To determine the efficacy of sitagliptin alone or in combination with metformin in women with polycystic ovary in terms of ovarian cyclicity, fertility and cardiometabolic profile compared to metformin alone.
Rationalepolycystic ovarian syndrome (PCOS) affects a percentage of 5–10% of women of reproductive age worldwide and has a prevalence of 6.6% (95% CI: 2.3–10.9%) in Mexican women and most common cause of infertility in developed countries.
Treatment with insulin sensitizing drugs (metformin and pioglitazone) has been shown to improve menstrual cyclicity and fertility in the metabolic profile with polycystic ovarian patients. Incretins and DPP-4 inhibitors have been shown to enhance pancreatic β cell activity, increasing weight loss by its anorexic effect and resulting in an adequate weight control and improved fertility.
Previous evidence has compared the effect of exenatide and alone or in combination with metformin in the treatment of PCOS, in this article we will compare sitagliptin and metformin alone or in combination.
Study designBlind, controlled and randomized clinical trial.
PatientsWomen between 18 and 40 years of age, with a BMI >20 and diagnosed with PCOS with the Rotterdam criteria.
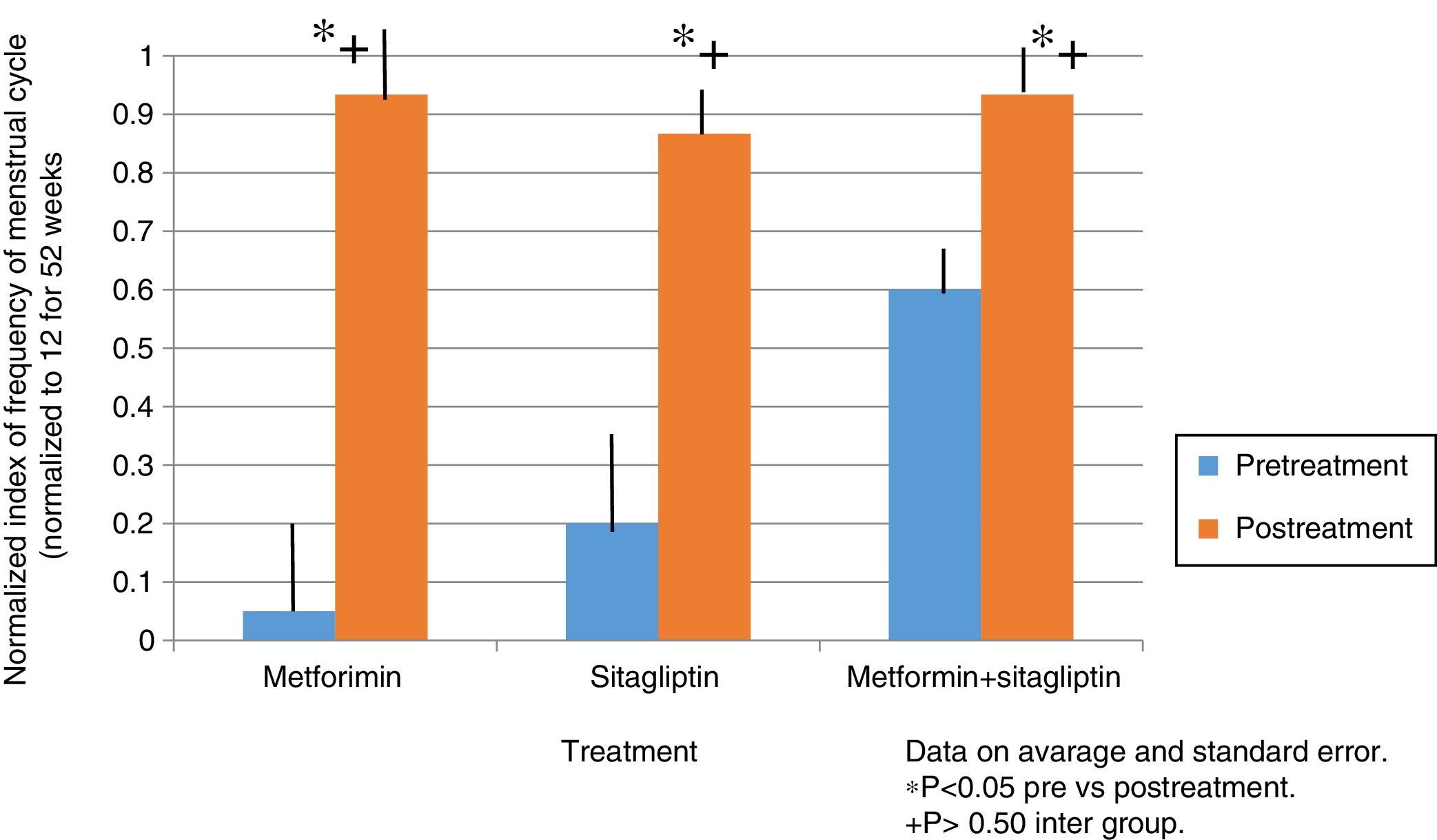
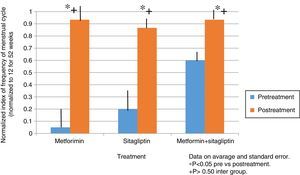
ResultsIn the normalized index of menstruations it was found that there was a statistically significant intragroup increase in each one of the treatments. With a higher percentage of change, that of metformin with 80%, followed by that of sitagliptin with 65% and then COMBO with 30%. No statistically significant differences were found between treatment groups.
ConclusionTherapeutic effect of sitagliptin was observed in patients with PCOS comparable to metformin and the combination of metformin-sitagliptin is more effective in terms of ovulation than the other two treatments alone.
Determinar la eficiencia de sitagliptina sola o en combinación con metformina en mujeres con ovario poliquístico en términos de ciclicidad ovárica, fertilidad y perfil cardiometabólico en comparación con metformina sola. Justificación: El síndrome de ovario poliquístico (SOP) afecta a un porcentaje entre el 5-10% de las mujeres en edad reproductiva en todo el mundo y tiene una prevalencia de 6,6% (IC del 95%: 2.3 a 10.9%) en mujeres mexicanas, siendo la causa más común de infertilidad en los países desarrollados.
Se ha observado que el tratamiento con fármacos sensibilizadores a la insulina (metformina y pioglitazona) mejora la ciclicidad menstrual y la fertilidad en el perfil metabólico de los pacientes con ovario poliquístico. Las incretinas y los inhibidores de la DPP-4 han demostrado mejorar la actividad de la célula β pancreática, aumentando la pérdida de peso por su efecto anoréxico y resultando en un control de peso adecuado y mejora de la fertilidad.
Las pruebas anteriores han comparado el efecto de exenatide solo o en combinación con metformina en el tratamiento de SOP, en este artículo vamos a comparar sitagliptina y metformina solas o en combinación.
Diseño del estudioEnsayo clínico cegado, controlado y aleatorizado.
PacientesMujeres entre 18 - 40 años de edad, con un IMC> 20 y con diagnóstico de SOP según los criterios de Rotterdam.
ResultadosEn el índice normalizado de menstruaciones se encontró que hubo un incremento estadísticamente significativo intragrupo en cada uno de los tratamientos. Teniendo un mayor porcentaje de cambio el de la metformina con un 80%, seguido por el de sitagliptina con un 65% y posteriormente el COMBO con un 30%. No se encontraron diferencias estadísticamente significativas entre grupos de tratamiento.
ConclusiónSe observó efecto terapéutico de sitagliptina en pacientes con SOP comparable con metformina y la combinación de metformina-sitagliptina es más eficaz en términos de la ovulación que los otros dos tratamientos solos.
Polycystic ovarian syndrome (PCOS) is a syndrome of ovarian dysfunction whose main characteristics are hyperandrogenism, hyperandrogenemia and the presence of polycystic ovaries. This syndrome affects 5–10% of women of reproductive age;1 however, a prevalence of 12.8% has been reported in Mexican American women. In 2010, Moran et al. conducted a prospective cross-sectional study on 150 Mexican women to determine the prevalence of PCOS in this population. According to Rotterdam criteria, a prevalence of 6.6% (95% CI: 2.3–10.9%) was found.2–4
Its etiology remains unknown and it is the most common cause of infertility in developed countries.1
Polycystic ovarian syndrome is associated with significant metabolic alterations. The prevalence of diabetes mellitus 2 is 10 times higher in women with PCOS than among women without PCOS. An alteration in glucose tolerance, or the development of diabetes mellitus 2 is found in 30–50% of obese women over 30 years of age with PCOS, so screening for glucose intolerance has been recommended in women with PCOS.3 The prevalence of metabolic syndrome is 2–3 times higher among women with PCOS than among women without PCOS and 20% of women with PCOS under 20 years old have metabolic syndrome.1 There is also a significant risk among patients with PCOS to develop gestational diabetes.5
A large number of patients with PCOS are overweight and many are obese; however, obesity is not considered a cause for the development of this syndrome.6
Regarding pathophysiology studies it is suggested that teak cells in women with polycystic ovary syndrome are more efficient in converting androgen precursors to testosterone than teak cells in normal women. The concentration of LH has a relative increase on FSH and the ovaries preferentially synthesize androgens. An increase in the pulse frequency of gonadotropin releasing hormone (GnRH) was observed. Increased frequency of GnRH pulses promotes the transcription of the beta subunit of LH over the beta subunit of FSH.5
The role of insulin in the pathophysiology of PCOS is very important because it acts in synergy with LH to increase the synthesis of androgens in teak cells, and the ovaries of women with PCOS appear to be more sensitive to the effect of insulin. Perhaps hypersensitivity to it, even when the classical target organs of insulin, such as muscle and fat, show resistance to its action.6–8
Insulin prevents ovulation both by direct involvement of follicular development and by the indirect increase in intra-ovarian androgen levels or alteration of gonadotropin secretion. A decrease in circulating insulin levels results in an increase frequency of ovulation or menstruation, a reduction in testosterone concentrations or both.1
Metformin is the most worldwide used biguanide for the treatment of type 2 diabetes mellitus. Its most important action is the inhibition of the production of hepatic glucose and also the increase in sensitivity of peripheral tissues to insulin. Increased insulin sensitivity, which contributes to the efficacy of metformin in the treatment of diabetes, has also been found in non-diabetic women with polycystic ovary syndrome.1
In women with PCOS, long-term treatment with metformin can increase ovulation, improve menstrual cycle and reduce androgen levels; the use of metformin may even improve hirsutism. However, it has not shown a change in risk for developing DM2.1,9
The results of a randomized clinical trial reported in 1998 that pretreatment with metformin compared to placebo increased the incidence of ovulation after subsequent clomiphene treatment. The meta-analysis by Lord et al. in 2002 included data from 13 trials and 543 women with PCOS and concluded that metformin is effective and increases the frequency of ovulation (odds ratio, 3.88, 95% confidence interval, 2.25–6.69).9,10
Signs derived from the bowel and stimulated by oral nutrient intake play an important role in insulin release. Studies suggest that glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) represent the dominant peptides in most intestinal insulin-stimulating hormones.
GIP and GLP-1 are members of the glucagon peptide superfamily and share amino acids.11
Incretins increase insulin secretion in a glucose dependent form by activation of other specific β-cell receptors.11
An intracerebroventricular injection of GLP-1, or GLP-1 receptor agonists, results in a reduction in food intake that is associated with weight loss in some but not in all studies.11
There are other actions of GLP-1 on β cell independent of acute stimulation of insulin secretion. GLP-1R agonists (GLP-1 receptor) also promote insulin biosynthesis, β-cell proliferation and stimulate exocrine or precursor cells to further differentiate the β-cell phenotype. Increased GLP-1 receptor-dependent cell volume has been demonstrated in various animal experiments. Expansion of the β-cell following administration of GLP-1R receptor agonists prevents or delays the incidence of diabetes mellitus in mice.12
GLP-1 also activates anti-apoptotic pathways, leading to a reduction in β-cell death. Studies in mice have shown a reduction in caspase 3 activation. The antiapoptotic action of GLP-1R agonists is probably directed to peroxide reduction induced by apoptosis of Min6 cells.12
Giovani Paacini et al. performed a study aimed at characterizing the secretion of GIP and GLP-1 after a loading of 75g of glucose in women with PCOS without glucose intolerance compared to healthy women.
GLP1 concentrations were the same in women with PCOS compared to control women in the initial phase of the tolerance curve up to 60min and were significantly lower in women with PCOS at 180min of the curve.11
A study by Pontikis et al. in 20 women with PCOS who underwent a glucose tolerance curve and isoglucose test after a night of fasting within two weeks, insulin, glucose, C-peptide, GIP and GLP-1 levels were measured. Obese women with PCOS showed low levels of GIP concentrations in response to the glucose tolerance curve compared to the control group. Age, insulin sensitivity (QUICKI), SHBG, and baseline GIP did not differ between the control group and patients with PCOS. However, baseline GLP-1 was significantly lower in obese women with PCOS compared to both control groups (p 0.023) and in lean women (p<0.02). The PCOS group showed a decrease in levels of GIP concentration after glucose loading compared to the control group.13,14
A novel drug, exenatide, is an incretin mimetic that mimics the glucoregulatory properties of GLP-1.12
Exenatide therapy often results in weight loss which may result in a decrease in insulin resistance. Optimal treatment of PCOS should not only improve anovulation but also decrease comorbidities such as obesity, insulin resistance and DM2, which are linked to this syndrome.11
Exenatide which is an analog of incretin glp-1 apparently has beneficial effects on the mass of the β-cell when given in pharmacological doses to rodents. The effect of DPP4 inhibitors on the β-cell mass is less clear. In mice in which diabetes was induced and treated with sitagliptin, this drug was observed to preserve β cells from apoptosis but there was no increase in β-cell mass.6
A study by Elkind-Hirsch et al. in patients with polycystic ovary syndrome, overweight and with insulin resistance evaluated the treatment with exenatide and metformin in terms of menstrual cycle, hormonal parameters, metabolic profile and inflammatory markers. 60 overweight women (BMI>27) and oligo-ovulation with PCOS, aged between 18 and 40 years,15 were included.
The study results showed a statistically significant increase in menstrual frequency in all treatment groups (p 0.001). More regular menses were reported with combination therapy compared to single drug therapy (p 0.018). Compared with baseline, ovulation periods improved in all groups, with a significantly higher proportion with combined therapy (p 0.01).15
Weight decreased significantly from the first to the last visit in all groups (p 0.001). The reduction in body weight was associated with a significant increase in menstrual frequency (p<006).15
HOMA-IR decreased significantly with all treatments (p 0.043). Similarly, insulin sensitivity, as determined by IS OGTT, improved significantly with treatment (p<0.002). The improvement in sensitivity was significantly higher with combined therapy than with exenatide alone (p<0.02) but not compared to metformin (p<0.085).16
The most frequent adverse effects were gastrointestinal of medium to moderate, nausea was the most frequent adverse effect and was greater during the combined therapy.15
Sitagliptin is a molecule belonging to the family of selective inhibitors of the enzyme dipeptidyl peptidase 4 (DDP-4) that normally degrades the endogenous incretin GIP and GLP-1.16
In humans, a daily dose of sitagliptin for 10 days has been observed to result in a nearly double increase of GLP-1 after food.
A study by Kazutaka Aoki et al. evaluated the effect of miglitol, sitagliptin and its combination on plasma concentrations of glucose, insulin and incretins in non-diabetic men. The results showed that the insulin sensitivity between the groups taking sitagliptin improved significantly, the endogenous concentrations of GIP and GLp1 increased and a statistically significant increase in pancreatic insulin secretion was observed.16
A systematic review and meta-analysis of drugs belonging to DDP-4 showed no risk of gastrointestinal adverse effects but there was an increased risk of urinary tract infections, headache and especially rhinopharyngitis.16
Treatment with insulin-sensitizing drugs (metformin and pioglitazone) has been shown to improve menstrual cycle, fertility and metabolic profile in patients with polycystic ovaries.17 However, they have no effect on beta cell activity and therefore on progression to DM2 or gestational diabetes.18,19 Incretins and DPP-4 inhibitors have been shown to enhance pancreatic β-cell activity, inhibit apoptosis, and promote weight loss because of their anorectic effect, thus providing adequate weight control and improved fertility.17 In addition, a deficit in the secretion and concentrations of GIP and GLP-1 was observed in women with PCOS.13,14 There is no work that has explored the possible therapeutic effect of the DPP-4 family in patients with PCOS.
HypothesisTreatment with sitagliptin alone or in combination with metformin in women with polycystic ovarian syndrome will be more efficient in terms of ovarian cyclicity, fertility and cardiometabolic profile compared to metformin alone.
Study designBlind, controlled and randomized clinical trial.
Objectives- •
Evaluate the change in menstrual frequency with the use of sitagliptin and metformin, alone and in combination, in obese and non-obese women with polycystic ovarian syndrome and assess the effect on the hormonal, metabolic and inflammatory profile.
- •
Evaluate changes in the menstrual pattern of patients with thin and obese PCOS with the use of sitagliptin and metformin, alone and in combination.
- •
Evaluate changes in anthropometry (absolute weight, BMI, waist circumference, waist hip index).
- •
Evaluate changes in insulin sensitivity and secretion.
- •
Evaluate changes in the concentration of reproductive hormones (FSH, LH, PRL, testosterone, androstenedione, DHEA, DHEAS, 17 OHP4, and TSH).
- •
Evaluate changes in ovulation rate (luteal phase progesterone).
- •
Evaluate changes in lipid profile (total cholesterol, HDL, LDL, VLDL, LDL, non-HDL cholesterol, triglycerides).
- •
Evaluate changes in markers of inflammation (C-reactive protein, VSG, adiponectin, IL6, SHBG).
- •
Age between 18 and 40 years.
- •
BMI >20.
- •
Diagnosis of PCOS by criteria of Rotterdam.
- •
Women with a diagnosis of diabetes mellitus.
- •
Smokers.
- •
Use of hormones in the 6 months prior to study entry.
- •
Drugs that affect bowel motility.
- •
Lipid-lowering consumption.
- •
Drugs that decrease weight in the last 3 months.
- •
Intake of metformin in the last 6 months.
- •
No previous history of assisted fertilization treatment in the previous 6 months.
- •
No signed letter of informed consent.
- •
There is no attachment to treatment.
- •
Do not go to scheduled appointments.
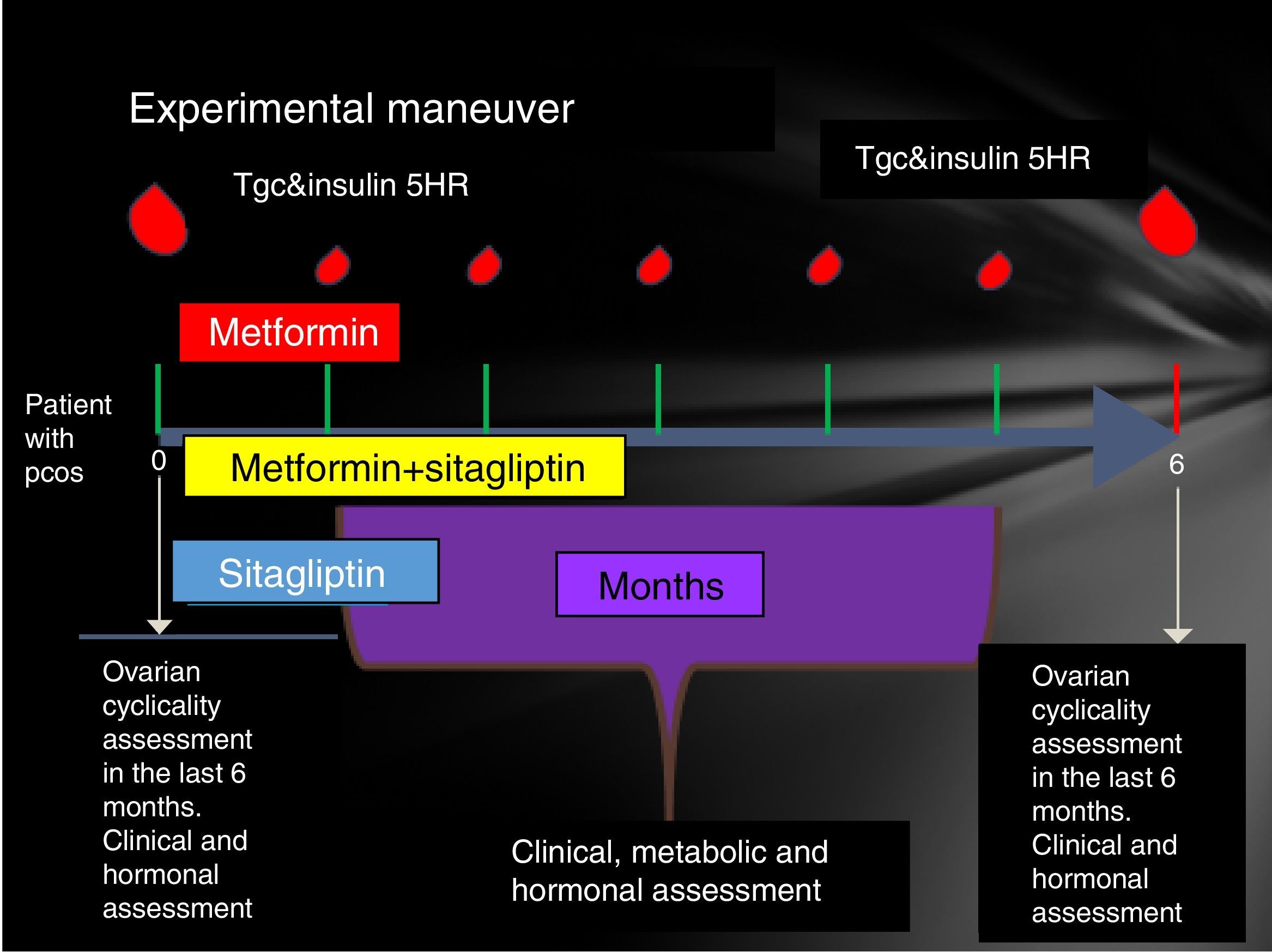
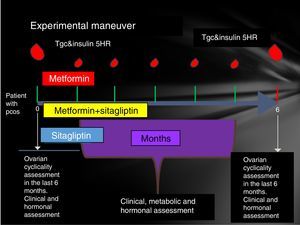
Participating patients were cited every Friday from 8 to 14h. The reasons for the study, their advantages and disadvantages were explained to them extensively and the signing of an informed consent was therefore considered. Patients who accepted to be admitted had a clinical evaluation (determination of menstrual pattern and application of the Ferriman Gallwey scale to determine the degree of hyperandrogenism), transvaginal USG and hormone quantification (LH, FSH, testosterone, androstenedione, dehydroepiandrosterone, Prolactin, cortisol, ACTH, TSH, T4, T3) in order to identify patients who meet the Rotterdam criteria and exclude other diseases with a clinical picture similar to PCOS.
Patients who were identified with PCOS were cited in the follicular phase of the menstrual cycle (from the 1st to the 5th day of menstruation), special mention is those patients who present with amenorrhea who were cited from the 1st to the 5th day of bleeding after the application of 5mg daily of medroxyprogesterone, at this time was randomized to assign them to one of three groups:
- •
Group 1, metformin with an initial dose 425mg VO before breakfast and before dinner until reaching a dose of 850mg every 12h.
- •
Group 2, sitagliptin 100mg v. every 24h.
- •
Group 3, sitagliptin plus metformin at the doses described above.
Prior to administration of the first dose, a 75-g glucose tolerance curve was programmed. In the first sample, 20ml were obtained to quantify: lipid profile (total cholesterol, HDL, LDL, VLDL, LDL, non-HDL cholesterol triglycerides) and markers of inflammation (C-reactive protein, VSG and adiponectin, IL6, SHBG) counts in the following times 0, 30, 60, 120, 180, 240 and 300min. At each time 3ml were taken to quantify glucose and insulin.
The same was done at 24 weeks post treatment compliance according to the assigned group with only 24h of suspension of the assigned medication. An individual with a normal menstrual pattern was considered if she presented 5 menses in 24 weeks of drug intervention (Fig. 1).
Statistic analysisIt was performed for quantitative variables, mean and standard deviation. Proportions were calculated for qualitative variables. Quantitative variables were compared with paired Student's t-test. Qualitative variables were compared with chi square tests. A p less than 0.05 will be considered as statistical significance.
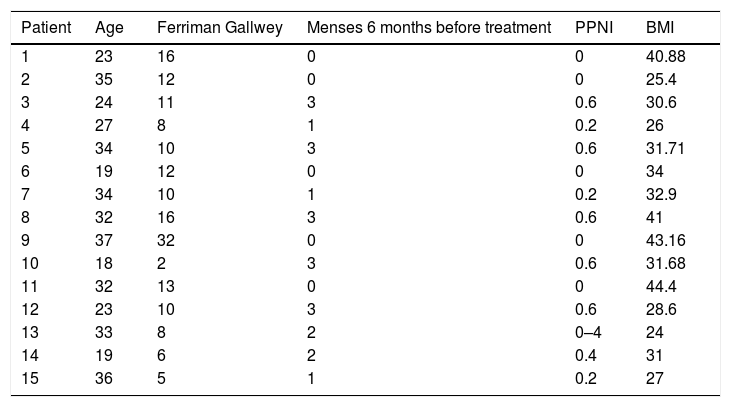
ResultsFifteen patients who were diagnosed with PCOS according to the Rotterdam criteria were included in this study. Fifteen other causes of hyperandrogenism were ruled out in the study protocol, and all of them were diagnosed with PCOS according to the Rotterdam criteria. Patients presented clinical or biochemical signs of hyperandrogenism and 12 (80%) had ultrasonographic images compatible with polycystic ovaries. The age range was between 18 and 37 years, 100% presented menstrual alterations, 7 patients (46%) presented opsomenorrhea and 8 (53.3%) amenorrhea. In terms of weight, 5 patients (33.3%) presented grade I obesity, 5 patients (26.6%) obesity grade II, 1 patient grade III obesity, 4 patients (26.6%) grade IV obesity. Considering that in a period of 6 months it is normal to present 5 menstrual cycles an index was created to normalize the number of menses per group according to their frequency (Table 1).
Clinical features of patients with polycystic ovary syndrome.
| Patient | Age | Ferriman Gallwey | Menses 6 months before treatment | PPNI | BMI |
|---|---|---|---|---|---|
| 1 | 23 | 16 | 0 | 0 | 40.88 |
| 2 | 35 | 12 | 0 | 0 | 25.4 |
| 3 | 24 | 11 | 3 | 0.6 | 30.6 |
| 4 | 27 | 8 | 1 | 0.2 | 26 |
| 5 | 34 | 10 | 3 | 0.6 | 31.71 |
| 6 | 19 | 12 | 0 | 0 | 34 |
| 7 | 34 | 10 | 1 | 0.2 | 32.9 |
| 8 | 32 | 16 | 3 | 0.6 | 41 |
| 9 | 37 | 32 | 0 | 0 | 43.16 |
| 10 | 18 | 2 | 3 | 0.6 | 31.68 |
| 11 | 32 | 13 | 0 | 0 | 44.4 |
| 12 | 23 | 10 | 3 | 0.6 | 28.6 |
| 13 | 33 | 8 | 2 | 0–4 | 24 |
| 14 | 19 | 6 | 2 | 0.4 | 31 |
| 15 | 36 | 5 | 1 | 0.2 | 27 |
PPNI: Pretreatment period normalized index; BMI: body mass index.
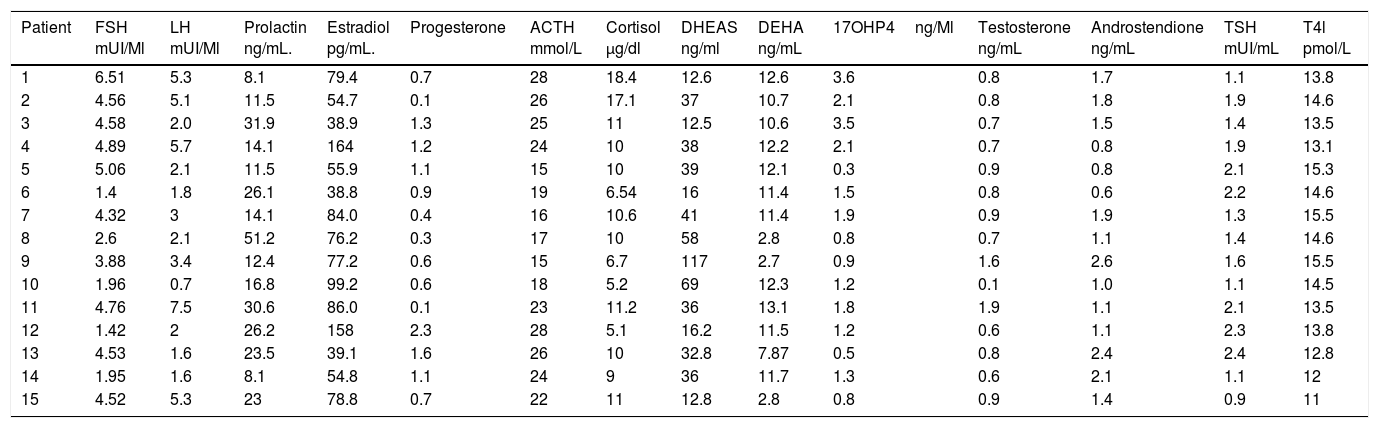
It was observed that only 2 patients (13.33%) presented the characteristic dissociation of LH and FSH, and 2 patients (13.33%) presented testosterone concentrations compatible with androgen producing ovarian tumor, which was discarded by ultrasonography; it is also noted that all patients had a TSH concentration lower than 2.5, which is the cut-off point currently used as a diagnosis of subclinical hypothyroidism. And also that all patients had a concentration of 17OHP4 (17 hydroxyprogesterone) below 4ng/mL which is the cutoff point for suspecting 21-hydroxylase deficiency (Table 2).
Hormonal features of patients with polycystic ovary syndrome.
| Patient | FSH mUI/Ml | LH mUI/Ml | Prolactin ng/mL. | Estradiol pg/mL. | Progesterone | ACTH mmol/L | Cortisol μg/dl | DHEAS ng/ml | DEHA ng/mL | 17OHP4ng/Ml | Testosterone ng/mL | Androstendione ng/mL | TSH mUI/mL | T4l pmol/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.51 | 5.3 | 8.1 | 79.4 | 0.7 | 28 | 18.4 | 12.6 | 12.6 | 3.6 | 0.8 | 1.7 | 1.1 | 13.8 |
| 2 | 4.56 | 5.1 | 11.5 | 54.7 | 0.1 | 26 | 17.1 | 37 | 10.7 | 2.1 | 0.8 | 1.8 | 1.9 | 14.6 |
| 3 | 4.58 | 2.0 | 31.9 | 38.9 | 1.3 | 25 | 11 | 12.5 | 10.6 | 3.5 | 0.7 | 1.5 | 1.4 | 13.5 |
| 4 | 4.89 | 5.7 | 14.1 | 164 | 1.2 | 24 | 10 | 38 | 12.2 | 2.1 | 0.7 | 0.8 | 1.9 | 13.1 |
| 5 | 5.06 | 2.1 | 11.5 | 55.9 | 1.1 | 15 | 10 | 39 | 12.1 | 0.3 | 0.9 | 0.8 | 2.1 | 15.3 |
| 6 | 1.4 | 1.8 | 26.1 | 38.8 | 0.9 | 19 | 6.54 | 16 | 11.4 | 1.5 | 0.8 | 0.6 | 2.2 | 14.6 |
| 7 | 4.32 | 3 | 14.1 | 84.0 | 0.4 | 16 | 10.6 | 41 | 11.4 | 1.9 | 0.9 | 1.9 | 1.3 | 15.5 |
| 8 | 2.6 | 2.1 | 51.2 | 76.2 | 0.3 | 17 | 10 | 58 | 2.8 | 0.8 | 0.7 | 1.1 | 1.4 | 14.6 |
| 9 | 3.88 | 3.4 | 12.4 | 77.2 | 0.6 | 15 | 6.7 | 117 | 2.7 | 0.9 | 1.6 | 2.6 | 1.6 | 15.5 |
| 10 | 1.96 | 0.7 | 16.8 | 99.2 | 0.6 | 18 | 5.2 | 69 | 12.3 | 1.2 | 0.1 | 1.0 | 1.1 | 14.5 |
| 11 | 4.76 | 7.5 | 30.6 | 86.0 | 0.1 | 23 | 11.2 | 36 | 13.1 | 1.8 | 1.9 | 1.1 | 2.1 | 13.5 |
| 12 | 1.42 | 2 | 26.2 | 158 | 2.3 | 28 | 5.1 | 16.2 | 11.5 | 1.2 | 0.6 | 1.1 | 2.3 | 13.8 |
| 13 | 4.53 | 1.6 | 23.5 | 39.1 | 1.6 | 26 | 10 | 32.8 | 7.87 | 0.5 | 0.8 | 2.4 | 2.4 | 12.8 |
| 14 | 1.95 | 1.6 | 8.1 | 54.8 | 1.1 | 24 | 9 | 36 | 11.7 | 1.3 | 0.6 | 2.1 | 1.1 | 12 |
| 15 | 4.52 | 5.3 | 23 | 78.8 | 0.7 | 22 | 11 | 12.8 | 2.8 | 0.8 | 0.9 | 1.4 | 0.9 | 11 |
FSH: follicle stimulating hormone; LH: leuteinising hormone; ACTH: adrenocorticotropic hormone; 17OHP4: 17-hydroxyprogesterone; TSH: thyroid stimulating hormone; T4l: free thyroxine; DEHA: dehydroepiandrosterone; DEHAS: dehydroepiandrosterone sulfate.
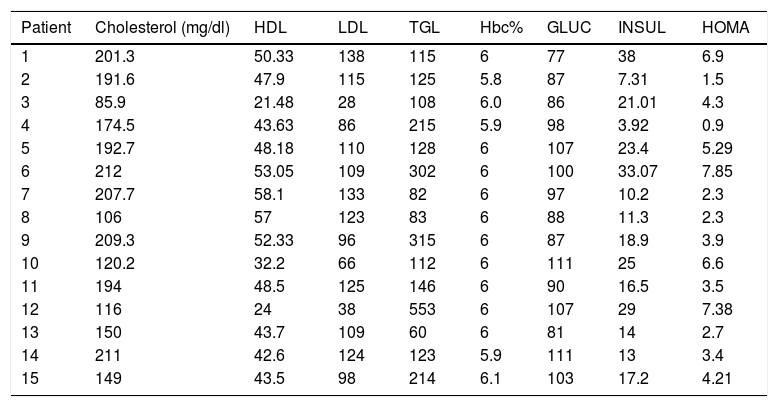
Five patients (33.33%) had hypercholesterolemia, 5 patients (33.33%) had hypertriglyceridemia, 7 patients (46.6%) had LDL hypercholesterolemia, and 3 patients (20%) had abnormally low concentrations of HDL cholesterol.
On the other hand, 5 patients (33.33%) had altered fasting glycemia, 10 patients (66.6%) had baseline hyperinsulinemia, 13 patients (86%) presented insulin resistance and 2 patients (13.33%) dysinsulinism.
As for glycosylated hemoglobin in the 15 patients (100%) the value was normal, so the diagnosis of diabetes mellitus was ruled out by this criterion (Table 3).
Metabolic features of patients with polycystic ovary syndrome.
| Patient | Cholesterol (mg/dl) | HDL | LDL | TGL | Hbc% | GLUC | INSUL | HOMA |
|---|---|---|---|---|---|---|---|---|
| 1 | 201.3 | 50.33 | 138 | 115 | 6 | 77 | 38 | 6.9 |
| 2 | 191.6 | 47.9 | 115 | 125 | 5.8 | 87 | 7.31 | 1.5 |
| 3 | 85.9 | 21.48 | 28 | 108 | 6.0 | 86 | 21.01 | 4.3 |
| 4 | 174.5 | 43.63 | 86 | 215 | 5.9 | 98 | 3.92 | 0.9 |
| 5 | 192.7 | 48.18 | 110 | 128 | 6 | 107 | 23.4 | 5.29 |
| 6 | 212 | 53.05 | 109 | 302 | 6 | 100 | 33.07 | 7.85 |
| 7 | 207.7 | 58.1 | 133 | 82 | 6 | 97 | 10.2 | 2.3 |
| 8 | 106 | 57 | 123 | 83 | 6 | 88 | 11.3 | 2.3 |
| 9 | 209.3 | 52.33 | 96 | 315 | 6 | 87 | 18.9 | 3.9 |
| 10 | 120.2 | 32.2 | 66 | 112 | 6 | 111 | 25 | 6.6 |
| 11 | 194 | 48.5 | 125 | 146 | 6 | 90 | 16.5 | 3.5 |
| 12 | 116 | 24 | 38 | 553 | 6 | 107 | 29 | 7.38 |
| 13 | 150 | 43.7 | 109 | 60 | 6 | 81 | 14 | 2.7 |
| 14 | 211 | 42.6 | 124 | 123 | 5.9 | 111 | 13 | 3.4 |
| 15 | 149 | 43.5 | 98 | 214 | 6.1 | 103 | 17.2 | 4.21 |
HDL: high density cholesterol; LDL: low-density cholesterol; TGL: triglycerides; Hbc%: glycosylated hemoglobin; HOMA: index of insulin resistance; GLUC: glucose; INSUL: insulin.
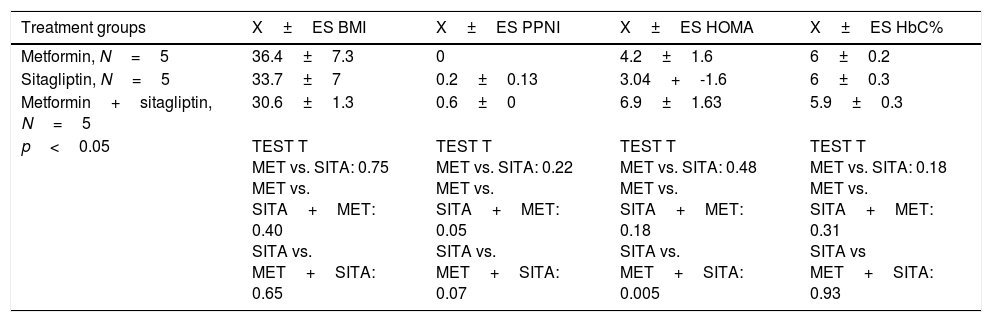
Five patients were in the metformin group, five patients in the sitagliptin group and five patients in the metformin+sitagliptin group. The hormonal, clinical and metabolic characteristics between the groups were homogeneous at the start of the study, except than in the combo group (MET+SITA), insulin resistance was higher (p<0.05) but with a higher index of menses (p<0.05), a condition that has to be taken into account when analyzing the results for this group (Table 4).
Clinical, hormonal and metabolic basal features by treatment group.
| Treatment groups | X±ES BMI | X±ES PPNI | X±ES HOMA | X±ES HbC% |
|---|---|---|---|---|
| Metformin, N=5 | 36.4±7.3 | 0 | 4.2±1.6 | 6±0.2 |
| Sitagliptin, N=5 | 33.7±7 | 0.2±0.13 | 3.04+-1.6 | 6±0.3 |
| Metformin+sitagliptin, N=5 | 30.6±1.3 | 0.6±0 | 6.9±1.63 | 5.9±0.3 |
| p<0.05 | TEST T MET vs. SITA: 0.75 MET vs. SITA+MET: 0.40 SITA vs. MET+SITA: 0.65 | TEST T MET vs. SITA: 0.22 MET vs. SITA+MET: 0.05 SITA vs. MET+SITA: 0.07 | TEST T MET vs. SITA: 0.48 MET vs. SITA+MET: 0.18 SITA vs. MET+SITA: 0.005 | TEST T MET vs. SITA: 0.18 MET vs. SITA+MET: 0.31 SITA vs MET+SITA: 0.93 |
PPNI: Pretreatment period normalized index; BMI: body mass index; X: average; SE: standard error; MET: metformin; SITA: sitagliptin.
Although the patients were given counseling for the aspects of nutrition and exercise; only recommendation was in accordance with international guidelines as part of the management of PCOS, and sought to have an adequate attachment. All patients in each group reported adhering to these recommendations in a percentage greater than 90% so that the effect of the absolute weight loss intragroup and intergroup and in BMI represents the effect of the drug in the corresponding group.
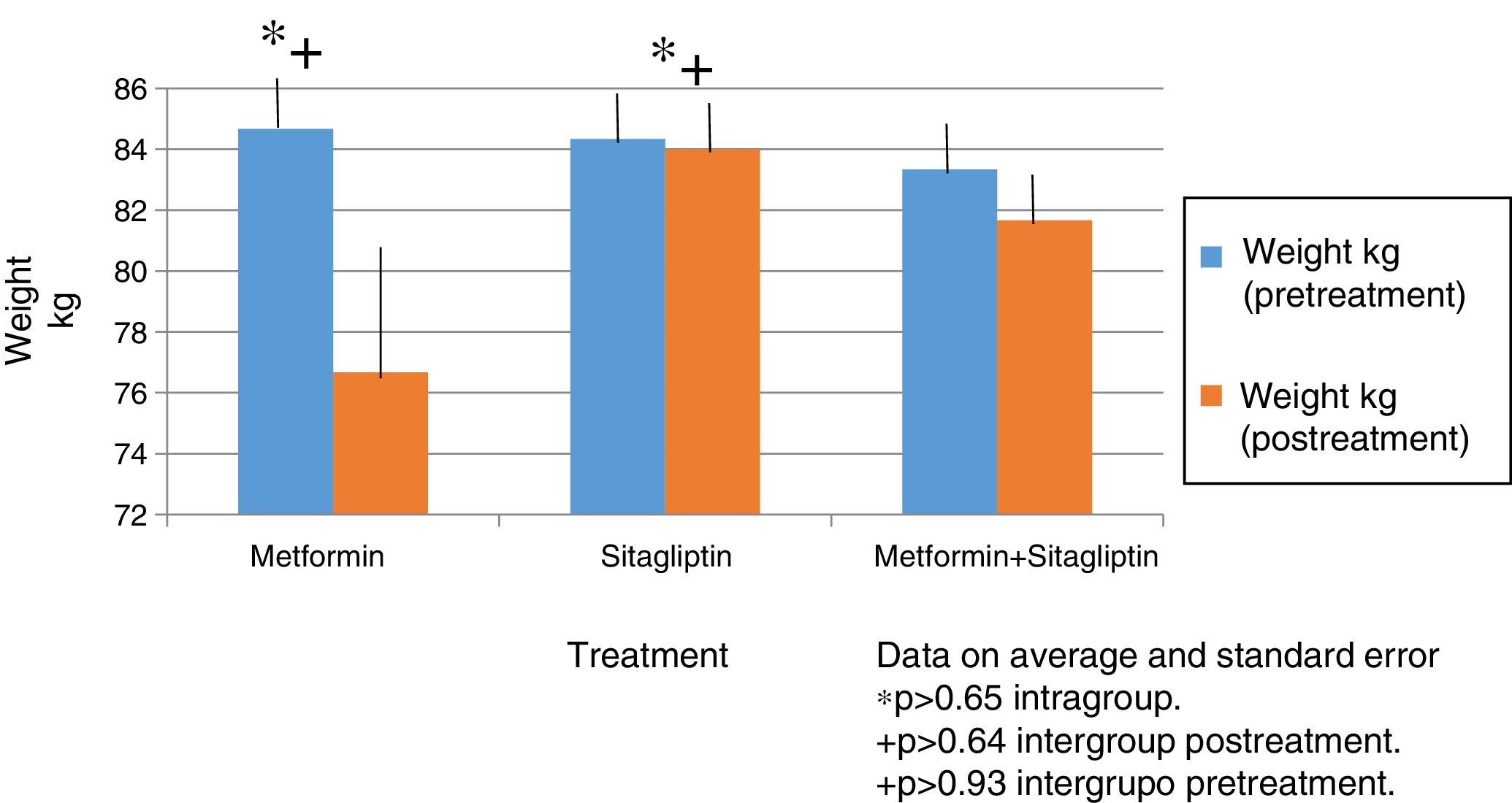
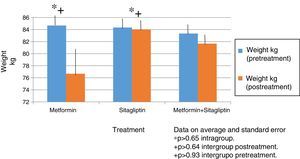
In all treatment groups there was a decrease in weight associated with the use of the drug, with the percentage of change (10%) being higher for the metformin group and lower for the sitagliptin group. The percentage change for the COMBO group was 2%. However, there were no significant differences in the intragroup reduction or in the comparison between groups associated with the drugs (Fig. 2).
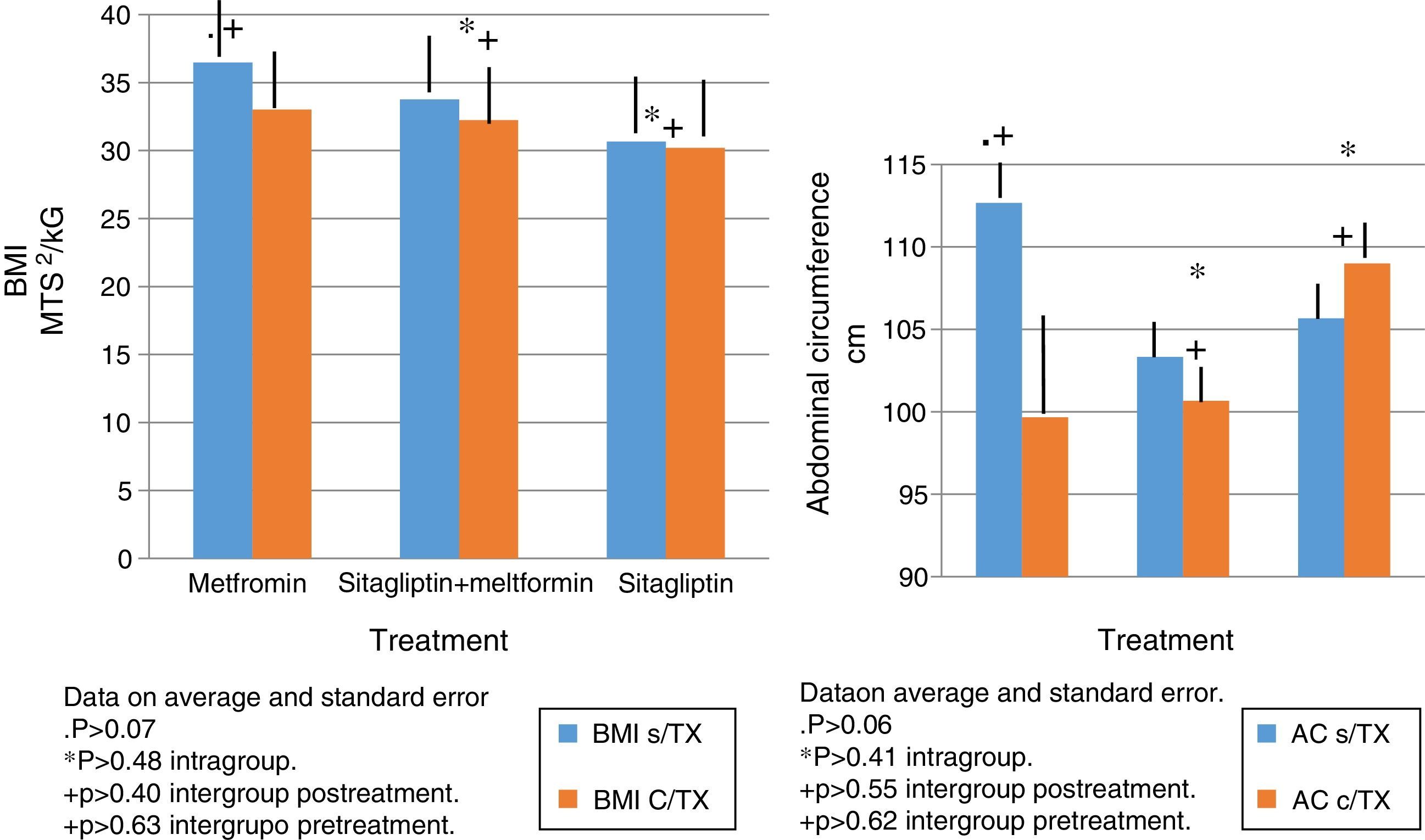
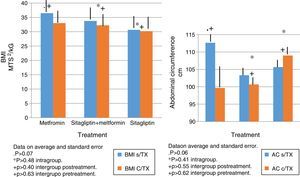
In terms of IMC correlates with what was found in the weight analysis however with more discrete changes in the metformin group with a percentage change of reduction of 5%. There were no significant differences in the reduction of intra- or inter-group BMI associated with treatment (Fig. 3).
The same behavior observed in the metformin group in terms of absolute weight and BMI is observed in the reduction in the abdominal perimeter up to 13cc. In the Sitagliptin group, there was also a reduction of the abdominal perimeter of 3cm and an increase of the abdominal perimeter in the COMBO group of up to 3cm.
As with the above variables, no statistically significant differences were observed in intragroup and intergroup comparisons (Fig. 3).
The same behavior observed in the metformin group in terms of absolute weight and BMI is observed in the reduction in the abdominal perimeter up to 13 cc. In the Sitagliptin group, there was also a reduction of the abdominal perimeter of 3cm and an increase of the abdominal perimeter in the COMBO group of up to 3cm.
As with the above variables, no statistically significant differences were observed in intragroup and intergroup comparisons (Fig. 3).
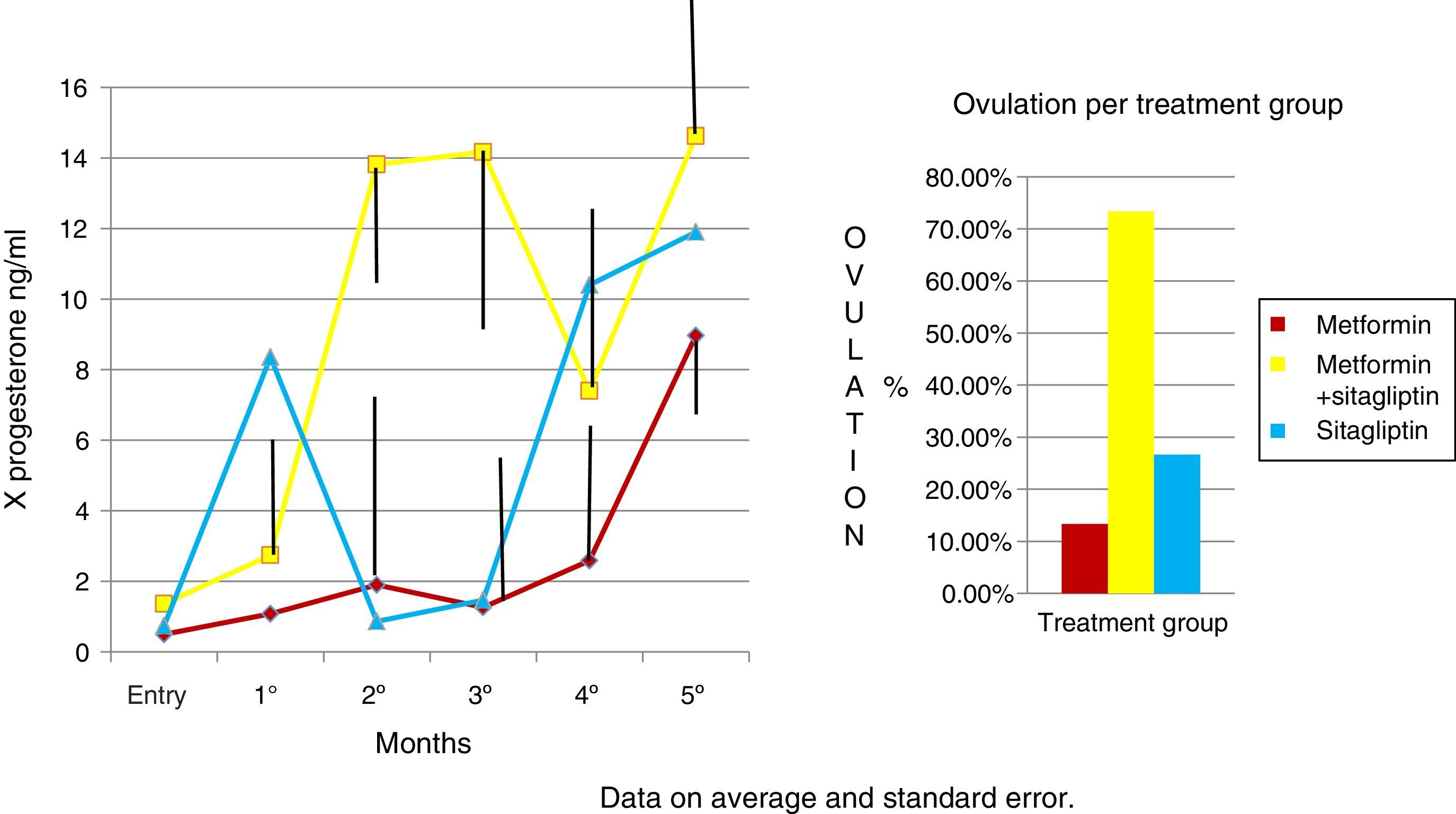
In the case of the normalized menstruation index, it was found that there was a statistically significant intra-group increase in each of the treatment groups. The group with the highest percentage of change was the metformin group with 80%, followed by sitagliptin with 65% and then COMBO with 30%. No significant differences were found between treatment groups (Fig. 4).
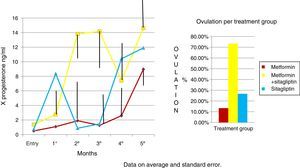
An increase in all groups of progesterone concentrations was observed as the treatment time was completed. Since the COMBO group had the greatest increase in concentrations and with a more regular behavior, the second group with the highest increase with progesterone concentrations was that of sitagliptin, however, with an ethical behavior and, thirdly, the group of metformin with a more regular behavior. Although the statistical tests did not show significant differences (Fig. 5).
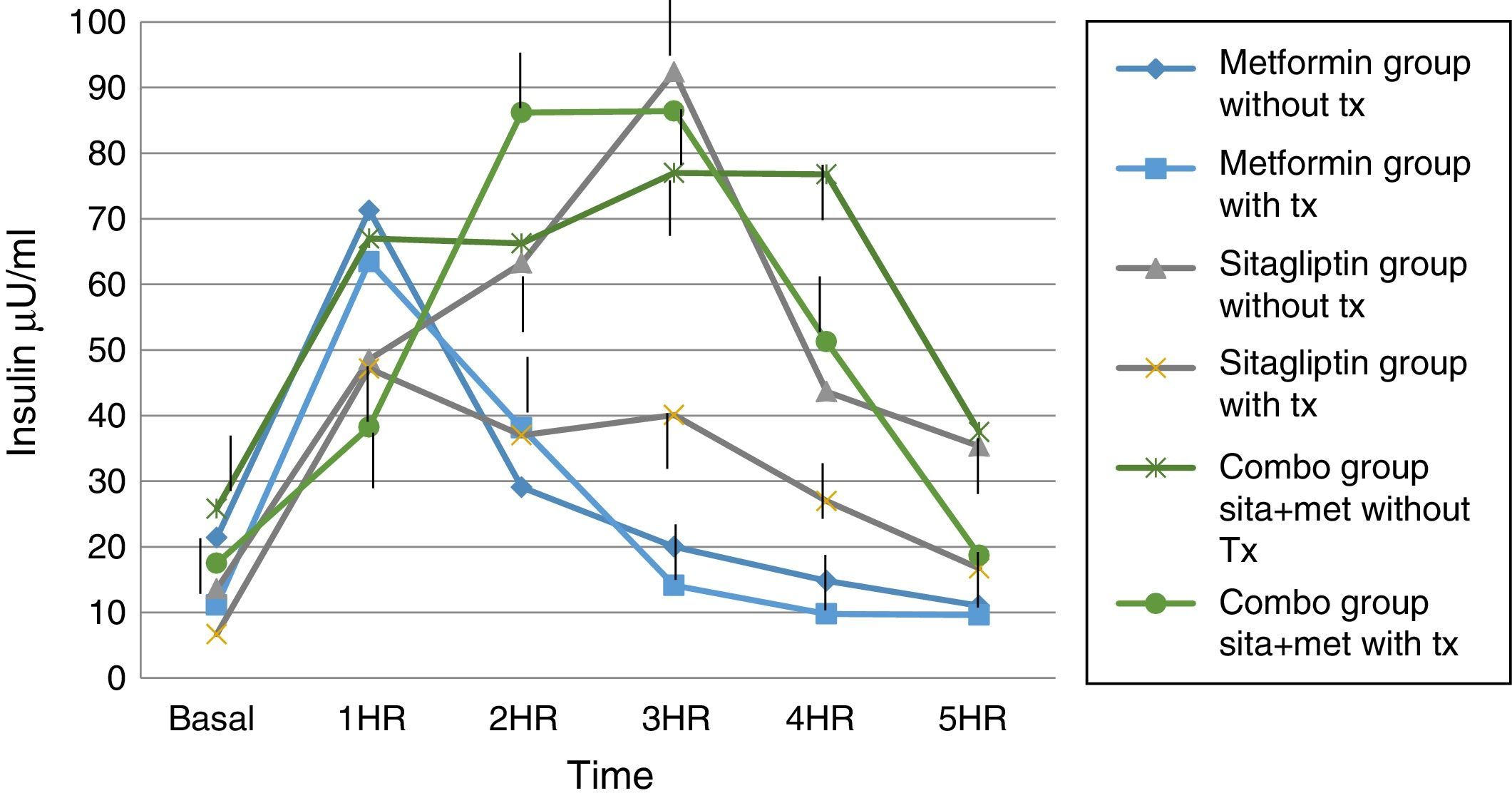
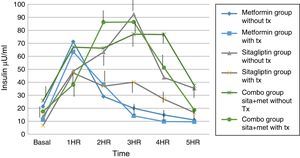
Regarding insulin secretion, it was observed that in both the metformin and sitagliptin groups there was a decrease in insulin concentrations, especially in the middle and final part of the curve of secretion. However, a greater delta shift was observed in the sitagliptin group especially in the 3rd, 4th and 5th hour of secretion (50, 10 and 20μU/ml insulin respectively). Comparing 10μU/ml insulin at the same times for metformin in the COMBO group, there was a reduction in secretion in the initial part of the secretion with a change delta of 10 and 20μU/ml of insulin in the first and second hour; however, in the middle and final part of the curve it seems to have lost the effect of reducing insulin secretion by the treatment. Statistical tests showed no difference in intragroup change (T paired p>0.05) as well as between each time of secretion between groups (ANOVA p>0.05) (Fig. 6).
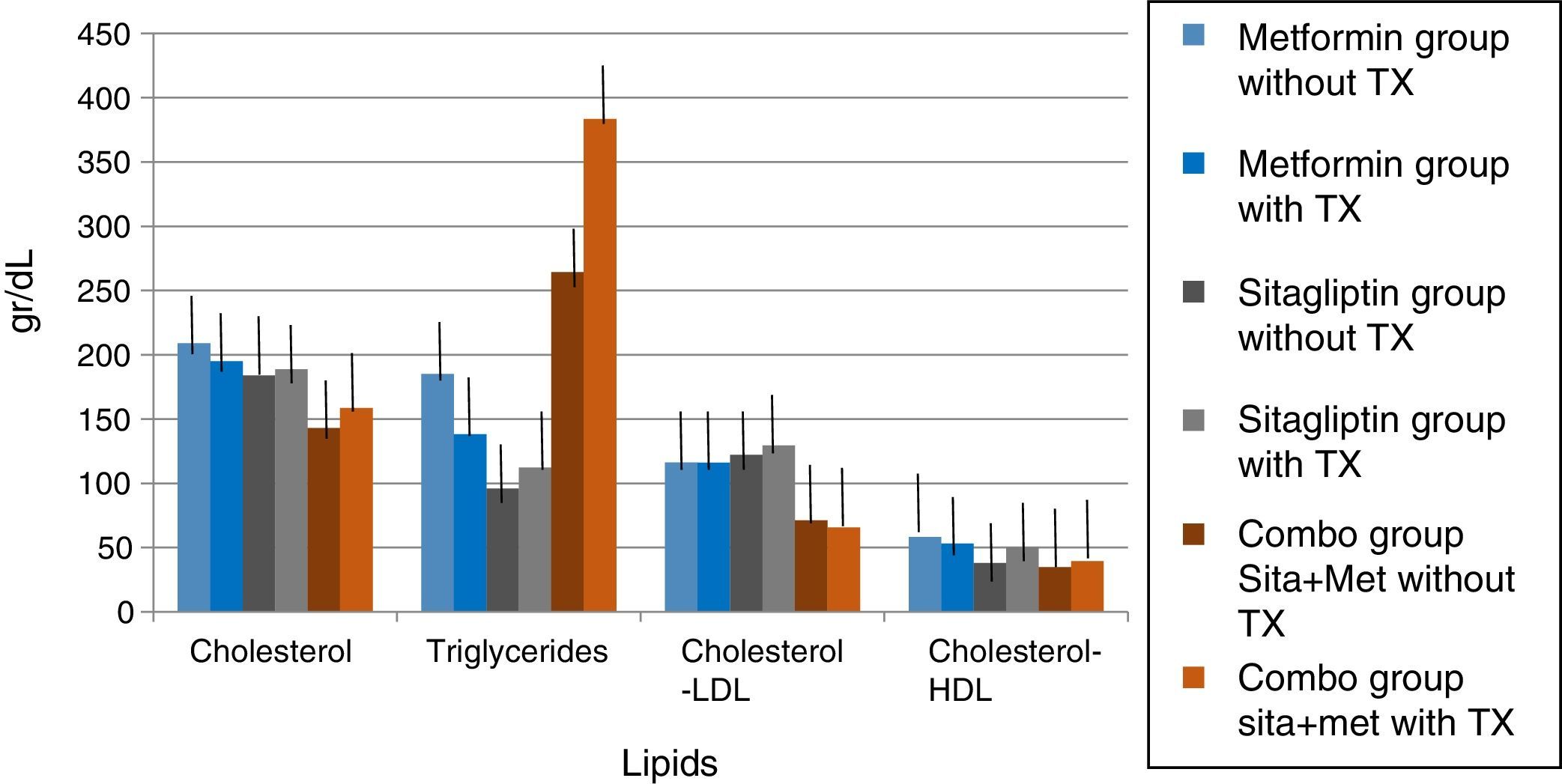
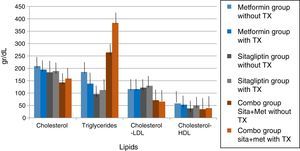
In lipids there was no effect of any of the treatments only in the case of metformin in terms of triglycerides if there was a reduction with a delta of 50g/dl decrease (33.3%). An increase in the COMBO group was also observed in the triglycerides with a change delta of 100g/dL (20%). However, the statistical tests showed no significant differences (Fig. 7).
DiscussionThe group of patients with PCOS had homogeneous characteristics and no statistically significant differences. In the case of the COMBO group at the beginning, significantly greater resistance to insulin was found, so even discrete changes in weight would be reflected in clinical consequences such as increased frequency of menses and increased progesterone concentrations in ovulation ranges. However, paradoxically, this group started with a higher index of menses with respect to the other groups. Therefore, the delta change in terms of menstruation frequency reflected in this group with respect to the previous ones may have been underestimated, which is due for future research to control this variable, as this being an open study it was not possible to control. It is noteworthy that within the groups of sitagliptin vs metformin the biochemical, clinical and hormonal characteristics did not show statistically significant differences and the effect on increased menstrual frequency is comparable to that observed in the metformin group, considering that the basal index of menses was higher in the metformin group. Although the trend indicates that metformin would have greater effect in increasing the frequency of its menstruations, no significant differences were found. The number of study participants should be increased to perhaps find such differences. In the case of the COMBO group, the effect on menstruation frequency also reached significance between groups; however, the number of sample sizes was insufficient to demonstrate differences between the treatment groups. In the case of the Elkind-Hirsch K. study comparing metformin vs. exenetide vs COMBO, an increase in the frequency of menses was observed in all groups, however, there were also significant differences between groups for weight loss that the effect of exenetide on the frequency of menstruations is in doubt that the observed result is secondary to the use of exenatide or to the weight loss that was presented between groups.16
In the case of our study we did not observe statistically significant weight differences before and after any treatment group as we observed in terms of increased frequency of menses and ovulation could be attributed directly to the treatment effect. It is noteworthy that both metformin vs sitagliptin groups had almost equal results in terms of increased progesterone concentrations which is an indirect measure of ovulation, slightly higher in the sitagliptin vs metformin group but with more ethical behavior, which would underpin fetal possible sitagliptin ovarian receptors. It is noteworthy that, although the COMBO group was less efficient in terms of increased frequency of menses, its effect on ovulation was greater than in other treatment groups, which could suggest a greater number of ovarian receptors for this treatment or greater Sensitivity of the same. In terms of insulin secretion, it should be noted that while sitagliptin is not considered an insulin sensitizer, it has been observed to decrease plasma insulin concentrations, perhaps by influencing better pancreatic beta cell function in our studies showed a significant decrease in insulin secretion, even with greater change delta than in the metformin group, however it must be considered that both the metformin group and the COMBO group started with increased insulin resistance and concentrations of insulin were not as low as those observed in the metformin group. This has also been observed in a study by Kazaka Aoki et al.15 In the case of the lipid profile there seems to be no impact of the drugs on this variable so that although the three groups improve the hormonal, insulinogenic and reproductive profile have no real impact on lipid metabolism, the latter has already been observed by multiple authors.8,9,10,11,15
ConclusionsSitagliptin improves ovarian cyclicity and ovulation in women with PCOS on comparable terms with regard to metformin and metformin sitagliptin combination.
The combination of sitagliptin metformin is more effective in terms of ovulation than the other two treatments alone. However, sitagliptin showed that it can influence the reproductive and intraovaric aspect. It also showed that it can improve insulin metabolism in patients with PCOS so it would be interesting to demonstrate if it could be a treatment that not only improves the clinical, metabolic and reproductive conditions of patients with PCOS but also prevents the development of diabetes mellitus, a highly frequent consequence of these patients.
The weight did not modify the results of the findings and a tendency of the metformin to produce decrease of weight and of abdominal perimeter was observed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interests.