CIWA-Ar score is the gold standard for stratifying severity of alcohol withdrawal syndrome (AWS).
AimTo identify the correlation between the different scales used in patients with AWS, and with the biochemical–haematological profile.
Material and methodsProspective, analytical observational study in 49 patients with AWS diagnosed between February 2011 and July 2012. Maddrey, Glasgow, MELD, CIWA-Ar scores and laboratory values were obtained at diagnosis.
ResultsThere was no correlation between CIWA-Ar and other scales (R2=0.001, p=0.823, 95%CI); however, a linear relationship (p=0.000, 95%CI) was found. On the correlation matrix, CIWA-Ar was not associated with any of the study parameters. There were no significant differences in length of stay when analyzed by type of treatment.
ConclusionCIWA-Ar is a good predictor of severity, albeit with no biochemical or hematic correlation.
El puntaje CIWA-Ar es el estándar de oro para estratificar la severidad del síndrome de supresión etílica (SSE).
ObjetivoIdentificar la correlación entre las diferentes escalas utilizadas en pacientes con SSE, así como con el perfil bioquímico-hemático.
Material y MétodosEstudio prospectivo, observacional-analítico, incluyendo 49 pacientes con SSE atendidos durante febrero 2011 y julio 2012. Se obtuvo el puntaje Maddrey, Glasglow, MELD, CIWA-Ar, y valores de laboratorios al momento del diagnóstico.
ResultadosNo se estableció correlación entre CIWA-Ar y las diversas escalas (R2=0.001, p=0.823, 95%IC), pero si entre ellas (p=0.000, 95%IC). En la matriz de correlación, CIWA-Ar no se asoció a ningún parámetro. No existieron diferencias significativas del tiempo de estancia al analizar por tipo de tratamiento.
ConclusiónCIWA-Ar es buen predictor de severidad, pero sin correlación bioquímica o hemática.
Alcohol withdrawal syndrome (AWS) is one of the main complications of alcohol abuse. Approximately 8% of all hospital admissions are due to manifestations or complications associated with AWS, and between 20% and 30% of patients are admitted to intensive care units.1 A number of mechanisms are involved in the physiopathology of AWS, including elevated catecholamine levels secondary to inhibition of presynaptic α 2 autoreceptor activity, homocysteine-mediated NMDA receptor over-stimulation, and diminished GABA receptor affinity.2 The first clinical manifestations of AWS, usually minor symptoms, such as tremor, anxiety, perspiration and palpitations, appear between 6 and 24h following the last intake of alcohol. Following this, symptoms increase and become more severe; some patients present delirium tremens, which are characterized by confusion, hallucinations, hypertension and fever.3 Most patients are treated in accordance with their symptoms, which are assessed using the CIWA-Ar (Clinical Institute Withdrawal Assessment for Alcohol, revised) protocol.4 The CIWA-Ar score is currently the gold standard for evaluating the manifestations and severity of AWS.5 Treatment is generally based on anticonvulsant, sympatholytic, and neuroleptic agents. Benzodiazepines, which reduce the risk of seizures, symptom severity, and the risk of developing delirium tremens, are still the treatment of choice.6,7 Haloperidol is the most commonly used neuroleptic, particularly in the presence of hallucinations.8
Particular care should be taken with patients presenting with AWS and a concomitant disease, such as pancreatitis, chronic obstructive pulmonary disease, or liver failure secondary to acute alcoholic hepatitis or cirrhosis, as any of these factors can increase the risk for complications and mortality.3,9 Patients with liver failure should be evaluated using the Glasgow Alcoholic Hepatitis score, and the MELD10 and/or Maddrey score (when liver failure is secondary to acute alcoholic hepatitis).11–14
Both MELD and Maddrey scores use biological markers synthetized in the liver to identify patients at greater risk for mortality.14,15 Zapata-Irissón et al., in a study comparing both scales in a Mexican population, conclude that both are equally effective for determining prognosis in clinical practice.15 As the CIWA-Ar is a subjective, clinical score, its relationship with the foregoing scales and with the patient's biochemical and haematology profile is unclear.
The aim of this study is to determine the correlation, if any, between the CIWA-Ar score and the MELD, Maddrey and Glasgow scores used to assess prognosis in patients with hepatic failure and AWS. We also evaluated the correlation between the CIWA-Ar score and blood count and biochemistry findings at the time of admission.
Patients, materials and methodsWe conducted an observational, prospective, descriptive and analytical study in a population of patients diagnosed with alcohol withdrawal syndrome admitted to the Internal Medicine Department of the General Hospital of Cuautitlán. All patients were initially examined in the emergency room. Patients requiring ventilatory support were excluded. The decision to start antipsychotic therapy or sedation was taken by the attending physician in each case. At admission, patients were assessed using the study scales and samples were drawn for biochemistry and complete blood count. All patients gave their informed consent at the time of admission.
TreatmentPatients were sedated with 10mg intravenous benzodiazepine (diazepam) every 4–8h, depending on individual requirements. Levomepromazine was administered intramuscularly at a dose of 25mg every 6–8h. Haloperidol was administered either intravenously or intramuscularly at a dose of 5mg every 6 or 8h until the psychotic episode was resolved. Patients presenting with seizures were treated with benzodiazepines. Steroids were only used in patients scoring higher than 32 on the Maddrey scale.
Statistical analysisSample size was calculated for a statistical power of 0.8 using Statistica version 7 (StatSoft, Inc., Tulsa, Oklahoma). The resulting size was 49 patients (95%CI, 2% margin of error). Statistical analysis was performed using IBM SPSS Statistics for Windows, version 20 (Armonk, New York, USA). First, descriptive statistical methods were used to calculate the frequency of qualitative variables and the mean values of quantitative variables. A correlation analysis was performed to compare the Maddrey, MELD and Glasgow scales with the CIWA-Ar assessment protocol. Following this, linear regression was used to analyze correlated variables. We also performed factor analysis on the 29 blood chemistry and complete blood count parameters to obtain a matrix of principal components using a Varimax rotation. Log10 transformation was used on all values to reduce variance. The nonparametric U-Mann Whitney test was used to determine the existence of a significant difference between mean CIWA-Ar scores at 24h after start of antipsychotic therapy and length (days) of hospital stay. Significance was set at p≤0.05 (95%CI). Finally, we compared mean hospital stay (days) of each subgroup with their Maddrey, MELD and CIWA-Ar scores.
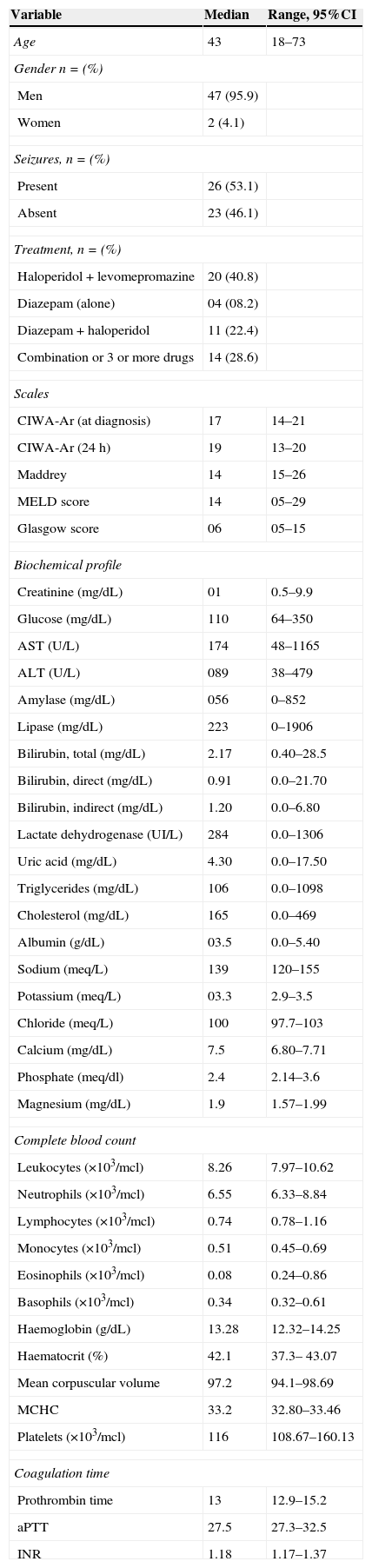
ResultsA total of 49 patients were included between February 2011 and July 2012; most were men (n=47, 95.9%). Mean time from last alcohol intake prior to hospitalization was 3.4 days (range 1–15 days). Approximately 46.9% (n=23) of patients presented with convulsions at the time of admission. Table 1 shows the general characteristics of the patients and the treatment given.
General characteristics of patients at the time of admission to the accident and emergency department.
| Variable | Median | Range, 95%CI |
|---|---|---|
| Age | 43 | 18–73 |
| Gender n=(%) | ||
| Men | 47 (95.9) | |
| Women | 2 (4.1) | |
| Seizures, n=(%) | ||
| Present | 26 (53.1) | |
| Absent | 23 (46.1) | |
| Treatment, n=(%) | ||
| Haloperidol+levomepromazine | 20 (40.8) | |
| Diazepam (alone) | 04 (08.2) | |
| Diazepam+haloperidol | 11 (22.4) | |
| Combination or 3 or more drugs | 14 (28.6) | |
| Scales | ||
| CIWA-Ar (at diagnosis) | 17 | 14–21 |
| CIWA-Ar (24h) | 19 | 13–20 |
| Maddrey | 14 | 15–26 |
| MELD score | 14 | 05–29 |
| Glasgow score | 06 | 05–15 |
| Biochemical profile | ||
| Creatinine (mg/dL) | 01 | 0.5–9.9 |
| Glucose (mg/dL) | 110 | 64–350 |
| AST (U/L) | 174 | 48–1165 |
| ALT (U/L) | 089 | 38–479 |
| Amylase (mg/dL) | 056 | 0–852 |
| Lipase (mg/dL) | 223 | 0–1906 |
| Bilirubin, total (mg/dL) | 2.17 | 0.40–28.5 |
| Bilirubin, direct (mg/dL) | 0.91 | 0.0–21.70 |
| Bilirubin, indirect (mg/dL) | 1.20 | 0.0–6.80 |
| Lactate dehydrogenase (UI/L) | 284 | 0.0–1306 |
| Uric acid (mg/dL) | 4.30 | 0.0–17.50 |
| Triglycerides (mg/dL) | 106 | 0.0–1098 |
| Cholesterol (mg/dL) | 165 | 0.0–469 |
| Albumin (g/dL) | 03.5 | 0.0–5.40 |
| Sodium (meq/L) | 139 | 120–155 |
| Potassium (meq/L) | 03.3 | 2.9–3.5 |
| Chloride (meq/L) | 100 | 97.7–103 |
| Calcium (mg/dL) | 7.5 | 6.80–7.71 |
| Phosphate (meq/dl) | 2.4 | 2.14–3.6 |
| Magnesium (mg/dL) | 1.9 | 1.57–1.99 |
| Complete blood count | ||
| Leukocytes (×103/mcl) | 8.26 | 7.97–10.62 |
| Neutrophils (×103/mcl) | 6.55 | 6.33–8.84 |
| Lymphocytes (×103/mcl) | 0.74 | 0.78–1.16 |
| Monocytes (×103/mcl) | 0.51 | 0.45–0.69 |
| Eosinophils (×103/mcl) | 0.08 | 0.24–0.86 |
| Basophils (×103/mcl) | 0.34 | 0.32–0.61 |
| Haemoglobin (g/dL) | 13.28 | 12.32–14.25 |
| Haematocrit (%) | 42.1 | 37.3– 43.07 |
| Mean corpuscular volume | 97.2 | 94.1–98.69 |
| MCHC | 33.2 | 32.80–33.46 |
| Platelets (×103/mcl) | 116 | 108.67–160.13 |
| Coagulation time | ||
| Prothrombin time | 13 | 12.9–15.2 |
| aPTT | 27.5 | 27.3–32.5 |
| INR | 1.18 | 1.17–1.37 |
AST, aspartate aminotransferase; ALT, alanine-aminotransferase; MCHC, mean corpuscular haemoglobin concentration; aTTP, activated partial thromboplastin time; INR, international normalized ratio.
The first step consisted in establishing whether a correlation existed between the main scales used in patients with AWS and liver failure. A direct correlation was found between the Maddrey scale and the MELD and Glasgow scales (p=0.000, 95%CI). There was no correlation between severity assessed on the CIWA-Ar scale and the Maddrey, MELD and Glasgow scales (Pearson correlation=0.033, −0.08, 0.029; p=0.823, 0.955, 0.842, respectively). Regression analysis showed that the Maddrey score at diagnosis has no correlation with the CIWA-Ar score (R2=0.001, p=0.823, 95%CI); the same was true of the Glasgow score (R2=0.001).
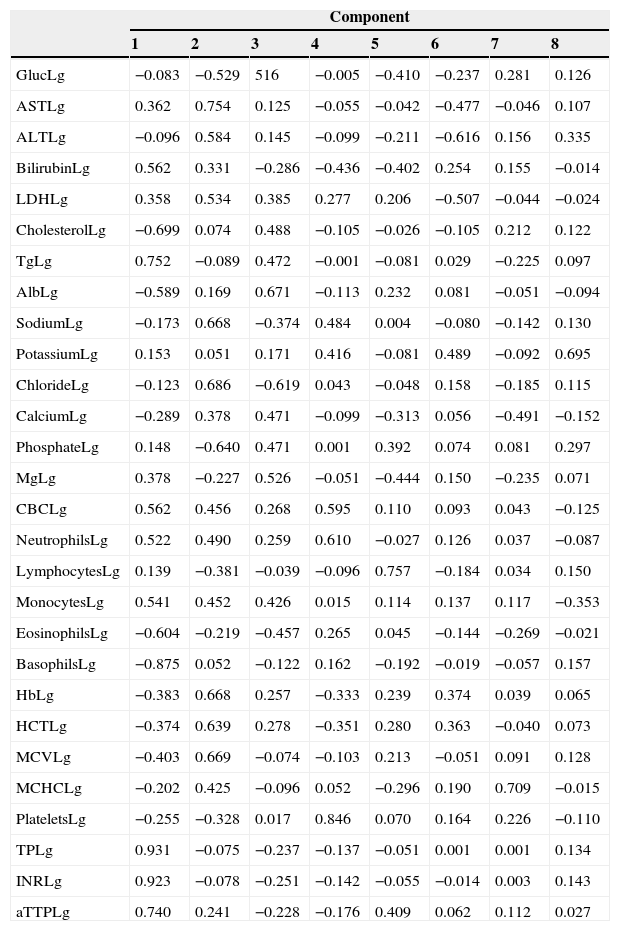
Factor analysis of the biochemical and haematology profileFactor analysis was performed using biochemical and haematology profiles determined at the time of diagnosis. The result of the components is shown in Table 2. We extracted 8 components, the principal ones being component 1 (glucose, bilirubin, cholesterol, triglyceride, albumin, leucocyte count, neutrophil count, monocytes, eosinophils, prothrombin time, INR, and activated partial thromboplastin time) and component 2 (AST, ALT, LDH, sodium, chloride, phosphate, haemoglobin, haematocrit, and mean corpuscular volume). The remaining components only include a few isolated variables (magnesium, platelet count, and calcium). When the CIWA-Ar score was added to the component matrix, it was placed in component 7, together with mean corpuscular haemoglobin concentration (MCHC) and calcium.
Components matrix of biochemical profile and blood count of patients with alcohol withdrawal syndrome at diagnosis.
| Component | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| GlucLg | −0.083 | −0.529 | 516 | −0.005 | −0.410 | −0.237 | 0.281 | 0.126 |
| ASTLg | 0.362 | 0.754 | 0.125 | −0.055 | −0.042 | −0.477 | −0.046 | 0.107 |
| ALTLg | −0.096 | 0.584 | 0.145 | −0.099 | −0.211 | −0.616 | 0.156 | 0.335 |
| BilirubinLg | 0.562 | 0.331 | −0.286 | −0.436 | −0.402 | 0.254 | 0.155 | −0.014 |
| LDHLg | 0.358 | 0.534 | 0.385 | 0.277 | 0.206 | −0.507 | −0.044 | −0.024 |
| CholesterolLg | −0.699 | 0.074 | 0.488 | −0.105 | −0.026 | −0.105 | 0.212 | 0.122 |
| TgLg | 0.752 | −0.089 | 0.472 | −0.001 | −0.081 | 0.029 | −0.225 | 0.097 |
| AlbLg | −0.589 | 0.169 | 0.671 | −0.113 | 0.232 | 0.081 | −0.051 | −0.094 |
| SodiumLg | −0.173 | 0.668 | −0.374 | 0.484 | 0.004 | −0.080 | −0.142 | 0.130 |
| PotassiumLg | 0.153 | 0.051 | 0.171 | 0.416 | −0.081 | 0.489 | −0.092 | 0.695 |
| ChlorideLg | −0.123 | 0.686 | −0.619 | 0.043 | −0.048 | 0.158 | −0.185 | 0.115 |
| CalciumLg | −0.289 | 0.378 | 0.471 | −0.099 | −0.313 | 0.056 | −0.491 | −0.152 |
| PhosphateLg | 0.148 | −0.640 | 0.471 | 0.001 | 0.392 | 0.074 | 0.081 | 0.297 |
| MgLg | 0.378 | −0.227 | 0.526 | −0.051 | −0.444 | 0.150 | −0.235 | 0.071 |
| CBCLg | 0.562 | 0.456 | 0.268 | 0.595 | 0.110 | 0.093 | 0.043 | −0.125 |
| NeutrophilsLg | 0.522 | 0.490 | 0.259 | 0.610 | −0.027 | 0.126 | 0.037 | −0.087 |
| LymphocytesLg | 0.139 | −0.381 | −0.039 | −0.096 | 0.757 | −0.184 | 0.034 | 0.150 |
| MonocytesLg | 0.541 | 0.452 | 0.426 | 0.015 | 0.114 | 0.137 | 0.117 | −0.353 |
| EosinophilsLg | −0.604 | −0.219 | −0.457 | 0.265 | 0.045 | −0.144 | −0.269 | −0.021 |
| BasophilsLg | −0.875 | 0.052 | −0.122 | 0.162 | −0.192 | −0.019 | −0.057 | 0.157 |
| HbLg | −0.383 | 0.668 | 0.257 | −0.333 | 0.239 | 0.374 | 0.039 | 0.065 |
| HCTLg | −0.374 | 0.639 | 0.278 | −0.351 | 0.280 | 0.363 | −0.040 | 0.073 |
| MCVLg | −0.403 | 0.669 | −0.074 | −0.103 | 0.213 | −0.051 | 0.091 | 0.128 |
| MCHCLg | −0.202 | 0.425 | −0.096 | 0.052 | −0.296 | 0.190 | 0.709 | −0.015 |
| PlateletsLg | −0.255 | −0.328 | 0.017 | 0.846 | 0.070 | 0.164 | 0.226 | −0.110 |
| TPLg | 0.931 | −0.075 | −0.237 | −0.137 | −0.051 | 0.001 | 0.001 | 0.134 |
| INRLg | 0.923 | −0.078 | −0.251 | −0.142 | −0.055 | −0.014 | 0.003 | 0.143 |
| aTTPLg | 0.740 | 0.241 | −0.228 | −0.176 | 0.409 | 0.062 | 0.112 | 0.027 |
Alb, albumin; ALT, alanine aminotransferase; aPPTa, activated partial thromboplastin time; AST, aspartate aminotransferase; CBC, complete blood count; Gluc, glucose; Hb, haemoglobin; HTC, haematocrit; INR, international normalized ratio; LDH, lactate dehydrogenase; Lg, logarithm; Mg, magnesium; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; PT, prothrombin time; Tg, triglycerides.
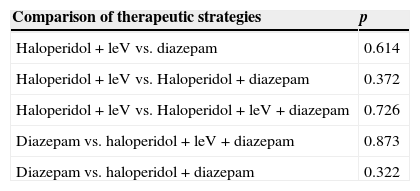
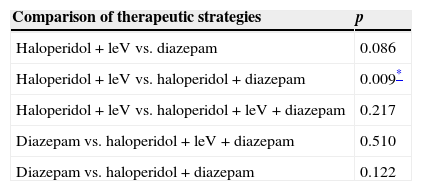
There was no difference between mean CIWA-Ar score at admission and at 24h in any of the 4 different therapeutic strategies used (Table 3). Mean length of stay was 6 days (6.1–8.7, 95%CI). A significant difference in this parameter was only found between patients treated with haloperidol+levomepromazine and those given haloperidol+diazepam (p=0.009, 95%CI) (Table 4). There was no difference in length of stay between patients with a Maddrey score of < or > 32 (p=0.665, 95%CI) and those with a MELD score of < or > 21 (p=0.807, 95%CI). However, difference in length of stay between patients with a CIWA-Ar score of < 8 and > 20 (p=0.028) was significant, although this was not the case in scores between 9 and 20 points (p=0.984). Mortality rate during follow-up was 6.1% (n=3).
Differences in mean CIWA-Ar scores at diagnosis and at 24h between different drug therapies.
| Comparison of therapeutic strategies | p |
|---|---|
| Haloperidol+leV vs. diazepam | 0.614 |
| Haloperidol+leV vs. Haloperidol+diazepam | 0.372 |
| Haloperidol+leV vs. Haloperidol+leV+diazepam | 0.726 |
| Diazepam vs. haloperidol+leV+diazepam | 0.873 |
| Diazepam vs. haloperidol+diazepam | 0.322 |
LeV, levomepromazine.
Difference in mean hospital stay between each drug therapy.
| Comparison of therapeutic strategies | p |
|---|---|
| Haloperidol+leV vs. diazepam | 0.086 |
| Haloperidol+leV vs. haloperidol+diazepam | 0.009* |
| Haloperidol+leV vs. haloperidol+leV+diazepam | 0.217 |
| Diazepam vs. haloperidol+leV+diazepam | 0.510 |
| Diazepam vs. haloperidol+diazepam | 0.122 |
LeV, levomepromazine.
Correlation analysis identified a positive relationship between Maddrey, MELD and Glasgow scores. This is similar to the findings of Ali et al. in a study comparing Maddrey, Child–Pugh and Glasgow scores in respect of mortality at 28 days.16 Palaniyappan found similar results after creating a ROC curve at 30 and 90 days, 6 months, and 1 year to evaluate the prognostic utility of 5 of the most commonly used liver failure scales. In this case, the only exception was the Child–Pugh score, which failed to predict mainly short-term mortality.17 Adding CIWA-Ar score to the model showed no correlation with any of the study scales. This could be due to the fact that the CIWA-Ar scale mainly evaluates clinical manifestations, not biochemical parameters.
Factor analysisFactor analysis also failed to determine correlation between scores and biochemical parameters or blood count in the component matrix. As in the foregoing case, we believe that the absence of correlation is due to lack of relationship between AWS severity, mainly in early events, and existing liver failure, which affects liver synthesis tests. Correlation was only established between CIWA-Ar score and various polymorphisms, mainly oxidative stress markers such as malondialdehyde (MDA) and superoxide dismutase (SOD).18
Differences between therapeutic strategiesNo difference was found between the 4 therapies used in terms of mean hospital stay. Considering that 3 of the treatments administered included benzodiazepines, we found no evidence that length of stay was shortened when other therapeutic strategies were combined with these drugs. This is consistent with the evidence in the literature, where both symptom-triggered and fixed-dose benzodiazepines (mainly diazepam) are the treatment of choice.19,20 According to a Cochrane review of 64 studies (4309 patients), benzodiazepines protected against specific AWS manifestations, particularly seizures, when compared with placebo and even with other sedatives. This is why they continue to be the first line therapy in these patients.21,22 We also compared drug groups in terms of length of hospital stay. In this regard, we found no differences between benzodiazepines alone or in combination with one or more sedative or neuroleptic agents. Jaeger et al. reported similar findings with symptom-triggered benzodiazepines. They found no difference between the on-demand and fixed-dose group in terms of length of stay (days), seizures or delirium tremens).23 Haloperidol was only used in patients presenting with delirium tremens. Several studies have reported the benefit of this drug in controlling hallucinations, mainly when administered on the basis of symptom-orientated dose adjustment.24–27 Administration of benzodiazepines in combination with other drugs is not a novel approach in AWS,28 particularly diazepam+halopediol;25 however, this combination has not been shown to be superior to benzodiazepines alone.29 Levomepromazine is usually reserved for patients presenting with a schizophrenic break.30,31 Its usefulness in AWS therapy is based mainly on anecdotal findings. In our experience, combination therapy with haloperidol or diazepam gave similar results, both in terms of CIWA-Ar score at 24h and length of hospital stay, with no significant adverse events.
MortalityThe mortality rate was 6.1% overall, with no difference in the number of deaths in respect of severity on the CiWA-Ar (Log Rank [Mantel–Cox], 0.52), MELD score (Log Rank [Mantel–Cox], 0.973) and Maddrey (Log Rank [Mantel–Cox], 0.978) scores. Mortality was low in our cohort, a finding consistent with other similar series. Nevertheless, mortality was higher in patients requiring admission to intensive care units (1–60% of admissions to intensive care units).32
The results presented and discussed here were obtained from a series of patients admitted to the Hospital General in Cuautitlán, a referral hospital for Mexico State treating patients not eligible for national health insurance. The social and demographic characteristics of this population do not necessarily reflect those of the Mexican population as a whole, and our results could be biased by variables beyond our control and inherent to the type of population treated. These variables include age (mainly young adults), low socioeconomic level (which could be a risk factor for greater metabolic susceptibility due to malnutrition),33,34 to name just a few. As this study was not designed as a controlled clinical trial, we were unable to apply better selection criteria to study subjects. An interesting aspect to explore in future studies on this topic would be the possible correlation between length of alcohol addiction or number of prior AWS episodes and treatment outcomes.
In conclusion, alcohol withdrawal syndrome is one of the principal diseases associated with alcohol abuse. Clinical scales such as CIWA-Ar are useful for determining the appropriate sedation protocol, but show no correlation with other assessment scales or with the patient's biochemical profile or blood count. We believe that benzodiazepines should continue to be the treatment of choice. The addition of other neuroleptic drugs had no effect on the length of hospital stay or the degree of agitation. Researchers should continue to search for new strategies or explore the use of new drugs, such as 5-HT receptors blockers.
Conflict of interestThe authors declare that they have no conflict of interests.