Non-invasive procedures for prenatal diagnosis are the most frequently used methods in the first trimester of pregnancy in pregnant women under 35 years of age because they do not involve risk of pregnancy loss; however, they are not considered to be a definitive diagnosis method. During the first trimester the duo test (PAPP-A and free βhGC), cell-free fetal DNA in maternal blood and structural ultrasound scans are the principal tools used; the quadruple marker test (αFP, E3, β-hCG, inhibin A) is used in the second trimester. However, the definitive diagnosis is performed by cytogenetic analysis through amniocentesis.
MethodsThirty women, under 35 years of age with high-risk pregnancy, were studied with duo test and structural ultrasound in the first trimester and amniocentesis in weeks 15–18.
ResultsOnly five duo tests were positive: three showed risk of trisomy 18 and one of Turner syndrome, they all corroborated with the cytogenetic study; the fifth showed a risk of Down's syndrome, however it was a chromosomally normal product. Three patients with a negative duo test cytogenetically detected with karyotypes with structural abnormalities, which were: deletion of the short arm of chromosome 18 [46,XY, del(18)(p11)], Robertsonian translocation between chromosome 13 and 14 [45,XY, rob(13:14)] and a chromosome derived from X [46,X, der(X)]. The duo test is a very useful tool for the diagnosis of numerical chromosome abnormalities, but not for detecting structural chromosome aberrations. However, it is essential to perform amniocentesis to definitively rule out chromosomal aberrations in products of conception.
Los procedimientos de diagnóstico prenatal no invasivos están siendo los métodos más usados en el primer trimestre del embarazo en mujeres embarazadas menores de 35 años, ya que no implican riesgo de pérdida gestacional; sin embargo, no se consideran un método diagnóstico definitivo. Durante el primer trimestre de gestación se utiliza el dúo test (PAPP-A y βhGC libre), DNA fetal libre de células en sangre materna y la ultrasonografía estructural; durante el segundo trimestre se usa el cuádruple marcador (αFP, E3, β hGCH, Inhibina A). Sin embargo, el diagnóstico definitivo se lleva a cabo mediante el análisis citogenético a través de la amniocentesis.
MétodosSe estudiaron 30 mujeres menores de 35 años con embarazo de alto riesgo a las cuales se les realizó ultrasonido estructural y dúo test en el primer trimestre del embarazo y amniocentesis en la semana 15-18.
ResultadosÚnicamente cinco dúo test fueron positivos, de los cuales tres mostraron riesgo de trisomía 18 y uno para síndrome de Turner, todos corroborados con estudio citogenético; el quinto mostró riesgo de síndrome de Down, sin embargo fue un producto cromosómicamente normal. En tres pacientes con dúo test negativo, se detectaron citogenéticamente cariotipos con anomalías estructurales, las cuales fueron: pérdida parcial del brazo corto del cromosoma 18 [46,XY,del(18)(p11)], translocación robertsoniana entre el cromosoma 13 y 14 [45,XY,rob(13:14)] y un cromosoma derivado del X[46,X,der(X)]. El dúo test representa una herramienta muy útil para el diagnóstico de alteraciones cromosómicas numéricas, no así para detectar aberraciones estructurales de los cromosomas, sin embargo es imprescindible la realización de la amniocentesis para descartar definitivamente aberraciones cromosómicas del producto en gestación.
Prenatal diagnosis, using invasive procedures such as amniocentesis and chorionic villus sampling, in women with a high risk of having babies with chromosomal and structural abnormalities1 is a very useful tool which is widely used nowadays. However, these invasive procedures involve a significant risk of miscarriage (1 in 200).2 For this reason, these procedures are restricted only to pregnant women who are over 35 years of age or younger women with biochemical markers or positive ultrasound tests for chromosomopathy. Currently, non-invasive procedures are considered to be a very useful tool for making decisions with regard to the continuity of pregnancies with genetic diseases.3 These studies have become very relevant in recent years, although they are not considered to be diagnostic procedures, but only screening procedures. The triple marker test, which determines the beta fraction of human chorionic gonadotropin (βhCG), unconjugated estriol (E3) and alpha-fetoprotein (αFP), was one of the first biochemical markers used in screening for chromosomal abnormalities in the product of conception.4 At a later date, the determination of inhibin A was added, and it was then referred to as the quadruple marker test. The quadruple marker test is performed between weeks 15 and 20 of pregnancy. Subsequently, the duo test was established. This is performed in the first trimester of pregnancy (11–13.6 weeks). It determines the multiples of the median of the pregnancy-associated plasma protein (PAPP-A) and free βhGC. The duo test is complemented by the fetal ultrasound which measures nuchal translucency and nasal bone. A non-invasive prenatal diagnosis test based on next-generation sequencing (NGS), which consists of the analysis of cell-free fetal DNA in maternal blood, has recently been developed. This procedure is increasingly gaining importance in the screening for chromosomopathy, especially in trisomies 21, 18 and 13.5–8 All these screening procedures make it possible to select patients who require invasive procedures, such as amniocentesis and chorionic villus sampling, in a more appropriate manner. Amniocentesis should be directly indicated for patients over 35 years of age, in whom biochemical markers do not have a relevant use for predicting aneuploidy in products of conception. The objective of this study is to identify the use of the duo test in detecting numerical chromosomal abnormalities in patients under 35 years of age in a sample of Mexican women with high-risk pregnancy who came to the Hospital General de México. A high-risk pregnancy is defined as that in which there are conditions present which compromise the health or life of the pregnant mother and/or her baby.
Material and methodsThis project consisted of a descriptive, comparative study, with analysis, using a two-by-two table, between the duo test and amniocentesis. The determination of free βhGC and pregnancy-associated plasma protein (PAPP-A) in serum was performed using chemiluminescence on 30 pregnant women, under 35 years of age and at 11–13.6 weeks of pregnancy, who came to the Department of Genetics with a diagnosis of high-risk pregnancy essentially due to structural morphological abnormalities observed by ultrasound. The sonographic markers of chromosomopathies were used in accordance with the criteria of the Fetal Medicine Foundation. In all cases, the karyotype test was performed in amniotic fluid using amniocentesis between weeks 15 and 18 of pregnancy. 15ml of amniotic fluid supplemented with the culture medium, CHANG Medium® In Situ, was obtained and incubated in a CO2-enriched environment at 37°C. The GTG banding technique and a chromosome analysis using conventional methods were performed. The statistical analysis consisted of descriptive statistics and a two-by-two table to determine sensitivity and specificity.
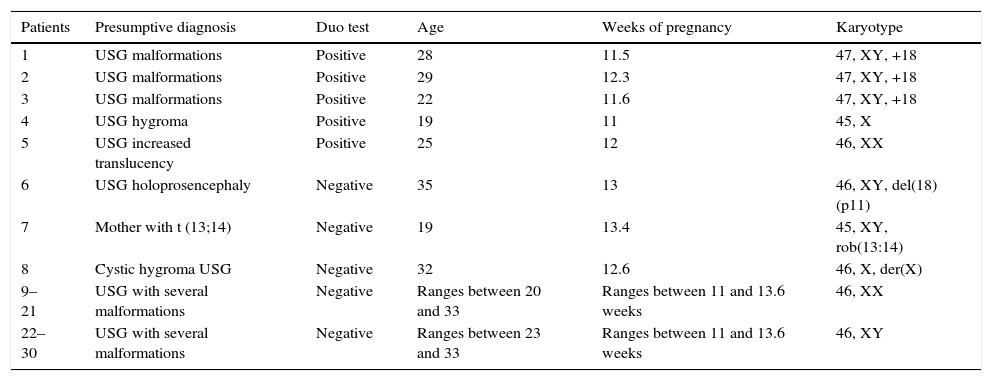
ResultsFive of the 30 duo tests performed on women with high-risk pregnancy showed an increased risk of chromosomopathy. Of the five cases, three showed a risk of trisomy 18, one of Down's syndrome (trisomy 21) and one of Turner syndrome (X chromosome monosomy). These conditions were confirmed in four of the five cases using cytogenetics: three of trisomy 18 and one of Turner syndrome; the other (potentially Down's syndrome) was a normal chromosomal fetus. Of the remaining patients with a normal duo test (n=25), three cases had structural abnormalities which included partial deletion of the short arm of chromosome 18, [46,XY, del(18)(p11)], Robertsonian translocation between chromosome 13 and 14 [45,XY, rob(13:14)] and a derived X chromosome X[46,X, der(X)]. It is worth mentioning that the duo test does not make it possible to differentiate between these types of structural chromosomal abnormalities. The sensitivity and specificity of the duo test with regard to the result of the karyotype is summarized in Table 1.
Patients with positive chromosomal abnormalities detected using cytogenetics and their relation with the analysis of the duo test.
| Patients | Presumptive diagnosis | Duo test | Age | Weeks of pregnancy | Karyotype |
|---|---|---|---|---|---|
| 1 | USG malformations | Positive | 28 | 11.5 | 47, XY, +18 |
| 2 | USG malformations | Positive | 29 | 12.3 | 47, XY, +18 |
| 3 | USG malformations | Positive | 22 | 11.6 | 47, XY, +18 |
| 4 | USG hygroma | Positive | 19 | 11 | 45, X |
| 5 | USG increased translucency | Positive | 25 | 12 | 46, XX |
| 6 | USG holoprosencephaly | Negative | 35 | 13 | 46, XY, del(18)(p11) |
| 7 | Mother with t (13;14) | Negative | 19 | 13.4 | 45, XY, rob(13:14) |
| 8 | Cystic hygroma USG | Negative | 32 | 12.6 | 46, X, der(X) |
| 9–21 | USG with several malformations | Negative | Ranges between 20 and 33 | Ranges between 11 and 13.6 weeks | 46, XX |
| 22–30 | USG with several malformations | Negative | Ranges between 23 and 33 | Ranges between 11 and 13.6 weeks | 46, XY |
USG, ultrasonography.
Prenatal analysis by means of a karyotype in amniotic fluid is a reliable method for checking for aneuploidy and other structural chromosomal abnormalities. It is a method which should be indicated directly for women over 35 years of age, given the increased risk of them having babies with numerical chromosomal abnormalities. As for women under 35 years of age, it is preferable that a non-invasive method be carried out initially, since performing an amniocentesis carries the risk of miscarriage (1 in 200).9 Some of the non-invasive procedures which are used between weeks 11 and 13.6 of pregnancy are structural ultrasound and determination of the biochemical markers, PAPP-A and free βhGC (known as the duo test). These two procedures have a detection range of up to 85% for chromosome aneuploidy. When a woman reaches weeks 15–18 of pregnancy, the quadruple marker test is used, which has a detection range of around 80%. The results obtained in this study, presented in a contingency table, show a sensitivity of 100% and a specificity of 96% among patients with karyotypes 47, XY, ±18 (trisomy 18) and the duo test (Table 2). However, no increased risk of chromosopathies in patients whose karyotype presented a structural abnormality was found. This means that it is not possible to rule out structural chromosomal abnormalities using the duo test determination. Furthermore, even when a duo test was found to be positive for trisomy 18 in a healthy patient, this was to be expected since the specificity of the duo test is estimated at 85%.10,11
There are currently other non-invasive techniques which enable the screening of another series of defects present in the fetus. These are not covered in this study. We therefore only mention these techniques, such as NanoString technology, BACs-on Beads (BoBs) technology and the cell-free fetal DNA analysis method.
In conclusion, in this study we have managed to identify an increased risk of having babies with numerical and structural chromosomal abnormalities in patients under 35 years of age using the duo test and amniocentesis. This denotes the importance of performing these types of studies in pregnant women regardless of their age, since offering an accurate diagnosis of the baby's normality gives a couple peace of mind during the pregnancy. If an abnormality is detected, genetic counseling will allow the couple to make the decision that is best for them.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.