The World Health Organisation indicates that the number of people with type 2 diabetes mellitus is more than 366 million and is expected to increase to 552 million by 2030, i.e., 8.3% of the total adult population. Each year, 14.9% of patients with this disease experience plantar ulcers, which in most cases are resistant to standard treatments. Of these, 15–20% require radical amputation within five years of diagnosis.
Material and methodsThis is a retrospective study reviewing the records of patients with type 2 diabetes mellitus and complications from lower limb injuries. We selected 220 records of patients with type 2 diabetes mellitus and lower limb injuries, undergoing hyperbaric oxygen therapy. We grouped wounds according to the Wagner Grading System and classified them according to the Skeik Modified Scale to determine the effect of hyperbaric oxygen therapy (HBOT) on the healing time of wounds. By way of comparison, the study was divided a second time, adjusting the initial population so as to include only cases that benefited from HBOT.
ResultsOverall improvement was achieved in 149 patients (67.7%), with a greater response in Wagner stages II and III. In patients who received 16–30 sessions, it was found that 77 (64.16%) maintained their improvement and only 43 (28.85%) had a relapse.
ConclusionsAt the end of the study, we found that HBOT is favourable and permanent in 52.72% of patients, seeing temporary improvement in 15%. This opens up the possibility of new developments that successfully determine suitable selective parameters and lead to a speedy recovery.
La Organización Mundial de la Salud, indica que el número de personas con diabetes mellitus tipo 2 es superior a 366 millones y se espera que para el año 2030, aumente a 552 millones de la población global adulta, correspondiente al 8.3%. Cada año, el 14.9% de pacientes con esta enfermedad, presentan úlceras plantares que en la mayoría de los casos son resistentes a tratamientos estándares, de estos, 15–20% requieren amputaciones radicales en los siguientes cinco años de ser diagnosticados.
Material y métodosSe trata de un estudio retrospectivo con revisión de expedientes de pacientes con diabetes mellitus tipo 2, complicados con heridas en extremidades inferiores. Seleccionamos 220 expedientes de pacientes con diabetes mellitus tipo 2, y lesiones en extremidades inferiores, sometidos a oxigenación hiperbárica. Agrupamos heridas por grado de Wagner clasificándolas por escala Skeik modificada, para determinar el efecto del oxígeno hiperbárico (OHB) sobre el tiempo de curación de estas. Para fines comparativos se divide el estudio en un segundo tiempo, ajustándose la población inicial, solo con casos beneficiados.
ResultadosSe obtuvo mejoría general en 149 pacientes (67.7%), presentando mayor respuesta en estadios II y III de Wagner. En quienes recibieron de 16 a 30 sesiones, se comprueba que 77 (64.16%) mantuvieron su mejoría alcanzada y solo 43 (28.85%) presentó recaída.
ConclusionesAl término del estudio, establecemos que la OHB, es favourable y permanente en el 52.72%, identificándose un 15% con beneficio temporal, dejando posibilidades de nuevos desarrollos para precisar parámetros selectivos con idoneidad exitosa, e impacto en su pronta recuperación.
Type 2 diabetes mellitus (T2DM) is considered a global public health epidemic.1 Its effect on Mexico's National Health System also represents a health emergency that significantly affects work productivity, school performance and economic development.2 The World Health Organisation (WHO) estimates that the number of people with T2DM in the global adult population is more than 366 million, estimating that by 2030 the global population will increase to 552 million adults, accounting for 8.3%.3 Moreover, according to the International Diabetes Federation, China, India, the United States, Brazil, Russia and Mexico are the countries with the greatest number of diabetics.4 Data from the National Health and Nutrition Examination Survey 2012 (NHANES 2012) identify 6.4 million Mexican adults with diabetes, i.e., 9.2% of adults in Mexico have already been diagnosed with diabetes.5 The cost of caring for diabetics in Mexico varies greatly, with care costs per patient ranging from 700 to 3200 dollars per year.6 Between 4.7% and 6.5% of the healthcare budget of the Mexican Social Security Institute (IMSS) is allocated to T2DM.7 It has been estimated that the life expectancy of individuals with diabetes decreases by 5–10 years, and in Mexico, the average age of people who died from diabetes in 2010 was 66.7 years, i.e., a decrease of 10 years.8
Due to its early asymptomatic course, T2DM combined with late diagnosis and poor management leads to serious complications such as premature disability, myocardial infarction, blindness, kidney failure, venous insufficiency, lower limb injuries and amputation, and premature death.9,10
Each year, 14.9% of patients have plantar ulcers, which in most cases are resistant to standard treatments. Of these, 15–20% require radical amputation within five years of diagnosis.11
Currently, these lesions are considered to be a severe chronic clinical syndrome of T2DM, 85% of which are caused by multiple factors, including the presence of motor-sensory neuropathy, angiopathy, oedema and impaired immunity, leading to infection, ulceration and gangrene of the lower limbs and requiring prolonged hospitalisation and debilitating amputations.12
Patients with these complications have a 15–40 times higher risk of amputation than non-diabetics, with men more prone to amputation than women. Lozano12 estimates that the total annual incidence is 2–3%, while the incidence is 7% in patients with neuropathy with a prevalence of 2–10%.13
International consensus documents have reported that after lower limb amputation, the incidence of a new ulcer and/or contralateral limb amputation at 2–5 years is 50%, while survival after radical surgery is 50% and 40% at 3 and 5 years, respectively.14
The costs of this complication are high. In the US various articles show an annual cost of these injuries that exceeds 4 billion dollars a year.15
Therapeutic alternatives such as hyperbaric oxygen therapy (HBOT) are currently available to prevent, treat and, at best, reduce the complications of this disease. Clinical studies show that the mechanism of action of HBOT contributes to their improvement, reversing the hypoxaemic state in the tissues, sometimes reaching up to 300–400mmHg.16 HBOT also modulates the mitochondrial oxidative stress in the signalling pathway of endothelial growth factors and17 increases the vascular-tissue concentration differential. In addition, HBOT has been reported to increase the expression of growth factors (and their receptors) that are essential for the development of angiogenesis.18
Based on the frequency of these lesions and the benefits of HBOT described in the literature, we decided to conduct our study to: Determine the effect of the number of HBOT sessions on the improvement of these lesions based on the Skeik scale, taking into account Wagner's classification, and the incidence of amputation of the affected limb after using HBOT in the same patients.
Material and methodsThis is a retrospective study reviewing the records of patients with T2DM and complications from lower limb injuries.
The patients underwent sessions at a pressure of 2.3–2.5 absolute atmospheres (ATA) for 60 or 90min in a multiplace hyperbaric chamber (MISSA, M800 2008 model, with serial number MIS 34-07).
For the analysis, patients who did not initiate HBOT, were evaluated and did not start treatment excluded. Patient records were grouped according to the Wagner classification.22 The Skeik classification23 was used to assess treatment outcomes and was adapted based on the following criteria: (a) completely healed, with full closure of the wound; (b) significantly healed, with more than 50% improvement; (c) partially healed, with less than 50% improvement; and (d) failure to treat (no significant changes in the wound) based on clinical impression. The WHO Body Mass Index (BMI) classification was used, while vascular obstruction was reported according to the attending physician's subjective assessment. For comparative purposes, the study was divided a second time, adjusting the initial population so as to include only cases that benefited from HBOT. In these cases, the Social Work Service conducted a telephone survey to learn the outcome of the patient and his/her wound, starting from the last day of HBOT until the date of the survey, by asking four questions: (a) was the patient still alive?; (b) was the affected limb saved from amputation?; (c) did the patient undergo another treatment?; and (d) did relapse occur? A response was obtained from 184 patients. Data were processed with the SPSS statistical software (version 15.0.1, 2001), using data analysis (contingency tables, Pearson correlation coefficient and chi-squared test) to compare the results obtained.
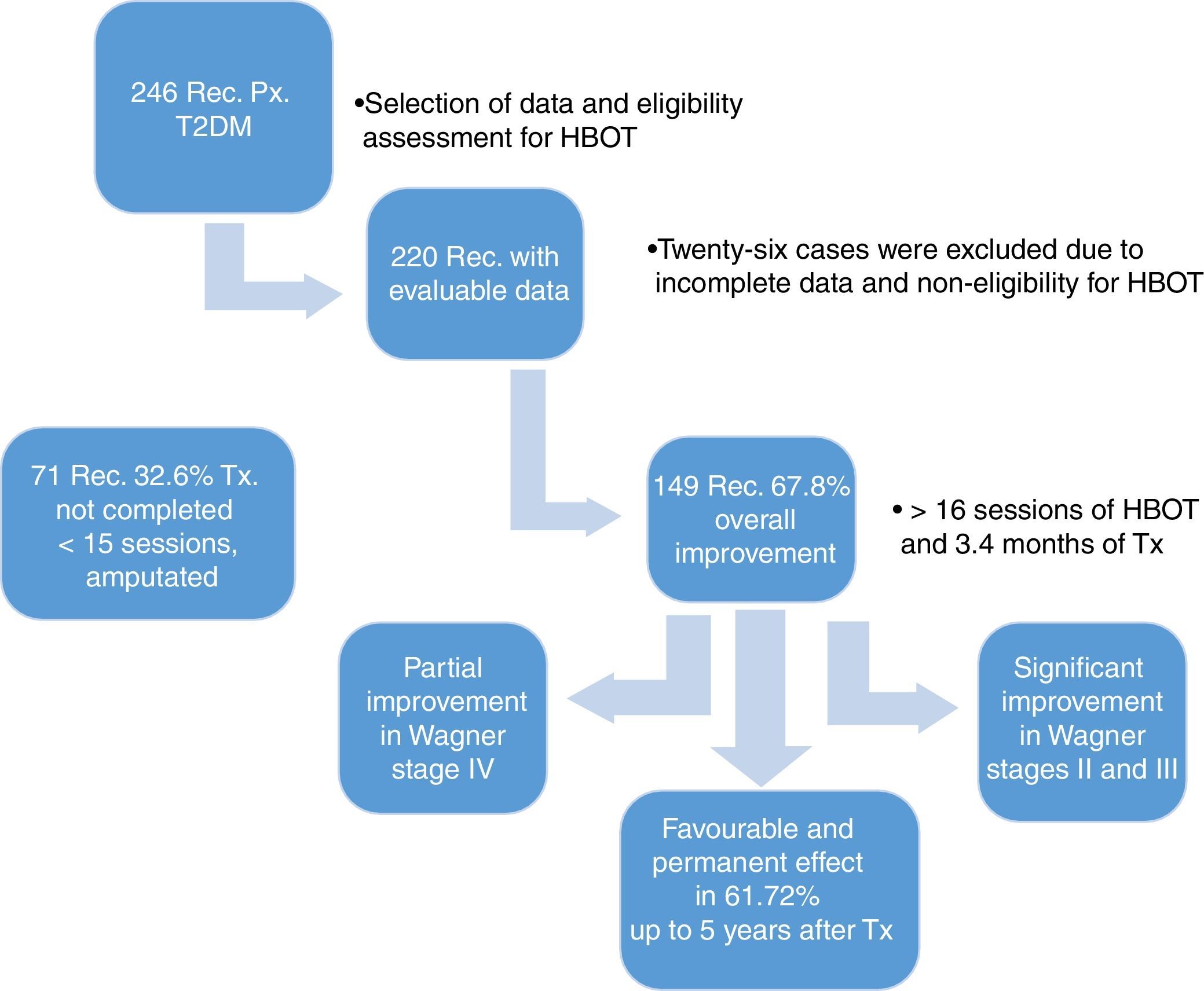
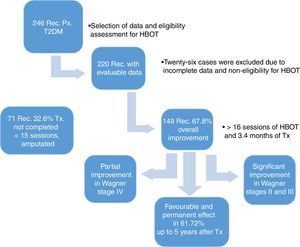
ResultsWe reviewed 246 records of patients diagnosed with T2DM and late manifestations of the disease, such as necrobiosis lipoidica of soft tissue, stump dehiscence, plantar ulcers and fasciotomies that heal with difficultly, treated between 2009 and 2015. We excluded 26 records of patients whose study-related information was incomplete, leaving us with 220 evaluable records. All 220 patients underwent a comprehensive assessment that included a chest X-ray, taking into account lung pattern, ear examination for tympanic membrane integrity, general metabolic status and characteristics of the lesions to determine HBOT eligibility.
The demographic characteristics of the general sample of patients are shown in Table 1. During treatment, the affected limb was amputated in 11 patients (5%), due to complications of the disease, without a response to HBOT. Of the patients who showed overall improvement, 149 (67.7%) were divided according to the Skeik scale: 79 (35.9%) healed completely; 62 (28.2%) improved significantly; 8 (3.6%) improved partially; and 71 (32.3%) did not heal (Fig. 1).
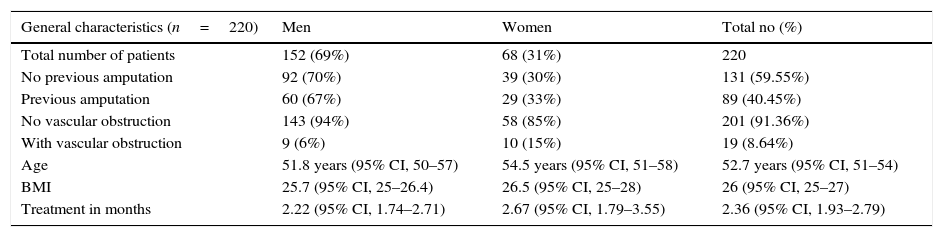
Demographic data of patients, 2009–2015.
| General characteristics (n=220) | Men | Women | Total no (%) |
|---|---|---|---|
| Total number of patients | 152 (69%) | 68 (31%) | 220 |
| No previous amputation | 92 (70%) | 39 (30%) | 131 (59.55%) |
| Previous amputation | 60 (67%) | 29 (33%) | 89 (40.45%) |
| No vascular obstruction | 143 (94%) | 58 (85%) | 201 (91.36%) |
| With vascular obstruction | 9 (6%) | 10 (15%) | 19 (8.64%) |
| Age | 51.8 years (95% CI, 50–57) | 54.5 years (95% CI, 51–58) | 52.7 years (95% CI, 51–54) |
| BMI | 25.7 (95% CI, 25–26.4) | 26.5 (95% CI, 25–28) | 26 (95% CI, 25–27) |
| Treatment in months | 2.22 (95% CI, 1.74–2.71) | 2.67 (95% CI, 1.79–3.55) | 2.36 (95% CI, 1.93–2.79) |
Data, patient records – Hyperbaric Chamber Department.
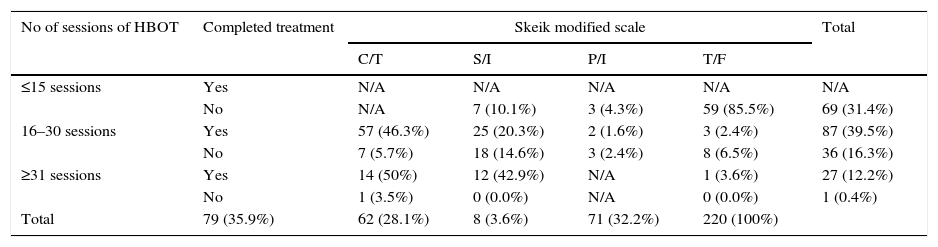
The distribution based on gender was as follows: for women, 28 (12.7%) healed completely; 14 (6.4%) improved significantly; 4 (1.8) improved partially; and 22 (10%) did not heal. For men, 51 (23.2%) healed completely; 48 (21.8%) improved significantly; 4 (1.8%) improved partially; and 49 (22.3%) did not heal. It was observed that the time (in months) for obtaining results based on the Skeik scale was as follows: complete closure within 3.47 months; significant improvement within 2.75 months; partial improvement within 1.20 months; and no healing within 1.0 month. Response to number of treatment sessions completed is described in Table 2 (p=0.000).
Distribution of patients based on the number of sessions, treatment completion and Skeik modified scale.
| No of sessions of HBOT | Completed treatment | Skeik modified scale | Total | |||
|---|---|---|---|---|---|---|
| C/T | S/I | P/I | T/F | |||
| ≤15 sessions | Yes | N/A | N/A | N/A | N/A | N/A |
| No | N/A | 7 (10.1%) | 3 (4.3%) | 59 (85.5%) | 69 (31.4%) | |
| 16–30 sessions | Yes | 57 (46.3%) | 25 (20.3%) | 2 (1.6%) | 3 (2.4%) | 87 (39.5%) |
| No | 7 (5.7%) | 18 (14.6%) | 3 (2.4%) | 8 (6.5%) | 36 (16.3%) | |
| ≥31 sessions | Yes | 14 (50%) | 12 (42.9%) | N/A | 1 (3.6%) | 27 (12.2%) |
| No | 1 (3.5%) | 0 (0.0%) | N/A | 0 (0.0%) | 1 (0.4%) | |
| Total | 79 (35.9%) | 62 (28.1%) | 8 (3.6%) | 71 (32.2%) | 220 (100%) | |
p=0.000 C/T=completed treatment, S/I=significant improvement, P/I=partial improvement, T/F=treatment failure.
Taking into account the BMI of patients, according to the WHO guidelines and the behaviour of the lesion based on the Skeik scale, it was observed that: 7 (3.2%) of the patients were malnourished and only 4 (1.8%) had healed completely; 85 (38.6%) had a normal weight, of whom 35 (15.9%) had healed completely; 91 (43.2%) were overweight, of whom 28 (12.75) had healed completely; 12 (15%) were obese, of whom 12 (5.45) had healed completely.
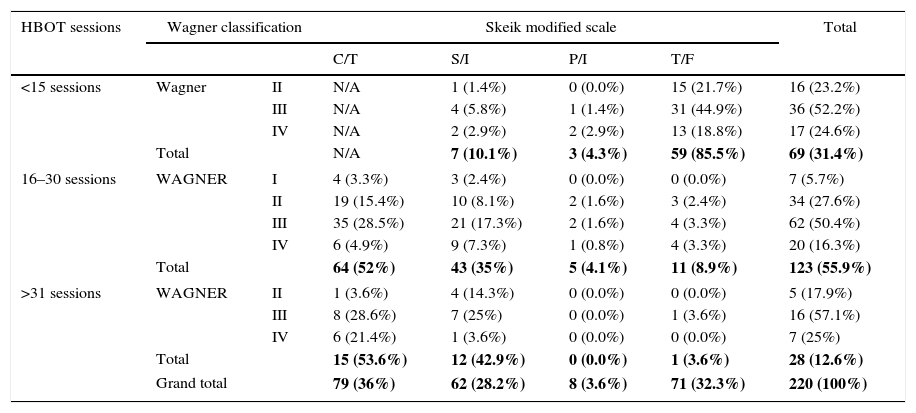
Comparison of the number of HBOT sessions vs. the Wagner classification and state of lesion according to the Skeik scale.
| HBOT sessions | Wagner classification | Skeik modified scale | Total | ||||
|---|---|---|---|---|---|---|---|
| C/T | S/I | P/I | T/F | ||||
| <15 sessions | Wagner | II | N/A | 1 (1.4%) | 0 (0.0%) | 15 (21.7%) | 16 (23.2%) |
| III | N/A | 4 (5.8%) | 1 (1.4%) | 31 (44.9%) | 36 (52.2%) | ||
| IV | N/A | 2 (2.9%) | 2 (2.9%) | 13 (18.8%) | 17 (24.6%) | ||
| Total | N/A | 7 (10.1%) | 3 (4.3%) | 59 (85.5%) | 69 (31.4%) | ||
| 16–30 sessions | WAGNER | I | 4 (3.3%) | 3 (2.4%) | 0 (0.0%) | 0 (0.0%) | 7 (5.7%) |
| II | 19 (15.4%) | 10 (8.1%) | 2 (1.6%) | 3 (2.4%) | 34 (27.6%) | ||
| III | 35 (28.5%) | 21 (17.3%) | 2 (1.6%) | 4 (3.3%) | 62 (50.4%) | ||
| IV | 6 (4.9%) | 9 (7.3%) | 1 (0.8%) | 4 (3.3%) | 20 (16.3%) | ||
| Total | 64 (52%) | 43 (35%) | 5 (4.1%) | 11 (8.9%) | 123 (55.9%) | ||
| >31 sessions | WAGNER | II | 1 (3.6%) | 4 (14.3%) | 0 (0.0%) | 0 (0.0%) | 5 (17.9%) |
| III | 8 (28.6%) | 7 (25%) | 0 (0.0%) | 1 (3.6%) | 16 (57.1%) | ||
| IV | 6 (21.4%) | 1 (3.6%) | 0 (0.0%) | 0 (0.0%) | 7 (25%) | ||
| Total | 15 (53.6%) | 12 (42.9%) | 0 (0.0%) | 1 (3.6%) | 28 (12.6%) | ||
| Grand total | 79 (36%) | 62 (28.2%) | 8 (3.6%) | 71 (32.3%) | 220 (100%) | ||
p=0.000. H/C=healed completely, S/I=significant improvement, P/I=partial improvement, T/F=treatment failure.
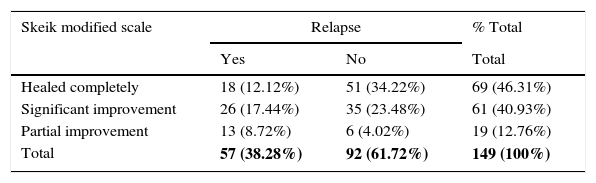
Patients who had a relapse of the lesion in the second part of the study, i.e., up to 6 years after completing their treatment, are shown in Table 4 (p=0.000). Table 5 Shows patients in the second part, with behaviour of the lesion according to the Sheik scale and the affected limb being saved from amputation after treatment (p=0.000).
Cases in the second part of the study where a survey was conducted and relapse of the lesion occurred based on the Skeik scale.
| Skeik modified scale | Relapse | % Total | |
|---|---|---|---|
| Yes | No | Total | |
| Healed completely | 18 (12.12%) | 51 (34.22%) | 69 (46.31%) |
| Significant improvement | 26 (17.44%) | 35 (23.48%) | 61 (40.93%) |
| Partial improvement | 13 (8.72%) | 6 (4.02%) | 19 (12.76%) |
| Total | 57 (38.28%) | 92 (61.72%) | 149 (100%) |
p=0.000.
Cases in the second part of the study where the affected limb was saved from amputation according to the Skeik scale.
| Skeik modified scale | Healed completely | Significant improvement | Partial improvement | Total |
|---|---|---|---|---|
| Lost limb | 3 (2.01%) | 1 (0.70%) | 3 (2.01%) | 7 (4.69%) |
| Limb saved | 65 (43.62%) | 60 (40.26%) | 17 (11.40%) | 142 (95.30%) |
| Total | 68 (45.63%) | 61 (40.96%) | 20 (13.41%) | 149 (100.0%) |
p=0.000.
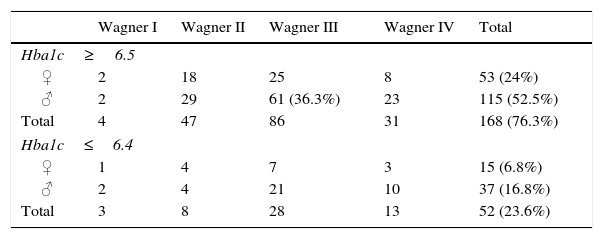
In the studied group, disease control was generally considered to be poor: 73.6% of the patients had a glycosylated haemoglobin (HbA1c)>6.5; 61 male patients in Wagner stage III had the worst treatment adherence; and only 23.6% of the patients had adequate treatment adherence (with HbA1c<6.4), as shown in Table 6.
DiscussionIn a study of 20 patients with complicated ulcers, the initial measurement of transcutaneous partial pressure of oxygen (TcPO2) was less than 10mmHg in ambient air around the lesion.18 After administering HBOT at 2.5 absolute atmospheres (ATA), if TcPO2 was less than 50mmHg, the lesion would consistently fail to heal. On the other hand, in patients whose TcPO2 increased to more than 100mmHg, success rates were higher than in the control group.18 HBOT never replaces surgical debridement since its effect would be null, because if there is no “surgery-related bacterial discharge”, oxygen will continue to be used by bacteria through direct competition.19
In 2005, Hopf et al.,20 evaluated angiogenesis and HBOT in a marine model, as well as vascular growth, with significant results due to hyperoxia. They also showed reduced tissue lactate and necrosis levels, inhibited growth of anaerobic bacteria, increased bactericidal activity of phagocytes and healing.20 Studies such as the one by Cianci21 have shown that in deep ulcers there is a lower incidence of amputation in patients receiving more than 30 HBOT sessions.21
In the first part of our study, of the 71 (32.2%) patients who did not heal, 69 (31.4%) did not complete the treatment, undergoing fewer than 15 HBOT sessions due to lack of treatment adherence. When comparing the Skeik modified scale and the Wagner classification, we can see that 149 (67.8%) patients had overall lesion improvement, with significant improvement observed in Wagner stages II and III and partial improvement in Wagner stage IV (Table 3, p=0.000). Results that can be contrasted with the collaboration group Cochrane Library has published two meta-analyzes on the use of hyperbaric chamber for the treatment of wounds. The first included 9 studies on chronic wounds, 8 of them in diabetic foot, accumulating a number of 471 patients. They concluded that the relative risk of wound healing was 5.20 being statistically significant in the short term. There were no significant differences in the long term nor in amputations.31
Cianci21 also achieved successful results (without amputation) in 78–89% of Wagner stage IV lesions treated with HBOT, demonstrating that the cost of HBOT is lower than that of amputation.22
No significant difference was found in patients with vascular obstruction, for which there are no reports on grade or surgical treatment, who underwent HBOT, observing good results in healing time in both cases. This was also the case with the study by Contreras-Méndez,24 which determined that the inflammatory process is regulated through the expression of the KLF2 protein based on the HBOT sessions, using Western blotting and immunohistochemistry.24 Similarly, the study by Michael CD17 showed that HBOT increases the concentration differential of vascular growth-tissue (vascular endothelial growth), as well as the expression of growth factors and their receptors.17
Regarding the total number of sessions undergone by all case groups, a greater response was observed in those who received 16–30 sessions (p=0.000), considering that treatment was incomplete in those who were not healed immediately after completing HBOT due to various external or patient-related reasons, since before and after these the response is very slow or null. In addition, Kranke's25 randomised trials showed that HBOT appeared to improve the healing of diabetes-related foot ulcers in less than 6 weeks when administered up to twice daily.25 Similarly, Kalani's26 prospective, controlled, long-term (3 years) randomised study, which compared two homogeneous groups with and without HBOT treatment, showed a lower rate of amputation (33% vs. 12%) and a short healing time.26 Allen's27 prospective study, with no control group, which included 85 patients with diabetic foot and risk of amputation, achieved good results in 78% of the cases (healing in 65% and improvement in 13%).27
In the second part of the study, 65.2% of patients had overall improvement, based on the Skeik scale, due to the reduced sample. Lesion relapse was associated with the following: patients did not heal in the first part of the study; they did not adhere to the hyperbaric protocol; they used other treatments; and/or causes specific to the underlying disease. Validating what is described by the diabetic foot care guidelines of the Infectious Diseases Society of America (IDSA), which rates HBOT as B-I level of evidence “moderate evidence based on more than one randomised, controlled trial, recommending its use”.28 The Canadian Agency for Drugs and Technologies in Health reported that HBOT together with treatment for diabetic foot ulcers was more effective, healing wounds and reducing the incidence of amputations (11% in the HBOT group vs. 32% in the standard care group).29 The Cochrane Library shows current evidence on the effectiveness of HBOT in improving the healing of diabetic leg ulcers in patients with concomitant ischaemia. Larger, higher-quality trials are needed before using HBOT in routine clinical practice in patients with diabetic foot ulcers.30
ConclusionsHBOT is an adjuvant to standard treatment for these lesions, without replacing and/or delaying surgical debridement; good results are obtained, provided this therapeutic tool is available, thus contributing to decreased amputation rates and cutting the cost of standard treatment, hospitalisation, drugs and use of operating theatres. The results obtained in the second part of the study show that HBOT had a favourable and permanent effect in 61.72% of patients, identifying a 6.08% (difference between the improvement obtained in the first and second parts of the study) with temporary improvement. This opens up the possibility of developing new research that will permit us to know precisely in which other areas of medicine HBOT could be applied as an effective adjuvant therapy, focusing on parameters such as:
This will allow for adapting HBOT, with a high degree of specificity, to the clinical needs of the Mexican population served at this hospital, thus tailoring screening parameters to achieve successful patient eligibility in the use of this type of therapy. This will lead to early recovery for patients, who can return to their daily activities in the shortest time possible. This is also what Bishop31 suggests, i.e., that HBOT may be a useful adjunct in the treatment of diabetic patients with lower limb ulcers, emphasising that TcPO2 measurements would likely offer greater benefit to patients with this type of therapy.31
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflicts of interest.
We would like to thank all the patients included in this study who, by placing their trust and health in our hands by undergoing HBOT, helped us to obtain results that we constantly strive to improve. We would also like to thank the hospital management for supporting this study.