Colorectal cancer is the fourth most common cancer and the second leading cause of death from cancer in the United States. Preoperative neoadjuvant therapy (chemotherapy and radiotherapy) is the gold standard in the management of rectal cancer before surgical resection. The literature includes reports of absence of neoplastic cells after neoadjuvant therapy. There are no reports on complete pathological response to this type of therapy in Mexico.
ObjectivesDetermine the percentage of patients with rectal adenocarcinoma with complete pathological response after neoadjuvant therapy. All patients were treated in a colorectal surgery department of a tertiary care hospital.
Materials and methodsA total of 64 patients with rectal adenocarcinoma diagnosed by biopsy were treated from January 2010 to December 2015. Demographic data, tumour localisation, pathological report, TNM stage, neoadjuvant therapy, surgical procedure and postoperative pathological report were collected from patient records.
ResultsMean patient age was 52.8 years (range of 26–73); 31 were women and 33 men. Twenty-seven patients (27) were stage II and 27 stage III. The preoperative biopsy results were classified as: well differentiated (10); moderately differentiated (48); and poorly differentiated/undifferentiated adenocarcinoma (6). Twenty patients received neoadjuvant therapy (31.2%). In these, 2 tumours were localised in the upper third of the rectum, 6 in the middle third, and 12 in the lower third. Six patients underwent abdominoperineal resection, 10 total mesorectal excision, and four posterior pelvic exenteration. Six patients undergoing neoadjuvant therapy had a complete pathological response.
ConclusionsThe percentage of patients with a complete pathological response is similar to that in other literature reports. More evidence is needed to define good prognosis factors in patients who might not require surgery after neoadjuvant therapy.
Antecedentes El cáncer colorrectal es el cuarto tipo más frecuente de cáncer y la segunda causa de muerte por cáncer en Estados Unidos.
El tratamiento neoadyuvante preoperatorio (quimioterapia y radioterapia) es el estándar de oro en el manejo de cáncer de recto, previo a una cirugía resectiva. En la literatura se reporta ausencia de células neoplásicas posterior a neoadyuvancia. En México no se tienen reportes sobre la respuesta patológica completa posterior a neoadyuvancia en cáncer de recto.
ObjetivoReportar el porcentaje de pacientes que presentó respuesta patológica completa posterior a neoadyuvancia por adenocarcinoma de recto, tratados en un servicio especializado de tercer nivel.
Materiales y Métodos: Se incluyeron 64 pacientes con biopsia de adenocarcinoma de recto atendidos de enero del 2010 a diciembre del 2015. Se obtuvieron de los expedientes los datos demográficos, localización del tumour, resultado histopatológico, estadio TNM, esquema de neoadyuvancia, cirugía realizada y diagnóstico histopatológico posquirúrgico.
ResultadosLa edad media de los pacientes fue de 52.8 años (rango 26 a 73); 31 fueron mujeres, 33 hombres. 27 se encontraban en estadio II y 27 en estadio III. La biopsia preoperatoria se clasificó como adenocarcinoma bien diferenciado (10), moderadamente diferenciado (48) y mal diferenciado/indiferenciado (6). Veinte pacientes recibieron neoadyuvancia (31.2%). De los pacientes que recibieron neoadyuvancia dos tumores se encontraban en tercio superior, seis en tercio medio y 12 tercio inferior. En seis casos se realizó resección abdominoperineal, en 10 escisión total de mesorrecto (ETM) y cuatro exenteración pélvica posterior. De los pacientes con neoadyuvancia, en 6 (30%) no se encontró tumour en la pieza quirúrgica.
ConclusionesEl porcentaje de pacientes con respuesta patológica completa es similar al reportado en la literatura. Falta definir factores de buen pronóstico para conocer que pacientes se beneficiarían de una conducta de observación sin llegar a un tratamiento quirúrgico.
Colorectal cancer is the fourth most common cause of cancer and the second leading cause of death from cancer in the United States. It is estimated that 39,220 new cases occurred in 2016 (23,110 men and 16,110 women). In the same period, it is estimated that 49,190 people died from this disease. Despite these alarming figures, the incidence dropped from 60.5 per 100,000 people to 46.4 per 100,000 people between 1976 and 2005 (3% per year in 2003–2012).1
Since the early 2000 studies by Ralf Sauer, adjuvant therapy has shown a lower recurrence after surgery, compared with surgery without the therapy.2 Andrade et al. have also shown an increase in disease-free survival in patients treated with neoadjuvant therapy.3
Neoadjuvant therapy is indicated in stage II (T3–4, N0) or stage III (Nx) patients. Although patients with T3N0 and good prognostic factors can be operated on and subsequently receive chemotherapy, it has been shown that up to 22% of these patients with T3N0, staged with endorectal ultrasound or magnetic resonance imaging (MRI), have metastatic lymph nodes in the surgical specimen. Therefore, it is recommended that these patients be treated with neoadjuvant therapy.1 Total mesorectal excision (TME) still plays a fundamental role in patients who undergo surgery.4–6
Dr Haber-Gama from Brazil initiated the “watch and wait” protocol, i.e., monitoring and holding off on surgery, and waiting for the natural progression of patients with rectal cancer who received neoadjuvant therapy. In the initial data reported by this group, 67 (39%) of the 173 patients treated with radiation doses of 50.4–54Gy—with a concomitant 5-FU regimen—had a clinically evaluated complete pathological response. Of these patients, 9 (13%) underwent a surgical procedure, while 58 (87%) were closely monitored. Their mean follow-up was 65 months and the 5-year disease-free survival was 96%, compared with 72% of patients who did not have complete pathological response.7–9 These findings are very encouraging, similar to those found in the 1970s by Nigro, who used neoadjuvant therapy to cure epidermoid anal cancer without having to perform surgical resection.10
Although, information is constantly being reported internationally from studies with patients who have a complete pathological response (CPR) after neoadjuvant therapy, we have not found any report on this issue in Mexico. This study aims at reporting the percentage of patients with rectal adenocarcinoma who had a CPR after treatment with neoadjuvant chemotherapy and radiotherapy in a tertiary care hospital.
Material and methodsAll patients diagnosed with rectal adenocarcinoma, whose records were complete and who were treated at our hospital between January 2010 and December 2015, were included. For the analysis, patients who did not undergo surgical resection at our hospital, or those in whom follow-up was performed at another centre, were excluded.
Admission files included the following information: demographic data; histopathological characteristics of the tumour; neoadjuvant regimen; duration of neoadjuvant therapy; time of surgery after neoadjuvant therapy; type of surgery performed; histopathological report of the surgical specimen; recurrence during follow-up; and immediate postoperative death. For tumour height, the upper third of the rectum was considered when the neoplasm was 11–12cm away from the margins of the anus, the middle third of the rectum when it was 5–10cm away, and the lower third of the rectum when the distance was lesser than 5cm, measured by rigid proctosigmoidoscopy.
The data obtained were entered in a database using Excel 2013. Patients were divided into two groups: those who received neoadjuvant therapy and those who did not. Central tendency and dispersion measurements were calculated for the quantitative endpoints. For the qualitative endpoints, frequencies and percentages were calculated.
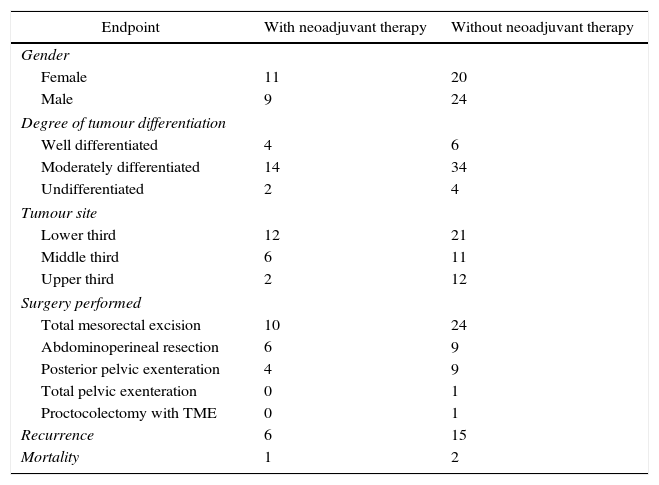
ResultsSixty-four patients were included, of whom 33 were men (51.5%) and 31 were women (48.4%). Patients had a mean age of 52.8 years (SD±11.57); patients with complete pathological response were 50 years old (±11.6), with a median of 49 years. In the group with no CPR, the average age was 53.1 years (±11.1), with a median of 51 years. 8. A total of five patients were younger than 40 years, of whom 2 received neoadjuvant therapy; the initial biopsy showed moderately differentiated adenocarcinoma, and no CPR was found in the surgical specimen after neoadjuvant therapy.
Most patients were staged as cT3 (37.5%) and cT4 (48.4%). Positive lymph nodes were found in 29 (45.3%) patients. 10.9% of patients were classified as stage I, 42.1% as stage II, 42.1% as stage III, and 4.6% as stage IV. Ten patients (41.6%) with cT3 had lymph nodes in the mesorectum, while 18 (75%) patients with cT4 had lymph nodes in the mesorectum.
Twenty (31.2%) of the 64 patients received neoadjuvant therapy at a dose of 37.5–50.4Gy in 25–28 sessions over an average period of 4 weeks. Chemotherapy regimens are shown in Table 1.
In 26.5% of the cases, the tumour was located in the middle third of the rectum and in 51.5% in the lower third of the rectum. Of the patients who received neoadjuvant therapy, 33.3% (2) of rectal tumours located in the middle third of the rectum and 3 (25%) located in the lower third of the rectum had a CPR. Three stage II patients and 2 stage III patients had a CPR (Table 2).
Patient characteristics.
| Endpoint | With neoadjuvant therapy | Without neoadjuvant therapy |
|---|---|---|
| Gender | ||
| Female | 11 | 20 |
| Male | 9 | 24 |
| Degree of tumour differentiation | ||
| Well differentiated | 4 | 6 |
| Moderately differentiated | 14 | 34 |
| Undifferentiated | 2 | 4 |
| Tumour site | ||
| Lower third | 12 | 21 |
| Middle third | 6 | 11 |
| Upper third | 2 | 12 |
| Surgery performed | ||
| Total mesorectal excision | 10 | 24 |
| Abdominoperineal resection | 6 | 9 |
| Posterior pelvic exenteration | 4 | 9 |
| Total pelvic exenteration | 0 | 1 |
| Proctocolectomy with TME | 0 | 1 |
| Recurrence | 6 | 15 |
| Mortality | 1 | 2 |
The surgical time between the end of neoadjuvant therapy and surgery with curative intent was 9 weeks (SD±22.1). 40% of patients (5) who had to wait more than 12 weeks had a CPR. Abdominoperineal resection (APR) was performed in 6 patients and TME in 10; of the APR patients, only 2 had a CPR, while 4 TME patients had a CPR.
CPR was observed in one patient with recurrence. Three (3) deaths were observed during follow-up: 1 in the group of patients with neoadjuvant therapy (local and distant recurrence) and 2 in the group of patients without neoadjuvant therapy (distant disease).
DiscussionThe first great unknown in patients who have received neoadjuvant therapy is when to perform surgery to obtain maximum benefits. Multiple studies establish different times.11 For example, the National Comprehensive Cancer Network (NCCN) guidelines recommend that surgery be performed within 5–12 weeks after the end of neoadjuvant therapy.1 The study by Zeng et al. compared neoadjuvant therapy/surgery intervals greater or less than 7 weeks, observing complete pathological response in 27.1% vs. 15.3% (p=0.029), respectively, decreased tumour penetrance in 65.6% vs. 50.5% (p=0.019), and decreased staging in 53.5% vs. 38.75% (p=0.026%), assuming that morbidity does not increase when performing a more complex surgery.12 Surgical complications from neoadjuvant therapy are minimised in the first few weeks, and inflammatory response is minimal within 4–8 weeks. However, according to other sources, it might be necessary to wait up to 10–12 weeks to test complete pathological response to treatment.6 A randomised multicentre study compared a 7-week vs. an 11-week wait to surgery. In the group undergoing surgery up to week 11, there was a higher rate of conversion of the laparoscopic procedure (8.6% vs. 9.5%, p=0.2638), with longer surgical times in the second group (291 vs. 305.8min, p=0.3577). A higher rate of morbidity (44.5% vs. 32%, p=0.04) and a higher rate of complications due to wound closure after APR (42.9% vs. 16.7%, p=0.216) were observed in the group undergoing surgery at 11 weeks. In this study no difference was found in complete pathological response rates.13 A population of 6397 patients was studied in a retrospective cohort of a national database in the United States. In periods shorter than 60 days after the end of radiotherapy, patients had a higher number of lymph nodes examined (10 vs. 9, p=0.0231), but a smaller rate of complete pathological response (6.6% vs. 8.2%, p=0.0414) and fewer positive surgical margins (4.8% vs. 6.7%, p=0.0091).14 Another study conducted on the same US national database found an optimal cut-off of 56 days between the end of chemotherapy and the surgical procedure, which decreased staging without increasing the risk of early readmission (OR 0.68, 95% CI 0.58–0.81, p<0.001) and with no difference in mortality. Regarding surgical margins, wait periods longer than 56 days increase the rate of circumferential margins (8.3% vs. 11.8%, p<0.001).15 The patients in our series were mostly operated on before week 10; therefore, according to the data analysed, it would be worth waiting longer to improve the CPR rate.
Another controversial issue is the change in surgical procedure after neoadjuvant therapy and complete clinical response. Current practice involves continuing with a specific surgical procedure for initial tumour signs; however, there are reports where post-neoadjuvant re-staging is considered possible by modifying the initial plan, even implementing the Watch and Wait protocol.16 After neoadjuvant therapy, it is expected that 50–60% of patients will have a decrease in staging, and about 20% of patients will have a complete pathological response.17
It should be noted that the tumour site with respect to the anal margin may be of importance when evaluating complete pathological response. Tumours in the lower and upper third of the rectum have been reported to be less likely to have a complete pathologic response.18 Another important prognostic parameter to obtain a complete pathological response is the degree of tumour differentiation. According to a retrospective, single-centre study of 53 patients who were treated with neoadjuvant therapy for well-differentiated or moderately differentiated tumours, these patient had lower rates of complete pathological response, compared with patients with poorly differentiated or undifferentiated tumours (75% vs. 15.3%, p=0.02).19 It has been proposed that the mucinous subtype could have a lower percentage of complete pathological response, since in a study of 111 patients, 25 of them had mucinous differentiation, none of whom achieved complete pathological response. On the other hand, their 5-year survival or disease-free survival rates were not lower, compared with patients with non-mucinous differentiation.20
The 5-year survival rate for patients with poor tumour response to neoadjuvant therapy is approximately 27%, which is much lower when compared to 72% of patients with 5-year survival with CPR. Patients are estimated to have a 5-year disease-free survival of 90.5%, 78.7% and 58.8% when they had a complete, partial and incomplete pathological response, respectively.1 In our series, recurrence was observed in 30% of the patients with neoadjuvant therapy, whereas in the group that did not receive neoadjuvant therapy, recurrence at follow-up was observed in 34% of the cases.
Postoperative chemotherapy is another paradigm in these patients. Routine practice is to initiate it as quickly as possible. A study by Patel et al. found that for every 4 weeks of delay in administering adjuvant therapy, there is a 14% decrease in overall survival. To date, it is unclear how long chemotherapy should last; it usually lasts 6 months with the FOLFOX regimen, with regimens under 4 months being justified when the patient has a complete pathological response to neoadjuvant therapy.21
The trickier part for deciding to only monitor a patient is how to identify those with a CPR. According to studies, only 25% of patients reporting a complete clinical response after surgery have a complete pathological response. This would lead to a higher surgery deferral rate, with a lower life expectancy.4 The evaluation of complete clinical response based on clinical and radiological criteria has a low sensitivity, since a complete pathological response often presents as an incomplete clinical response. Currently, better tools are needed to define which patients are likely to benefit from non-operative management after neoadjuvant therapy.22–24 Finding pathological response markers in clinical parameters has been the objective of several studies. A study by Zeng et al. published in 2015 found that fewer than 5 pre-treatment carcinoembryonic antigen (CEA) parameters (OR=2.17, 95% CI 1.195–3.939, p=0.011) and a time interval greater than 7 weeks between the end of neoadjuvant therapy and surgery (OR=2588, 95% CI 1.484–4.512, p=0.001) are independent parameters to predict complete pathologic response.25 Another retrospective study of 332 patients with stage II and III tumours in the middle and lower third of the rectum, who received neoadjuvant chemotherapy and radiotherapy, found pre-neoadjuvant CEA levels of 4.61 in the complete response group, compared with 10.49 in the incomplete response group. Regarding post-neoadjuvant parameters, in the complete pathological response group the levels were 1.4 vs 2.16 (p=0.014).26
In experienced hands, the follow-up protocol without surgical management could be an adequate strategy with close monitoring in well-selected patients. Establishing a Watch and wait protocol could mean an end to the surgical morbidity; however, long-term survival and local recurrence are not known.27 The patient should be the one deciding on whether to undergo this type of management after he/she has been told of its risks and benefits.5,28,29
ConclusionIn our population, treatment for rectal cancer with neoadjuvant regimens has complete pathological response rates similar to those reported in the literature. Currently, it is necessary to define good prognostic factors to know whether patients would benefit from monitoring without undergoing radical surgery after neoadjuvant therapy, as well as accurate diagnostic methods that help to screen patients with a complete clinical response so as to avoid delaying surgical resection.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.