | 35.94 | 45.44 | <0.05 |
| TBW | 24.68 | 31.30 | <0.05 |
| ICW | 12.64 | 15.75 | <0.05 |
| ECW | 12.05 | 25.55 | <0.05 |
| Fat % of wt | 40.53 | 39.94 | NS |
| FFM % of wt (Corrected with predicted TS formula) | 59.46 (57.69) | 60.05 (60.05) | NS (<0.05) |
| TBW % of wt | 40.84 | 41.47 | NS |
| ICW % of wt | 21.05 | 21.01 | NS |
| ECW % of wt | 19.77 | 20.47 | NS |
| TBW % of FFM | 68.65 | 68.98 | NS |
| ICW % of FFM | 35.31 | 34.83 | NS |
| ECW % of FFM | 33.34 | 34.15 | NS |
| ICW % of TBW | 51.43 | 50.44 | NS |
| ECW % of TBW | 48.57 | 49.55 | NS |
REE=resting energy expenditure.
NS=non-significant.
FFM wt=total fat-free mass.
TBW=total body water weight.
ICW=intracellular water weight.
ECW=extra cellular water weight.
Fat % of wt=fat mass percentage of total weight.
FFM % of wt=fat-free mass percentage of total weight.
TBW % of wt=total body water percentage of total weight.
ICW % of wt=intracellular water percentage of total weight.
ECW % of wt=extracellular water percentage of total weight.
TBW % of FFM=total body water percentage of fat-free mass.
ICW % of FFM=intracellular water percentage of fat-free mass.
ECW % of FFM=extracellular water percentage of fat-free mass.
ICW % of TBW=intracellular water percentage of total body weight.
ECW % of TBW=extracellular water percentage of total body weight.
TS is one of the most common sexual chromosome abnormalities, affecting 1/2500 live female births. Many clinical complications are observed during adulthood and are related to metabolic and autoimmune disorders.1,18 The metabolic damage that occurs in patients with TS is a topic due to complications observed in adulthood. This study analysed a consecutive group of 20 adult patients with TS and 20 healthy controls matched for waist circumference, waist circumference/height ratio, BMI and age. In a previous report, a skewed body composition between TS adolescents and controls was found.15 We included in the analysis two more anthropometric measures to verify this observation and confirm the homogeneity between our groups: waist circumference and the waist circumference/height ratio. These parameters are considered sensitive measures of metabolic risk.16 The average W/Hr was high in the entire study sample and was similar between groups, indicating high metabolic risk in both groups. Despite their anthropometric characteristics, body composition is altered in TS patients secondary to their short stature and anthropometric composition.11–15 Our observations in the TS group compared with the non-TS cases confirm these differences. However, the FFM % of wt value corrected with a specific equation for TS, showed a statistically difference after that, suggesting that FFM % is higher in TS and be a part of the metabolic risk observed in these patients. Overweight and obesity are common features in adult TS women, so it is important to analyse whether overweight or obesity is affected by the intrinsic energy expenditure and metabolic rate. Patients with TS had lower energy expenditure compared with the age-controlled women (p=0.05) (Fig. 1). Possibly as a direct consequence of this lower REE and free fat mass distribution, obesity is frequent in TS patients, accompanied by early metabolic co-morbidities.19,20 The REE modifications in TS could be explained by the lack of oestrogen's and poor compliance of the hormonal replacement therapy affecting eating behaviours, energy expenditure and insulin sensitivity. Turner syndrome patients exhibit chronic hypo-oestrogenism during pre-puberty that may induce early metabolic abnormalities that persist and worsen during adulthood. Oestrogens play a critical role in energy homeostasis and in determining body weight and body composition.21–23 Oestrogens may also modulate glucose disposal through its actions on the insulin-signalling pathway.24,25 Growth hormone (GH) treatment is considered the standard therapy used to treat TS, although they are not growth hormone deficient. GH promotes longitudinal bone growth and indirectly increases bone mineral density, and long-term treatment (>12 months) has been associated with an increased lean body mass and reduced abdominal adiposity.26,27 Modifications of these anthropometric indexes may contribute to metabolic improvement in the form of better glucose tolerance and decreased diastolic blood pressure. Most of these benefits persist after two years of GH discontinuation. It is important to highlight the fact that none of the TS patients in this study had growth hormone treatment during childhood, so GH could not have influenced the REE observed. The quality of life in adult TS has improved in recent years due to multidisciplinary management and medical follow-up; most TS patients require long-term monitoring and early treatment to avoid or treat early complications.28 After the first visit to our Clinic, only 5% of patients had been receiving continuous and multidisciplinary attention; this number is similar to previous reports in the literature.7 The search for distinctive metabolic parameters in TS could be helpful for medical management and providing beneficial advice concerning lifestyle behaviour, food intake and physical activity. The aim in these patients is to achieve a BMI below 25kg/m2 and a waist/height ratio of approximately 0.5 to decrease cardio-metabolic risk.2,10 Carbohydrate intolerance is more prevalent in TS patients, affecting 34%.15–29,30 In our group, 80% of the patients were obese, 15% overweight and only 5% normal-weight, suggesting that body composition predisposes TS patients to all the risks mentioned above. Our group of TS women had significantly higher levels of HDL (p=0.01) compared with the controls matched for anthropometric parameters, indicating that this elevation is characteristic of TS. However, there is no explanation in the literature to clarify whether there is a protective effect attributed to the elevated HDL in TS.31–33
In conclusion, this study indicates that the difference in REE and Fat Free Mass percentage, between patients with TS and controls is attributable to the syndrome and not to the body composition, highlighting the improvements in health care during the childhood-adulthood transition in TS patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
This work was performed in the Human Genetics Department, Hospital General de México, Facultad de Medicina, UNAM. It was supported by the Research Division of the Hospital General de México, CONACYT grant number 181798.