Atypical pneumonias, a group of diseases relatively unfamiliar to most clinicians, are caused by bacteria not normally associated with pneumonia and usually occur in patients with some kind of comorbidity. They can present as extrapulmonary infections, and prognosis can differ from typical pneumonias. Many clinicians are unfamiliar with the diagnosis of these diseases, and they are often incorrectly treated. In this review, we describe the clinical manifestations of the most common forms of atypical pneumonia, and outline the diagnostic procedures and therapies used in each case. In this way, hope to give physicians and other healthcare professionals the knowledge and diagnostic tools they need to deal with cases in which atypical pneumonia is suspected.
Las neumonías atípicas, un grupo de patologías relativamente desconocidas por los médicos, son causadas por microorganismos que no se consideran tradicionales. Este tipo de infecciones ocurren en un grupo de pacientes que generalmente tiene alguna comorbilidad y pueden presentar infecciones extrapulmonares y pronósticos distintos a los de las neumonías típicas. El diagnóstico de estas patologías no es muy conocido, por lo que el tratamiento no siempre es el adecuado. En este trabajo de revisión, mencionamos las manifestaciones clínicas causadas por los principales microorganismos que causan neumonías atípicas, así como las características particulares de los métodos diagnósticos disponibles y los respectivos tratamientos de las neumonías, en búsqueda de que los médicos y profesionales de la salud se familiaricen y obtengan un mayor conocimiento sobre este tema a la hora de enfrentarse a un caso sospechoso de una neumonía atípica.
The term atypical or walking pneumonia was first coined in 1938 to refer to a group of pneumonias caused by bacteria or other pathogens not usually associated with pneumonia. The symptomology and radiographic images of walking pneumonias differ from conventional pneumonia, and they occasionally present with extrapulmonary infection.1,2
The 5 most common pathogens that cause atypical pneumonia are: Legionella pneumophila, Chlamydophila pneumoniae, Mycoplasma pneumoniae, Coxiella Burnetti and Chlamydophila psitacci.3 Infection is most commonly associated with children in day care centres, elderly individuals, smokers, and patients with chronic diseases or immunodeficiency disorders.4
All physicians must be aware of these risk factors and of the clinical and radiological characteristics of atypical pneumonias. The following overview of these diseases and their 3 main causative pathogens will enable physicians to recognize the early signs of such infections and choose the most effective treatment.5
Legionella pneumophilaMorphological and structural characteristicsLegionella pneumophila is an aerobic, Gram-negative, nonencapsulated, non-spore-forming, flagellated, intracellular bacterium, often characterized as being a coccobacillus. Its cell wall contains fatty acid with high ubiquinone content and the capacity to form biofilms. It is oxidase, catalase and gelatinase-positive, has no nitrate-reducing capacity, and it can be observed with fascin staining.
L. pneumophila is an insidious bacterium that requires cysteine and iron to thrive. For this reason, it is detected by culture on buffered charcoal yeast extract (BCYE), polymyxin B, anisomycin and cefamandole agar. It can also be isolated by amoebal coculture.1–3
There are over 50 species of Legionella, but evidence has shown that only 19 of these cause infection in humans. There are also 64 subgroups of the Legionella genus, and 15 L. pneumophila serogroups.1,3
EpidemiologyL. pneumophila infection is uncommon, and usually occurs in immunocompromised individuals. Over 90% of infections in humans are caused by L. pneumophila serogroup 1.
The bacterium is ubiquitous, and is commonly found in fresh water, rivers, lakes, and in muddy, damp soil. It can also thrive in air conditioning, water heating, ventilation and shower systems. L. pneumophila can survive for prolonged periods under most climatic conditions. It can withstand temperatures ranging from 0 to 68°C, and is chlorine resistant.5
Epidemiological reports on the incidence of disease caused by this pathogen in Mexico and the rest of the world are somewhat out-dated. No outbreaks of Legionnaires’ disease have so far been reported in Mexico.6,9
Legionella causes between 2% and 9% of all cases of community-acquired pneumonia, and between 1% and 50% of nosocomial pneumonia11. Most epidemics occur in the summer and autumn. Approximately 20,000 cases of Legionnaires’ disease are reported annually in the United States. Infection usually occurs in middle-aged or elderly individuals, particularly those with cardiac or pulmonary complications. It also targets smokers, and immunocompromised individuals.1,7
Risk factorsThere is no evidence for person-to-person transmission of Legionnaires’ disease. The bacteria are transmitted via contaminated aerosols generated by nebulisers and humidifiers. They can also be transmitted directly during surgical interventions, and by drinking contaminated water.7
Immunocompromised individuals, solid organ transplant recipients, patients recovering from surgery or receiving endotracheal ventilation, patients admitted to critical care units, or individuals exposed to ventilation systems are most at risk for infection. Some studies have mentioned nasogastric intubation to be associated with risk for Legionella infection.
Other risk factors include contact with stagnant water, air conditioning systems, rivers, lakes, and domestic water heating systems.8
Clinical manifestationsSerology testing has shown that infection is often asymptomatic. Symptomatic L. pneumophila infection can present either as severe pneumonia, called Legionnaire's disease, or as a flu-like condition known as Pontiac fever.9
Legionnaire's disease is characterized by clinical and radiological signs consistent with pneumonia. Initial symptoms include anorexia, vomiting, myalgia and headache. Over 90% of patients present fever between 12 and 24h after onset of the first symptoms. Fever is high grade, usually over 39.4°C, and is accompanied by shivering and cough producing little or no sputum. Most patients complain of dyspnoea, and in 60% the mental state is altered, with symptoms ranging from lethargy to obtundation. Chest pain is common in immunocompromised patients, and can be confused with pulmonary thromboembolism. These patients can also present fever alone, with no other symptoms of pneumonia, despite radiological evidence of pulmonary infiltrates. Around 50% of patients present gastrointestinal symptoms, such as diarrhoea with blood and mucous, and intestinal colic. Other manifestations include hypotension, bradycardia, bronchospasm, toxic encephalopathy and rash. Radiological signs are similar to those of pneumonia caused by other pathogens.9,10,12
In Pontiac fever, patients present flu-like symptoms, with fever, headache, shivering, myalgia and poor general condition, with no clinical or radiological signs of pneumonia. The disease is self limiting, and resolves in 2–7 days.10
Cases of extrapulmonary infection have been documented, possibly due to haematogenous dissemination. These include prosthetic valve endocarditis, sternotomy wound infection, skin infections, abscess, cellulitis, sinusitis pericarditis, myocarditis, peritonitis, pancreatitis, acute pyelonephritis with renal abscess, and osteomyelitis, all of which are uncommon. Elevation of alkaline phosphatase and hepatic transaminase levels are a common finding. Hyponatraemia is more common in L. pneumophila infection than in other forms of bacterial pneumonia.12
DiagnosisDiagnosis is confirmed in the laboratory by culture, demonstration of bacterial antigens or DNA in body fluids, or evidence of a serologic response. Polymorphonuclear leucocytosis, azotaemia, acute liver failure, hyponatraemia, and hyperphosphataemia are all common findings.1,10,12
Legionella can be cultivated from sputum, endotracheal aspirate, bronchoalveolar lavage, open lung biopsy and pleural fluid. Sensitivity of expectorated sputum culture, however, is low (50%); bronchoalveolar lavage samples are known to have a higher sensitivity.12,13
The pathogen grows after 48h incubation at 37°C in aerobic conditions. Colonies are characterized by their blue colour and frosted glass appearance.13 On Gram staining, they are shown as long, thin, Gram-negative bacilli. Samples must be incubated and regularly checked for at least 10 days before the culture is reported negative.14
Legionella can be isolated in respiratory tract and tissue specimens in 2–4h using direct immunofluorescence. This method, however, can give false positives due to cross-reactivity, and the sensitivity of the test ranges from 25% to 66%.13,14
Immunochromatography, which can detect antigens in urine in around 15min, is considered to be a good L. pneumophila detection method. The test has 56–97% sensitivity, and 97% specificity, although it will only detect L. pneumophila serogroup 1.14,15
Serology using indirect immunofluorescence can take between 3 and 4 weeks to detect antibodies. A fourfold or higher rise in titre is considered diagnostic. In a single sample, a titre of 1:256 with documented pneumonia is considered diagnostic. These tests should be interpreted with caution due to the large number of patients with asymptomatic infection.2,15,20
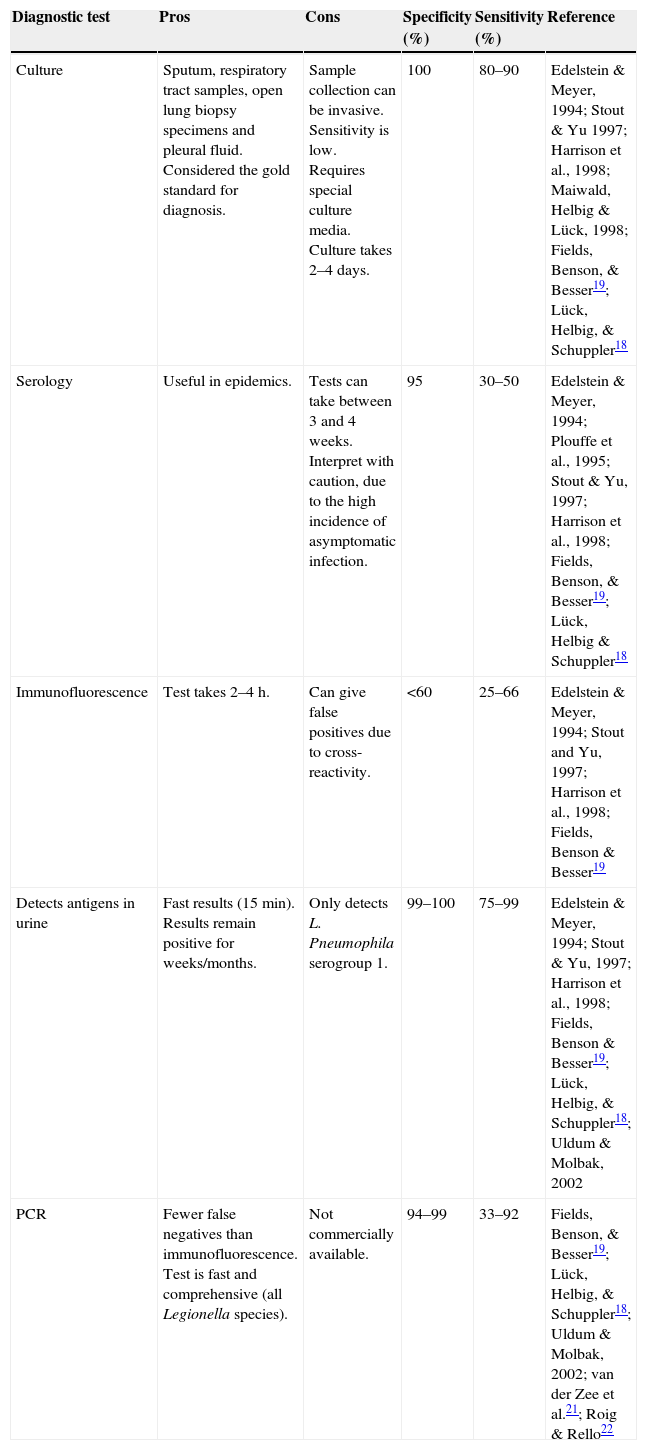
Nucleic acid amplification tests for detecting Legionella in respiratory tract, urine, serum and leukocyte samples have a sensitivity of between 30% and 86%.13Table 1 shows the different characteristics of the methods used to diagnose L. pneumophila infection.
Characteristics of diagnostic tests for L. pneumophila.
| Diagnostic test | Pros | Cons | Specificity (%) | Sensitivity (%) | Reference |
|---|---|---|---|---|---|
| Culture | Sputum, respiratory tract samples, open lung biopsy specimens and pleural fluid. Considered the gold standard for diagnosis. | Sample collection can be invasive. Sensitivity is low. Requires special culture media. Culture takes 2–4 days. | 100 | 80–90 | Edelstein & Meyer, 1994; Stout & Yu 1997; Harrison et al., 1998; Maiwald, Helbig & Lück, 1998; Fields, Benson, & Besser19; Lück, Helbig, & Schuppler18 |
| Serology | Useful in epidemics. | Tests can take between 3 and 4 weeks. Interpret with caution, due to the high incidence of asymptomatic infection. | 95 | 30–50 | Edelstein & Meyer, 1994; Plouffe et al., 1995; Stout & Yu, 1997; Harrison et al., 1998; Fields, Benson, & Besser19; Lück, Helbig & Schuppler18 |
| Immunofluorescence | Test takes 2–4h. | Can give false positives due to cross-reactivity. | <60 | 25–66 | Edelstein & Meyer, 1994; Stout and Yu, 1997; Harrison et al., 1998; Fields, Benson & Besser19 |
| Detects antigens in urine | Fast results (15min). Results remain positive for weeks/months. | Only detects L. Pneumophila serogroup 1. | 99–100 | 75–99 | Edelstein & Meyer, 1994; Stout & Yu, 1997; Harrison et al., 1998; Fields, Benson & Besser19; Lück, Helbig, & Schuppler18; Uldum & Molbak, 2002 |
| PCR | Fewer false negatives than immunofluorescence. Test is fast and comprehensive (all Legionella species). | Not commercially available. | 94–99 | 33–92 | Fields, Benson, & Besser19; Lück, Helbig, & Schuppler18; Uldum & Molbak, 2002; van der Zee et al.21; Roig & Rello22 |
Macrolides or quinolones are the treatment of choice. In patients with severe Legionnaire's disease, macrolides such as azithromycin or clarithromycin, or quinolones such as levofloxacin or moxifloxacin are recommended.16
Pontiac fever is self-limiting and does not require antibiotic therapy. Erythromycin was previously used, but reports have surfaced of treatment failure in immunocompromised patients.16
Quinolones are preferred in transplant patients receiving cyclosporine or tacrolimus, as macrolides interfere with the metabolism of immunosuppressants.16,17
Duration of treatment can vary. In immunocompetent patients, azithromycin for 5–10 days, or a quinolone for 10–14 days is usually sufficient. In immunocompromised patients, therapy should be extended to between 14 and 21 days to prevent recurrence.5,16,17
Despite treatment, Legionnaire's disease has a mortality rate of between 2% and 5%. Mortality among immunocompromised or inadequately treated patients is from 5% to 30%.1,2,11,17
Chlamydophila pneumoniaeMorphological and structural characteristicsChlamydophila pneumoniae is an obligate intracellular Gram-negative bacterium that can exist as either an elementary or reticulate body. Elementary bodies, which are biologically inactive and can survive in hostile environments, are the infective form of the bacteria. In this form, the bacterium enters the host cell, usually via the respiratory tract, where it transforms into a reticulate body. In this metabolically active, intracellular, non-infectious form, the bacterium undergoes binary fission and returns to its elementary body form. The elementary bodies are released from the host cell after cell lysis.23
Sometimes, due to external factors such as lack of nutrients, reticulate bodies remain in an aberrant form, which is a relatively larger reticulate body that is still viable but unable to infect other cells. In this form, it is highly drug-resistant. It has been suggested that C. pneumoniae may act as a chronic stimulus in the perpetuation of vascular inflammation, thus exacerbating the atherosclerotic process.23,24
EpidemiologyIn the United States, C. pneumoniae is estimated to cause over 300,000 cases of pneumonia each year. Anti-C. pneumoniae antibodies are detected in around half (50%) of individuals of 20 years of age. The rate increases with age, and antibodies are detected in 70–80% of elderly individuals. It is thought to cause 20% of all lower airway infections, and that 70% of these are either asymptomatic or not serious enough to compel the patient to seek medical attention. It has been suggested that between 3% and 10% of all community acquired pneumonias are caused by C. pneumoniae, and in Latin America it is thought to cause around 6% of all such diseases. The pathogen is also responsible for 20% of all upper respiratory tract infections.24,25
Risk factorsRisk factors for C. pneumoniae infection are thought to involve immune system functions and the genetic predisposition of the host. In studies carried out in Asia, researchers have observed that infection is extremely seasonal, and peaks in the summer months.26
Although many studies have associated C. pneumoniae infection with an increased risk for heart disease due to the inflammatory response elicited by the pathogen, the results remain inconclusive.26–30 The presence of this pathogen has also been associated, albeit insignificantly, with a number of diseases, including asthma exacerbations, multiple sclerosis, chronic fatigue syndrome, Alzheimer's disease, age-related macular degeneration, chronic skin lesions, cerebrovascular disease.26,27
Clinical manifestationsC. pneumoniae causes sinusitis, pharyngitis and pneumonia, although in most cases infection is asymptomatic. The first manifestation of infection can resemble a typical viral infection,23
C. pneumoniae infection can present as:
- •
Acute infection: Although in some immunocompromised patients symptoms can be severe enough to merit hospitalization, in most immunocompetent patients clinical symptoms are usually mild.
- •
Recurrent infection: Recurring infection and its associated symptoms will depend on the characteristics of the host's immunological system.
- •
Chronic infection: C. pneumoniae infection can cause exacerbation of COPD or asthma.
- •
C. pneumoniae carriers: Around 2.5% of the population are carriers of this pathogen. They are usually asymptomatic, although immune deficiencies can cause acute symptoms.
Pneumonia develops in 2 stages: at the onset, symptoms are similar to a cold (pharyngitis, laryngitis, sinusitis), which is followed by moderate pneumonia. Pneumonia lasting between 1 and 4 weeks is followed by persistent cough that can last several weeks.24
DiagnosisDiagnosis should be based on clinical suspicion. Certain radiological features are suggestive of C. pneumoniae infection, such as subsegmental, generally single patchy infiltrate, lobar or sublobar consolidation, or interstitial infiltrate with hilar adenopathy. In 20–25% of all cases, pleural effusion, generally bilateral, is also seen.28,31
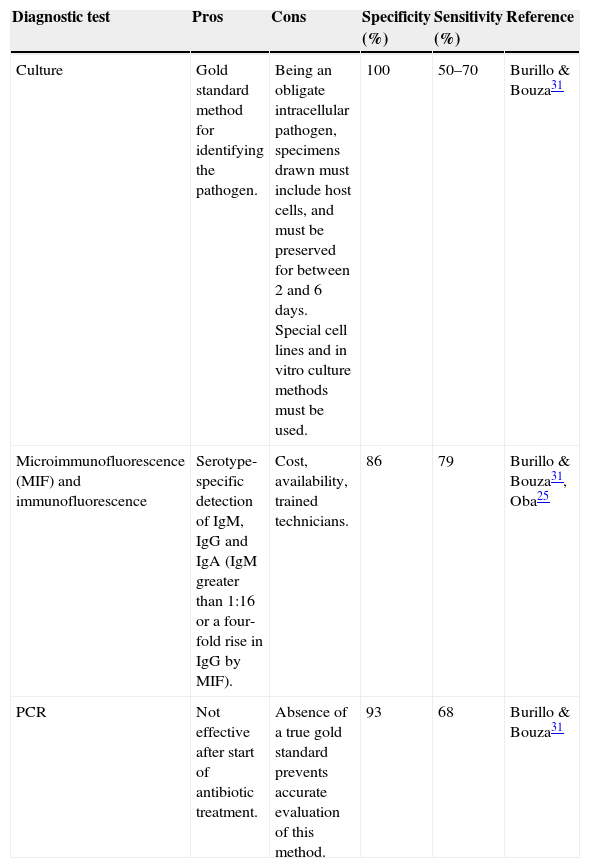
Laboratory studies will usually find no changes in leukocyte levels, inflammatory parameters can be slightly elevated, and serum antibodies may be detected. On histology, tissue samples of infected patients show slight intra-alveolar inflammation and cytoplasmic inclusion. It is important to note that absence of antibodies does not rule out C. pneumoniae.29Table 2 shows the characteristics of different C. pneumoniae diagnostic procedures.
Characteristics of diagnostic tests for C. pneumoniae.
| Diagnostic test | Pros | Cons | Specificity (%) | Sensitivity (%) | Reference |
|---|---|---|---|---|---|
| Culture | Gold standard method for identifying the pathogen. | Being an obligate intracellular pathogen, specimens drawn must include host cells, and must be preserved for between 2 and 6 days. Special cell lines and in vitro culture methods must be used. | 100 | 50–70 | Burillo & Bouza31 |
| Microimmunofluorescence (MIF) and immunofluorescence | Serotype-specific detection of IgM, IgG and IgA (IgM greater than 1:16 or a four-fold rise in IgG by MIF). | Cost, availability, trained technicians. | 86 | 79 | Burillo & Bouza31, Oba25 |
| PCR | Not effective after start of antibiotic treatment. | Absence of a true gold standard prevents accurate evaluation of this method. | 93 | 68 | Burillo & Bouza31 |
The treatment of choice is doxycycline, although it should not be given to children under the age of 9 years. Treatment should continue for 10–14 days. If symptoms persist, a second cycle should be administered. Second line therapies include erythromycin (500mg 4 times daily), azithromycin (500mg for between 7 and 10 days), and clarithromycin (1g once daily for 10 days).30,31 Antibiotic therapy is usually successful, although the cycle may need to be repeated or the treatment prolonged. Despite the large number of studies published to date, the role of this pathogen in other diseases has yet to be investigated and clarified.31,32
Mycoplasma pneumoniaeMorphological and structural characteristicsThis is the most important species in the Mycoplasmataceae family of bacteria. M. pneumoniae is an extracellular, free-living obligate aerobe that only infects humans. In its coccus morphology it measures between 0.2 and 0.3nm; in its bacillus morphology it is 1–2nm long and 0.2nm wide. It does not have a cell wall, which makes it resistant to penicillins, cephalosporins, vancomycin and other antibiotics that inhibit cell wall synthesis. M. pneumoniae contains sterols in its cytoplasmic membrane
It can only be grown in an artificial acellular medium, and takes 6h to replicate. Its main antigenic determinants are glycolipids and membrane proteins.33
EpidemiologyM. pneumoniae infection is more common in toddlers in day care centres and in children aged 5–15 years. It causes between 10% and 20% of all cases of community-acquired pneumonia, of which 60% occur in children. Extrapulmonary complications occur in 25% of all cases. It has recently started to be considered the main cause of bacterial pneumonia on military bases. It colonizes the nose, throat, and trachea, and is transmitted via respiratory aerosols. Respiratory disease caused by M. pneumoniae infection is marginally more common in the summer and autumn months.34
Each year, 2 million new cases of M. pneumoniae pneumonia are reported in the United States, with 100,000 hospitalizations. It reaches epidemic proportions every 4–8 years.35
M. pneumoniae is the leading cause of atypical pneumonia among children and adolescents, causing between 10% and 30% of all cases. Although it is usually associated with mild acute respiratory infections, such as sore throat, pharyngitis, rhinitis, and tracheobronchitis, it can also cause more serious infections, such as pneumonia or lung abscess. It is associated with acute asthma exacerbations, chronic obstructive pulmonary disease, and can even cause community outbreaks on a scale similar to flu epidemics.35,36
Clinical pictureInfection is usually asymptomatic. In the case of recurrent infection, or infection in children, the following symptoms may present:
It mainly presents as tracheobronchitis, followed 2 or 3 weeks later by fever, general malaise, headache and unproductive cough, which can be accompanied by acute pharyngitis. Symptoms can worsen and persist for up to 2 more weeks, and can even lead to pneumonia.36,37
Uncommon manifestations include myalgia and digestive symptoms. Secondary extrapulmonary complications can include meningoencephalitis, paralysis, myelitis, pericarditis, haemolytic anaemia, arthritis, and mucocutaneous lesions.34
DiagnosisEarly, accurate diagnosis of M. pneumoniae infection is essential in order to start the right antibiotic therapy. As it is impossible to reach a diagnosis based solely on the non-specific clinical signs and symptoms, laboratory tests must be performed.
Chest X-ray findings include unilateral or bilateral alveolar infiltrates, centrilobular peribronchovascular nodules, areas of ground glass attenuation, intrathoracic lymphadenopathy, and even pleural effusion.
Various diagnostic methods can be used, including culture, serology and molecular biology tests. Due to the slow growth rate of M. pneumoniae (colonies are only visible after 2–5 weeks), culture is rarely used.38
The most common diagnostic method used nowadays is serology, mainly complement fixation (CF) tests. Sensitivity, however, will depend on whether the first sample was drawn in the early or later stages of development of the disease, and also on the availability of paired serum samples drawn at an interval of 2 or 3 weeks. Serology also include immunoglobulin (IgM) tests, which are more sensitive than CF, although IgM response may be non-specific or even wholly absent, particularly in adults35,37 DNA hybridization testing has been suggested as a rapid, specific alternative to culture, although it lack sensitivity.36
In view of all these shortcomings, a growing number of clinicians rely on RT-PCR (reverse transcription polymerase chain reaction) for its higher sensitivity and specificity. RT-PCR testing is performed on samples drawn from nasopharyngeal and oropharyngeal swabs and bronchoalveolar lavage,33,35 and involves either isolating the bacteria or detecting DNA and immunoglobulin sequences in mucus or blood samples draw from infected patients.33
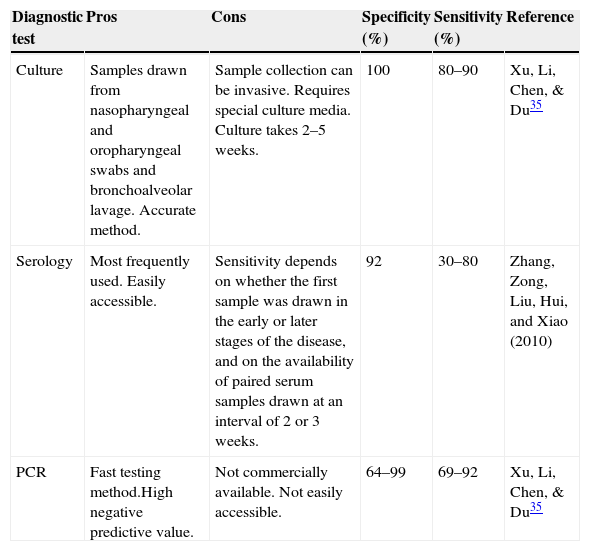
Although PCR testing is an effective M. pneumoniae diagnostic method, lack of standardization and commercial availability have restricted its use, and no official quality assurance guidelines are available to evaluate the effectiveness of the methods used.38 In addition to this, PCR equipment is often unavailable in some small hospitals and in less affluent countries and regions, above all developing countries. This is a major challenge in Mexico.37Table 3 shows the different characteristics of the methods used to diagnose M. pneumoniae.
Characteristics of diagnostic tests for M. pneumoniae.
| Diagnostic test | Pros | Cons | Specificity (%) | Sensitivity (%) | Reference |
|---|---|---|---|---|---|
| Culture | Samples drawn from nasopharyngeal and oropharyngeal swabs and bronchoalveolar lavage. Accurate method. | Sample collection can be invasive. Requires special culture media. Culture takes 2–5 weeks. | 100 | 80–90 | Xu, Li, Chen, & Du35 |
| Serology | Most frequently used. Easily accessible. | Sensitivity depends on whether the first sample was drawn in the early or later stages of the disease, and on the availability of paired serum samples drawn at an interval of 2 or 3 weeks. | 92 | 30–80 | Zhang, Zong, Liu, Hui, and Xiao (2010) |
| PCR | Fast testing method.High negative predictive value. | Not commercially available. Not easily accessible. | 64–99 | 69–92 | Xu, Li, Chen, & Du35 |
Experience has shown that many M. pneumoniae pneumonias are self-limiting. However, medical treatment is needed when symptoms appear.38 Definitive diagnosis of M. pneumoniae is usually treated with macrolides, which can also be used in children. Nevertheless, even when diagnosed, M. pneumoniae pneumonias are sometimes confused with Streptococcus pneumoniae infection, as M. pneumoniae precedes and aggravates infection caused by other pathogens.38,39
Macrolide therapy should be given in combination with fluoroquinolones, due to the increase in combined infection and macrolide-resistant strains. Among the foregoing antibacterials, the following are recommended: erythromycin, tetracyclines, mainly doxycycline, or fluoroquinolones. All these have been equally effective in treating M. pneumoniae, although doxycycline and fluoroquinolones are only used in adults.39 Erythromycin is occasionally associated with adverse effects, such as gastrointestinal symptoms, abdominal pain, nauseas, vomiting, diarrhoea, anorexia, rash, skin lesions, anaphylaxis and irreversible hearing loss.35 Early treatment is important, as therapy started at a later stage is far less effective.34
Studies on the effectiveness of adjuvant steroid treatment in M. pneumoniae infection have proved inconclusive. Despite this, most sources consulted conclude that they do not contribute any major benefits.39,40
ConclusionAtypical pneumonias are an important, worldwide health problem, and all physicians should be skilled in recognizing, diagnosing and treating the main forms of atypical pneumonia. There is scant reference in the literature to epidemiological studies on the prevalence of atypical pneumonias in Mexico, and what little information is available is out-dated. We believe, therefore, that this review should pave the way for future studies on this topic. It is also important to analyse the short- and long-term impact of atypical pneumonias in Mexico, and the systemic implications of infection by each pathogen.
Conflict of interestThe authors declare they have no conflict of interest.
FundingNo funding was received for this study.