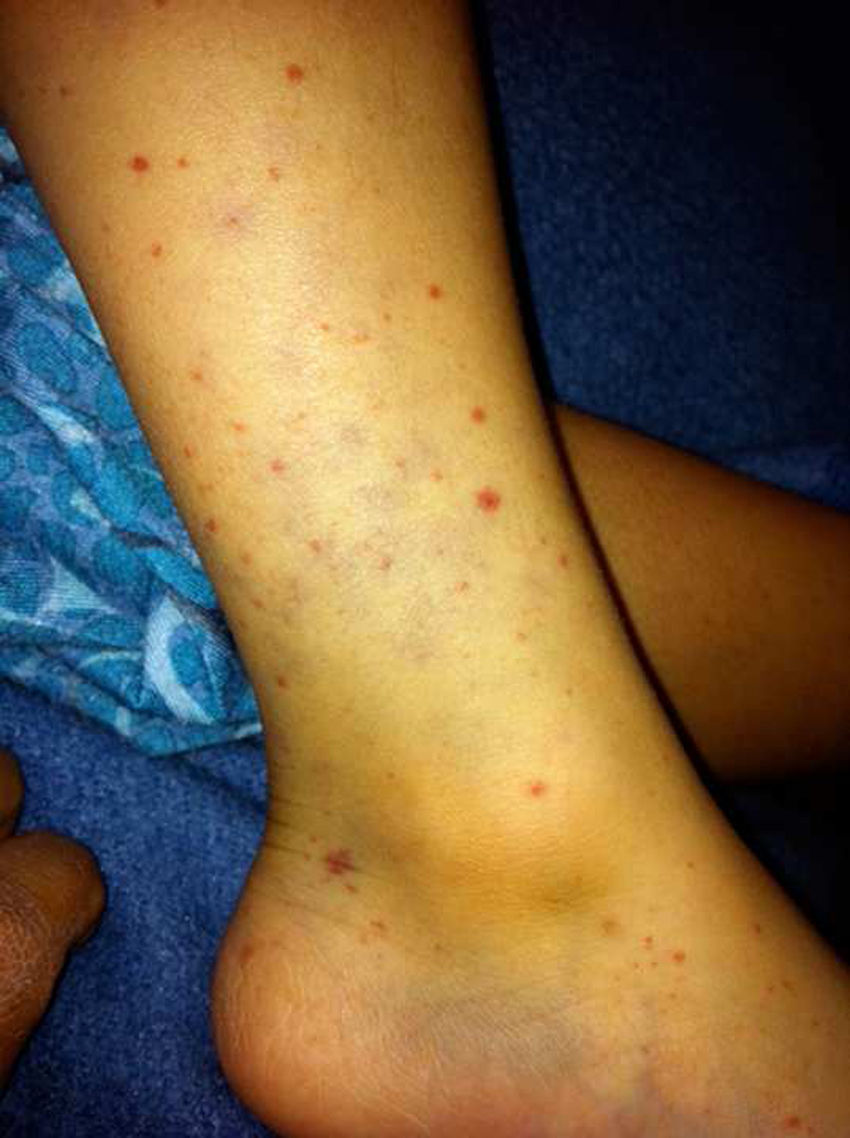
In immunocompetent patients, human cytomegalovirus (CMV) infections are generally asymptomatic. Infection by this virus takes on particular importance during pregnancy owing to a risk of congenital infection occurring as a result of fetal transmission. In this article, we report the case of two siblings with this infection. The younger sibling had cystic microphthalmia, which was believed to be secondary to prenatal CMV infection. Given her status as an infectious carrier, she infected her older brother, who had signs and symptoms similar to those of infectious mononucleosis. CMV infection has a wide clinical spectrum, and prenatal CMV infection must be considered in patients within the differential diagnosis of eye malformations.
En el paciente inmunocompetente las infecciones por el citomegalovirus (CMV) humano generalmente cursan asintomática, la infección por este agente adquiere especial relevancia durante la gestación debido al riesgo infección congénita producto de la transmisión fetal. En este reporte se presentan dos hermanos, el menor con microftalmia quística, la cual se adjudica a la infección prenatal por CMV, y por su estado de portador infecto a su hermano mayor, el cual presento un cuadro similar a mononucleosis infecciosa. La infección por CMV presenta un amplio espectro clínico, se debe considerar en pacientes la transmisión prenatal de CMV dentro del diagnóstico diferencial de malformaciones oculares.
Human cytomegalovirus (CMV) is an icosahedral virus belonging to the sub-family Betaherpesvirinae. Generally, infection with this virus is asymptomatic in immunocompetent subjects.1–3 Clinical manifestations, if any, are usually similar to those of infectious mononucleosis syndrome.4–8
CMV infection takes on particular importance during pregnancy owing to a risk of congenital infection, which generally occurs in the first or second trimester of pregnancy. Although congenital CMV infection is one of the most common infections worldwide, it has been proposed that in-utero transmission is low and only causes clinical manifestations in 1%–2% of infected fetuses, adverse effects may consist of fetal malformations and even fetal death,7,9,10 eye sequelae attributed to congenital CMV infection are uncommon7,11 and the clinical spectrum of eye malformations depends on the gestational age at which the infection occurs in the fetus.12–14 Although the vast majority of infected fetuses will be found to be asymptomatic at the time of birth, they may eventually develop at least one related complication. Therefore, CMV infection currently represents one of the most significant causes of congenital infection worldwide.3,7,8,10
The objective of this article is to report the case of two siblings who had different clinical manifestations after being infected by CMV.
Case report 1A healthy 5-year-old boy. He had clear nasal discharge, a dry cough and a persistent fever of 38.5°C, regardless of activities, for three days despite being administered antipyretic drugs; subsequently he had petechiae and purpura on the lower limbs (Fig. 1), arthralgia in the knees and ankles, and bilateral conjunctival hyperaemia. A physical examination revealed a pharynx with hyperaemia, grade 4 tonsillar hypertrophy and crypts covered with a whitish exudate, as well as cervical lymphadenopathies in front of and behind the ears that were in excess of 2cm, non-tender and soft in consistency. The abdomen was palpated: splenic pole, liver at 1.5cm below the right costal margin, non-tender. Laboratory studies showed 12,000 leukocytes at the expense of 3000 (40%) neutrophils and 24,500 (49%) lymphocytes, hemoglobin 14.3, haematocrit 42.2, platelets 155,000, prothrombin time 13.20, partial thromboplastin time 41.5 and thrombin time 23.1, with no other abnormalities. Anti-CMV immunoglobulins were reported as an IgG level of 46.2, an IgM level of 3.42 (reference 06.0) and a CMV viral load greater than 14,000 copies. Treatment was started with valganciclovir at a dose of 12mg/kg/day for six weeks, and gradual clinical improvement was observed. Once his treatment had been completed, a new viral load was obtained and found to be negative.
Case report 2A 9-month-old girl, the boy's younger sister, with congenital microphthalmia in her right eye. Given her older brother's condition, although she was asymptomatic, she underwent anti-CMV immunoglobulin determinations that reported an IgG level of 56, an IgM level of 6.1 and a CMV viral load of 4500 copies. Like her older brother, the patient received treatment with valganciclovir 12mg/kg/day for 6 weeks. She was referred to the ophthalmology department, where it was decided to perform an eye excision. The final histopathology report for the excision was cystic microphthalmia.
DiscussionCMV excretion through saliva and urine has been observed to occur for months, or even occur episodically or persist for years after the primary infection.4 In neonatal infections, the virus may continue to be excreted for at least five or six years. In adults, viral excretion occurs for shorter periods; however, the virus will always be found to be latent.3,4 It has been established that less than 3% of adults are pharyngeal excreters; even so, excretion tends to recur in situations of immunosuppression and during pregnancy.7,10
CMV may infect any organ or tissue2; despite this, the most common clinical manifestations are neurological.7 Although eye abnormalities due to CMV are uncommon,7,11 this virus has the potential to cause a wide range of eye malformations, including, but not limited to anophthalmia, synophthalmia and cystic microphthalmia.12–14 Recently, Llorente-González et al. estimated that the prevalence of anophthalmia and microphthalmia is 0.2 to 3 per 10,000 births13; however, to date, microphthalmia has been associated with CMV in only a few publications.11,15
We believe that congenital CMV infection was responsible for the younger sister's ophthalmic malformation. We also believe that her chronic carrier status was the source of contagion to her older brother. This belief is based on a study by Pass et al.,16 who found that children under 2 years of age are most often able to excrete the virus, and may even account for 80% of cases. This is supported by research studies conducted by Joseph et al.17 It is also possible that the patients acquired the infection at school. This theory is backed by a study published by Staras et al.,18 who reported a prevalence of CMV in the United States in children between the ages of 6 and 11 of 36.3%. It should be noted that the same study found a prevalence in children from a Mexican background of up to 54.7%. Given this, it may be that the older brother was infected elsewhere such as at school, although we believe this to be less likely.
Currently, the gold standard for diagnosing congenital CMV infection is determining viral DNA through a polymerase chain reaction (PCR) using saliva samples, since this has shown greater specificity and sensitivity than performing a urine viral culture.8–10
CMV infection is generally asymptomatic and self-limiting. Therefore, in many cases, the only evidence of the infection is the presence of specific antibodies. However, in some cases, it may cause significant clinical manifestations in immunocompromised patients, newborns and pregnant women.19 Although there are antiviral drugs against CMV disease, their use is generally not recommended during pregnancy, owing to the risk of causing teratogenic effects.8 In newborns, there are treatment regimens using intravenous ganciclovir (6mg/kg/dose every 12h) and/or oral valganciclovir (16mg/kg/dose every 12h) for at least 6 weeks, and sometimes up to 6 months.1,3,15 Using foscarnet is also an option, but there has been little experience with this drug and it carries a high risk of nephrotoxicity.15 In light of the above, the clinical improvement described in this report cannot be attributed in full to the use of antiviral drugs, given that CMV infection tends to be self-limiting in non-immunocompromised patients. Other treatment strategies are currently being developed. Among these, anti-CMV vaccines8,16 and anti-CMV immunoglobulin G use8 have had promising preliminary outcomes.
ConclusionCMV infection has a wide clinical spectrum, and we believe that the younger sister's congenital CMV infection was responsible for her ophthalmic malformation. We also believe that her chronic carrier status was the source of contagion to her older brother. Given this, we believe that it is essential that all physicians be aware of the clinical spectrum of congenital CMV infection, and deliberately look for the presence of CMV in individuals born with microphthalmia.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThis article meets the requirements on informed consent and the authors report no conflicts of interest.