Violent behavior is influenced by genetic factors, and the MAOA-uVNTR polymorphism has been associated with violent behavior, specifically the low activity variant. It has been suggested that this polymorphism impacts on grey matter concentration in structures associated with behavioral inhibition and emotion processing, however in previous imaging studies well defined violent subjects have not been explored.
ObjectiveTo investigate the effect of MAOA-uVNTR polymorphism on brain structure of violent subjects.
MethodsThe grey matter concentration of 47 adult male subjects from a community sample classified as violent or controls, was assessed through DARTEL-voxel-based morphometry technique.
ResultsA significant genotype by behavior interaction was found in which violent-low activity allele carriers had decrease of grey matter concentration in right superior temporal pole compared to controls of the same allelic variation.
DiscussionThis findings suggests that grey matter integrity in superior temporal pole could be a neurobiological correlate of the allelic association between MAOA-uVNTR polymorphism and violent behavior due to its implication in socio-emotional processing.
La conducta violenta tiene una influencia genética importante, el polimorfismo MAOA-uVNTR se ha asociado con la conducta violenta. Se ha sugerido que dicho polimorfismo impacta la concentración de materia gris en estructuras asociadas con la inhibición conductual y el procesamiento emocional, sin embargo, no se han explorado estos efectos en sujetos violentos.
ObjetivoInvestigar el efecto del polimorfismo MAOA-uVNTR sobre la estructura cerebral en sujetos violentos.
MétodoSe comparó la concentración de materia gris mediante la técnica de morfometría basada en voxel con el procedimiento DARTEL en 47 hombres adultos miembros de la comunidad clasificados como controles o violentos.
ResultadosSe encontró una interacción significativa entre genotipo y conducta en la cual los sujetos violentos portadores del alelo de baja actividad presentaron reducciones de materia gris en el polo temporal superior derecho, al ser comparados con los controles de la misma variación alélica.
DiscusiónEstos hallazgos sugieren que la integridad de la materia gris en polo temporal superior podría subyacer a la asociación alélica entre MAOA-uVNTR y violencia, debido a la implicación de esta estructura cerebral en el procesamiento socio-emocional.
At present, violent behavior is a public health problem due to socially negative outcomes. The study of its genetic and neurobiological basis allows to understand its etiology and to develop evidence-based intervention programs to decrease its frequency.
It has been suggested that impulsive violence is associated with alterations in emotion regulation that depends on the integrity of brain structures such as temporal pole, orbitofrontal cortex, ventromedial cortex, dorsolateral cortex, anterior cingulate and amygdala.1
Structural brain imaging studies have demonstrated that violent, antisocial and violent psychopathic individuals show alterations in grey matter concentration. In one study of subjects with early onset of antisocial personality disorder that committed violent crimes, reductions in grey matter concentration in post central gyrus, superior fronto-temporal areas, medial frontal gyrus and orbitofrontal cortex and mainly in left posterior cingulate and right insula cortices were found. When individuals with both high psychopathy and antisocial traits were compared to a control group, reductions in grey matter concentration in medial temporal gyrus and parahippocampus were found. These brain alterations could be related with the onset and maintenance of persistent violent behavior.2 The increase of psychopathy traits is a risk factor for violent behavior, whereas in two studies of psychopathic individuals, grey matter reductions in medial and lateral areas of orbitofrontal cortex and superior and anterior temporal areas3,4 were found; according to these studies the reductions in these brain structures could underlie the emotional dysfunction that characterizes these violent populations. In a recent study it has been reported that youth homicide offenders, compared to non-homicide offenders and after controlling brain volumes, psychopathy scores and substance dependence, had grey matter reductions in right superior and middle temporal gyrus, left parahippocampus, fusiform gyrus and inferior temporal gyrus. A classification through supported vector machine, in which prefrontal and temporal cortices were included as predictors, showed that the structural alterations classified offenders in the two groups with 81% accuracy.5
The anatomical alterations found in violent individuals could be a result out of genetic influences. The findings about genetic contribution to violent behavior are consistent, it has been suggested that genetic factors explain about 50% of variance of violent, aggressive and impulsive behavior.6–10 Seemingly male subjects are more vulnerable to the effects of genetic factors on heritability and stability of violent traits throughout life, which can be related to the high prevalence of violent behavior in men.9,11,12
The variable number tandem repeat (VNTR) functional polymorphism in the promoter region of monoamine oxidase A (MAOA) coding gene (Xp11.4-11.3) has been proposed as a candidate for violent behavior. MAOA is an enzyme that mainly degrades serotonin (5HT) in brain, but also degrades norepinephrine and dopamine.13 The MAOA-uVNTR has two common alleles that impact on the enzymatic transcription, so it has been suggested that carriers of 3.5 or 4 repeats have high enzymatic expression of MAOA, whereas carriers of 2, 3 or 5 repeats have lower enzyme expression.14 MAOA enzyme plays a fundamental role in neurodevelopment regulating the pattern of neural differentiation and maturation, but the lack or low activity of MAOA in prenatal stages leads to an abnormal neurodevelopment.15
Several molecular genetic studies have shown that the low activity variant, both as a main effect and as a gene-environment interactions (e.g. early trauma, biological sex), is associated with traits related to violent behavior such as antisocial personality disorder, impulsivity, aggression, gang membership, weapon use and emotional dysfunction.16–22 It has been suggested that the low activity allele confers risk for a hypersensitivity to socially negative or stressful life events that could lead to a bias to choose violent behaviors.23 On the other hand, other studies showed a lack of effect of the interaction between maltreatment and MAOA genetic variation on aggression,24 and on hyperactivity and conduct problems.25 These contradictory findings about the moderating role of MAOA polymorphism on maltreatment–aggression relationship could be influenced by other factors such as victimization levels and the moment of the behavioral assessment.26
There are few neuroimaging studies that explore the effect of MAOA-uVNTR on brain structure. In two voxel based morphometry studies in healthy subjects, low activity allele male carriers showed bilateral grey matter increases in lateral orbitofrontal cortex (BA 47) that could be related to an altered neurodevelopment derived from the increase in serotonergic tone,27 and to other polymorphisms that could affect brain structure.28 Studies in which other structural techniques were used showed that low activity carries had lower cortical thickness in anterior cingulate and orbitofrontal cortex. That cortical thinning suggested that MAOA genetic variation has an important biological effect on brain structures that are fundamental for the development of neuropsychiatric disorders.29 On the other hand, there were no volumetric differences in amygdala between high and low MAOA activity carriers.30
Despite the evidence about the association between MAOA-uVNTR and violence, and its effect on brain structure, to the best of our knowledge, the effect of this polymorphism on brain structure in violent individuals has not been investigated; therefore, the objective of the present study was to investigate the effect of MAOA genetic variation in a community sample of male subjects phenotypically well defined as violent. Due to our subjects were violent men from community and they had not committed extremely violent acts such as murder, we hypothesized no structural differences between violent and control groups would exist. We also hypothesized that low activity allele carriers would have differences (increases or decreases) in brain structure specifically in lateral orbitofrontal areas (BA 47), compared to high activity allele carriers. Due to the proposed hypersensitivity that confers the low activity allele we further hypothesized a gene by behavior interaction in which violent subjects with low activity allele would have decreases in brain structures that are essential for the control of emotional behavior, specifically those areas related to negative affectivity.
MethodsSubjectsA total of 230 adult males were recruited from the community and completed the Spanish version of the Reactive and Proactive Aggression Questionnaire (RPQ31). This screening questionnaire allowed to obtain 109 aggressive males, and 121 non-aggressive subjects (RPQ cutoff≥832). From 109 aggressive males 37 volunteers were selected; and from 121 non-aggressive subjects 32 volunteers were selected. Seven aggressive males and two non-aggressive males were excluded from the study due to the following reasons: refused to participate, non-availability for examination, inability to see small objects without glasses, metal implants, neurological or psychiatric background, etc. The resulting sample of 60 volunteers (30 aggressive, 30 non-aggressive males) were assessed through a battery of questionnaires that included many scales of violence related traits: Spanish version of the Buss–Durkee Hostility Inventory (BDHI33), Spanish version of the Plutchik's Impulsivity Scale (PIS34), the Novaco Anger Scale (NAS35), and screening version of the Hare's Psychopathy Checklist (PCL:SV36). In order to well characterize the violent phenotype a K-means cluster analysis was carried out in SPSS 20 for windows (SPSS, Chicago, IL) to obtain two clusters based on Reactivity scale of RPQ and total scores of BDHI, PIS, NAS and PCL:SV.7 subjects were excluded because they did not complete the battery. Based on the results of the cluster analysis, the final sample consisted of 53 male subdivided in a “violent group” (n=25) characterized by high scores in aggression, hostility, impulsivity, anger and psychopathy traits; and a “control group” (n=28) characterized by low scores in aggression, hostility, impulsivity, anger and psychopathy traits (Table 1). Because of artifacts or structural alterations (e.g. cysts) two violent and four controls were excluded from voxel-based morphometry analysis, thus the images of 47 subjects (23 violent and 24 controls) were analyzed. All subjects provided written informed consent and were guaranteed confidentiality.
Centroids of clusters.
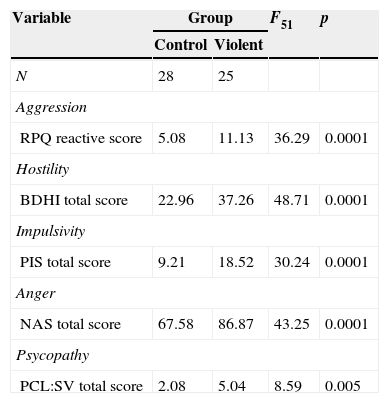
| Variable | Group | F51 | p | |
|---|---|---|---|---|
| Control | Violent | |||
| N | 28 | 25 | ||
| Aggression | ||||
| RPQ reactive score | 5.08 | 11.13 | 36.29 | 0.0001 |
| Hostility | ||||
| BDHI total score | 22.96 | 37.26 | 48.71 | 0.0001 |
| Impulsivity | ||||
| PIS total score | 9.21 | 18.52 | 30.24 | 0.0001 |
| Anger | ||||
| NAS total score | 67.58 | 86.87 | 43.25 | 0.0001 |
| Psycopathy | ||||
| PCL:SV total score | 2.08 | 5.04 | 8.59 | 0.005 |
Notes: RPQ, proactive and reactive aggression questionnaire; BDHI, Buss–Durkee Hostility Inventory; PIS, Plutchik's Impulsivity Scale; NAS, Novaco Anger Scale; PCL:SV, screening version of Hare's Psychopathy Checklist.
Brain images were collected on a General Electric 1.5T (Signa, GE Medical Systems, Milwakee, WI, USA). A sagittal MPRAGE T1-weighted structural image was acquired with the following parameters: echo time (TE)=3930ms, repetition time (TR)=3000ms, flip angle=15°, voxel size=1mm3 and FOV=256mm×256mm×160mm.
Voxel-based morphometryVBM-DARTEL analysis was used to compare the grey matter concentrations. The morphometric analysis was carried out using SPM8 software37 implemented in Matlab 2013b (Math Works, Natick, MA, USA). DARTEL procedure38 was used to obtain grey matter probability maps as follows: manual reorientation of the images to AC-PC axis (to facilitate the normalization process), segmentation of images in different tissues, grey matter, white matter and cerebrospinal fluid, creation of study-specific template, normalization of images to MNI space, modulation of the images through Jacobian determinants derived from normalization process, the resulting modulated grey matter maps contained 1.5mm×1.5mm×1.5mm isotropic voxels which were smoothed using isotropic Gaussian kernel of 10mm FWHM. Smoothed, modulated and normalized grey matter maps were used for the statistical analysis.
DNA extraction and genotyping procedureDNA was extracted from buccal cells using the Buccal Cell Kit GentraPuregen (Qiagen). Polymorphism analysis of MAOA-uVNTR was performed by the polymerase chain reaction (PCR). The sequences of the oligonucleotides used in this study were sense orientation: 5′-ACA GCC TGA CCG TGG AGA AG-3′, antisense direction: 5′-GAA CGG ACG ACG CTC CAT TCG GA-3′. The PCR reaction was performed in a final volume of 12.5μl containing 1.5mM MgCl2, 200μM of each primer, 0.2mM of dNTPs (dATP, dCTP, dGTP, dTTP), 0.25U of Taq Flexi Promega Go and 50ng genomic DNA. After 4min of denaturing at 95°C, 35 cycles were performed with the following conditions: 1min at 95°C, 1min at 62°C and 1min at 72°C. It ended with a step of 4min at 72°C. The PCR products were analyzed by agarose gel electrophoresis/Metaphor 2.5% and visualized under UV light after staining with ethidium bromide. The subjects were divided according to the genotypes of MAOA as high activity allele or low activity allele.
Statistical analysisTo explore the differences in age and years of education between control and violent groups an independent samples T test was performed using SPSS 20 for Windows. To explore the MAOA allele distribution between control and violent groups a chi-square test was performed using EPIDAT 3.1. Significance level of p≤0.05 was adopted.
Statistical design of brain images was carried out using SPM8, a two-way full factorial analysis of variance was estimated, the factors included were violent behavior with two levels (control vs. violent) and MAOA genotype with two levels (high activity allele vs. low activity allele). Total intracranial volumes (TIV) were included as a nuisance effect, TIV were obtained from the non-smoothed segmented grey matter, white matter and cerebrospinal fluid images via the “get totals” Matlab script.39 Proportional global normalization was used. Age and years of education were also included as covariates. An exclusive average-based mask was used for the model estimation40 and was created using Masking toolbox for SPM.41
The statistical analysis of grey matter maps was carried out in SPM8, the main effects were estimated as follows: for violent behavior (control group < or > violent group) and for MAOA genotype (high activity allele < or > low activity allele). F contrasts for gene by behavior interaction were also estimated, then the following T contrasts were estimated to identify the direction of interaction term between control and violent group of the same allele variation: control-high activity allele>violent-high activity allele; control-high activity allele<violent-high activity allele; control-low activity allele>violent-low activity allele; control-low activity allele<violent-low activity allele. Significance level was set at p≤0.05 with family-wise error (FWE) correction for multiple comparisons.
The MNI coordinates’ anatomical location was identified using Automatic Anatomical Labeling Atlas (AAL42) included in SPM toolbox WFU PickAtlas 2.4.43,44
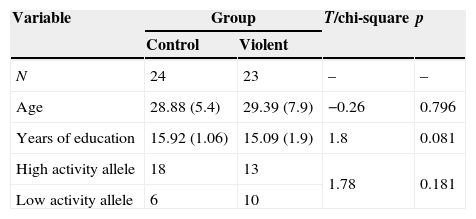
ResultsDemographic characteristics and MAOA allele distributionThere were no differences in age, years of education and frequency of MAOA allele distribution between control and violent group (Table 2).
Descriptive characteristics of the groups and MAOA allele distribution.
| Variable | Group | T/chi-square | p | |
|---|---|---|---|---|
| Control | Violent | |||
| N | 24 | 23 | – | – |
| Age | 28.88 (5.4) | 29.39 (7.9) | −0.26 | 0.796 |
| Years of education | 15.92 (1.06) | 15.09 (1.9) | 1.8 | 0.081 |
| High activity allele | 18 | 13 | 1.78 | 0.181 |
| Low activity allele | 6 | 10 | ||
Notes. Age is presented in years, mean (standard deviation).
In the total sample, the distribution of MAOA alleles was similar to the distributions described in other studies of Mexican population (chi-square=0.11, p=0.944).45,46
Voxel-based morphometryAt this SPM8 significance level (p≤0.05, FWE corrected), there were no main effects of violent behavior assessed with control < or > violent, and no main effects of MAOA genotype assessed with high activity allele < or > low activity allele contrasts, namely there were no areas of increased or decreased grey matter concentration between control and violent group and between MAOA high activity allele vs. low activity allele genetic groups.
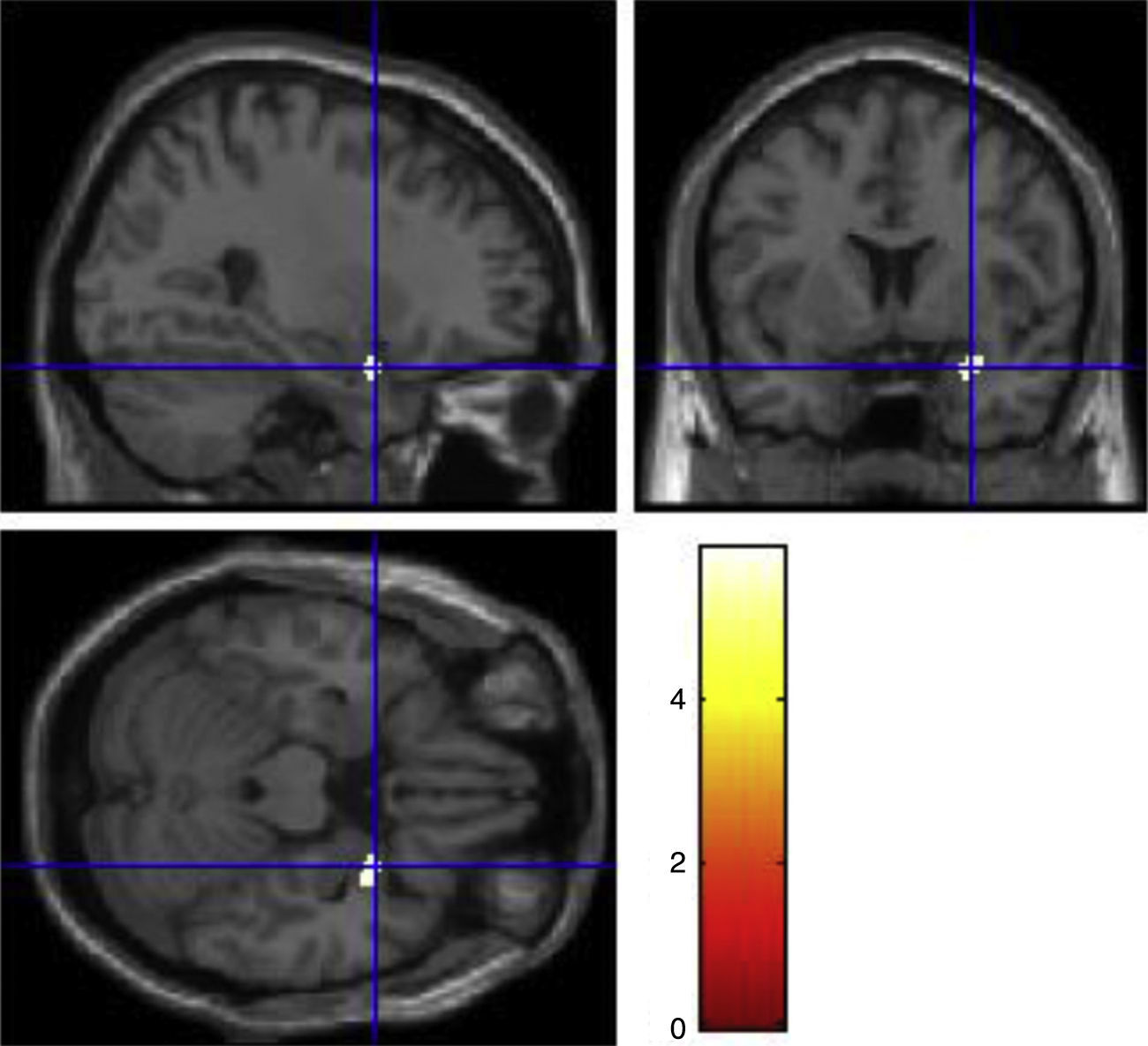
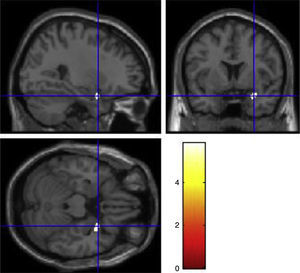
There was a significant gene by behavior interaction in right superior temporal pole (F1, 51=29.65, p=0.043). The post hoc comparison were carried out between control and violent group of the same allele variation, the results showed that there were grey matter reductions in the violent-low allele carriers group compared to the control-low allele carriers group in right superior temporal pole (MNI coordinates of the voxel of maximum statistical significance; x=28.5, y=6, z=−22.5, t=5.87, p=0.006, cluster size=102 voxels) (Fig. 1). There were no increases or decreases in the other contrasts between control and violent group of the same allelic variation.
DiscussionThe results of the present study suggest that MAOA genotype variation is an important factor that impacts in the brain structure of violent individuals. We did not found main effects of MAOA genotype, this result disagree with previous voxel-based morphometry studies carried out in healthy individuals.27,28 In the aforementioned studies MAOA genotype effects on violent behavior were not explored, which could be an important source of variation. To the best of our knowledge the present study is the first to explore the genotype by behavioral pattern (violence) interaction, therefore the results might be closest to the effect of the MAOA genotype in violence expression, instead of exploring the genetic risk to impulsivity and violence conferred by this polymorphism.
The main finding of the present study was the gene by behavior interaction expressed as reductions in violent-low activity allele carriers, compared to controls of the same allele variation, in grey matter concentration of right superior temporal pole. The reason why we expected decrements in grey matter concentrations in violent-low activity allele carriers was based mainly in the evidence about the role of 5HT and MAOA in neurodevelopment, it has been suggested that 5HT-like substances regulate the rhythm of cell division during proliferation process.47 In studies of MAOA-KO mice it has been shown that in pups there is a central increase of serotonin due to the low degradation of this neurotransmitter that leads to an abnormal development of cerebral cortex expressed as a low number of mature neurons in adult stages.48 5HT regulates several processes during neurodevelopment such as neural migration, cortical differentiation and the refinement of thalamo-cortical connections. In a study of MAOAneo mice in which the activity of MAOA enzyme during embryonic stage is disrupted, it was found a low number of mature neurons and also a delay in cell differentiation.15 Therefore, in violent-low activity allele carriers there could be a prenatal mechanism dependent of 5HT brain concentration that could affect the neurodevelopmental processes that could be expressed as grey matter reductions in adult stages in structures related with socio-emotional processing such as superior temporal pole.
Atrophies in superior temporal pole have been implicated in the expression of violent behavior in different samples such as antisocial and psychopathic individuals.2–4 This implication could be related to the functional and anatomical connectivity between superior temporal pole and other structures such as amygdala and orbitofrontal cortex through uncinate fasciculus.49 In a Diffusion Tensor Imaging study it was reported that violent psychopaths had a reduced white matter integrity in uncinate fasciculus which suggest that the lack of activity inhibition of amygdala through cortical structures such as orbitofrontal cortex and superior temporal pole could underlie the commission of violent acts.50
Studies about Klüver–Bucy's syndrome have proposed that temporal pole plays an important role in socio-emotional processing, injuries in this brain structure result in abnormal social behavior characterized by social withdrawal and aggression.51,52
Another source of evidence about the role of superior temporal pole in socio-emotional processing is the fronto-temporal dementia in which there is a rapid degeneration of frontal and/or anterior temporal pole. In the temporal variant of this dementia apparently the lateralization of the lobar degeneration is important for the expression of symptoms specifically, it has been suggested that the degeneration of right temporal pole is associated with inappropriate social behavior such as hypersexuality, aggression and lack of behavioral inhibition.52
Studies of functional magnetic resonance imaging have shown that right superior temporal pole is fundamental in the processing of social abstract concepts, namely, concepts that define social behaviors such as kind, honorable, friendly,53 the accurate processing of these social concepts in addition to emotional recognition and expression are fundamental for the establishment and maintenance of interpersonal relationships.51–54
It has been suggested that the integrity of superior temporal pole plays an important role in the activity of a neural network composed by insula and medial temporal pole. This neural network has been implicated in affective negativity regulation and in emotional attribution that could be related to the social negative and stressful events processing.55,56 Several MAOA allelic association studies have proposed that the link between violence and MAOA-uVNTR polymorphism depends on the hypersensitivity of low activity allele carriers to face negative and stressful events,16–23 so it is very likely that the reductions in superior temporal pole could underlie this hypersensitivity, however this hypothesis must be tested in future studies.
This study had a limitation about the sample size, as well as the relatively reduced sample size did not allow us to detect an allelic association between violence and MAOA genotype variation, since association studies used much larger samples. It is important to consider that we only explored the effect of one polymorphism; however it is necessary to explore the effect of other genes and polymorphisms that have been associated to violent behavior, and also complement the VBM analysis with other structural techniques such as cortical thickness for a better understanding of the effect of the MAOA polymorphism on brain structure. On the other hand, the strengths of this study were the method to define the violent phenotype, we included some components of violent behavior (aggression, hostility, impulsivity, anger and psychopathy) so the construct of violence was well assessed. Finally we consider that the gene by behavior design led us to a better understanding of the association of MAOA genetic variation and violent behavior.
ConclusionsThe results of the present study suggest that the genetic association of MAOA-uVNTR polymorphism has neurobiological basis, namely the polymorphism has a direct effect on cognitive and effective components of violent behavior. Due to the implication of superior temporal pole in socio-emotional processing this structure could be a neurobiological correlate of the MAOA-uVNTR polymorphism and violent behavior allelic association.
FundingThis research was partially supported by the Institute of Science and Technology of Mexico CityICYTDF 422.01PICDS08-19.
Conflict of interestThe authors declare that they have no conflict of interests.
The authors thank Dr. Beatriz Camarena-Medellín for the help in genotyping analysis. The authors also thank Dr. Luis Jiménez Ángeles from the National Center of Imaging and Medical Instrumentation Research of the Autonomus Metropolitan University-Iztapalapa for the help in image preprocessing.